Abstract
Enhanced understanding of the rheumatoid arthritis (RA) pathophysiology and the role of cytokines has enabled the development of innovative biological agents in the last 10 years that target specific parts of the immune response. Failure to achieve adequate response with traditional disease modifying anti-rheumatic drugs (DMARDs) and increasing evidence of ongoing radiographic deterioration of the affected joints despite seemingly clinical response were essential stimuli for the development of biologics. The current and upcoming biological agents are primarily aimed at neutralizing circulating and cell-bound pro-inflammatory cytokines, interfering in the interaction of antigen-presenting and T-lymphocytes, eliminating circulating B-lymphocytes or by interfering with the intracellular signaling mechanisms of immuno-competent cells that lead to inflammation. These agents have improved the currently available treatments due to greater efficacy, fast action and greater tolerability. However, use of these agents has also been associated with significant, although rare, adverse events and considerable cost. Therefore, these agents should be used with caution by experienced clinicians. The present work aims to provide a global and updated review of the current and in-development biological DMARDs for the treatment of RA.
Introduction
The introduction of biological agents has dramatically altered the therapy for patients with rheumatoid arthritis (RA). Advances in the current knowledge of cytokine milieu in RA pathogenesis have contributed to the development of biological agents, and translated research findings into clinical practice.
Well established currently available biological agents include three tumor necrosis factor alpha (TNF-α) inhibitors (infliximab, etanercept, adalimumab), an interleukin-1 (IL-1) receptor antagonist (anakinra), a B-cell-depleting agent (rituximab), and an inhibitor of T-cell costimulation (abatacept). TNF inhibitors were the first biologics to be added to the therapeutic arsenal; more recently the US Food and Drug Administration (FDA) approved biologic agents with different modes of action. TNF inhibitors have proved to be very effective in patients not responding to traditional disease-modifying anti-rheumatic drugs (DMARDs). However, about 20% to 40% of patients treated with a TNF inhibitor fail to achieve a 20% improvement in American College of Rheumatology (ACR20) criteria, and more lose response over time, due to secondary failure or acquired therapeutic resistance and some experience adverse events following treatment with a TNF inhibitor.Citation1 Two recently approved agents, certolizumab pegol and golimumab, have increased the number of available choices in the already existing class of TNF inhibitors.
Recently, several new agents have been reported to be in various stages of development and, if approved by regulatory authorities, may cause a major shift in the therapeutic paradigm of RA.
This paper provides a comprehensive review of the current and in-development biological DMARDS.
Methods
Information was derived from PubMed, and the clinical trials registered in Clinicaltrials.gov and database of systematic reviews and relevant congress abstracts up to and including May 2009. We systematically reviewed all the published and ongoing randomized controlled trials, to evaluate the efficacy, safety, and tolerability of the current and in-development biologics. Animal studies were excluded. The primary measure of outcome evaluated in most of the studies included improvement according to the ACR20 criteria, 28-joint Disease Activity Score (DAS28) score, Health Assessment Questionnaire (HAQ) score, and health related quality of life (HRQoL). Evidence of effectiveness has been summarized using the primary end points used in the studies identified. In addition, the most frequently reported adverse events have also been weighed against the benefits of the current and upcoming biologics.
No external sponsor was involved in this study. No persons other than the authors of this manuscript were involved in the design, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
First-generation TNF-α inhibitors
Infliximab (Remicade®)
Infliximab is a chimeric antibody that binds both transmembrane and circulating TNF-α. Its half-life of 8 to 10 days prompts its administration every 4 to 8 weeks at a dose of 3 to 10 mg/kg infused intravenously. Based on the ATTRACT trial which included 428 patients resistant to MTX (MTX) with a mean disease duration of 11 years, infliximab proved to be beneficial when used in combination with MTX as it halted progression of joint damage both in clinical responders and non-responders. An obvious mechanism justifying the results is the hindering of TNF action on bone resorption and the blunting of TNF effect on synthesis of cartilage proteoglycan.Citation2 Moreover, it was recently shown that impedance of joint damage in RA can also be attributed to infliximab’s effect on decreasing synovial infiltrates early after initiation of treatment by inhibiting cell migration and not by inducing apoptosis.Citation3 Subgroup analysis of ATTRACT trial showed that radiographic stabilization of the disease was evident even in patients with early RA (ie, less than 3 years).Citation4,Citation5 Furthermore, greater baseline joint damage correlated with poorer physical function at baseline and less improvement in physical function after treatment suggesting that early intervention may be required. This question was to be addressed by the ASPIRE trial that included 1004 patients with disease duration less than 3 years. Indeed, infliximab infusion along with MTX in a treatment naïve population improved clinical signs and symptoms, functional outcomes and prevented structural damage of the joints significantly more than MTX monotherapy.Citation4
An important Dutch study (BeSt), examined the efficacy of initial combination therapy versus monotherapy, involving 508 patients with newly diagnosed RA and comparing different treatment strategies. Sequential monotherapy (Group 1) and step-up combination therapy (Group 2), both starting with MTX, were compared with initial combination therapy consisting of a tapered high-dose prednisone, MTX, and sulfasalazine (SSA) (Group 3) and with initial combination therapy consisting of MTX and infliximab (Group 4). After 2 years of treatment, the goal of a DAS44 2.4 was reached by 82% in infliximab group comparing to 75% of patients in group 1, 81% in group 2, and 78% in group 3. More patients in combination groups 3 and 4 had been able to taper and discontinue drugs of the initial combination therapy because of continuous low disease activity, given 54% of patient receiving infliximab + MTX combination therapy tapered their treatment to monotherapy, compared to 36% in prednisone/MTX/SSA group. After 2 years of treatment, 80% of all patients achieved the goal of DAS 2.4, and 42% reached clinical remission (DAS < 1.6).Citation6
Another randomized controlled trial (RCT) in 20 patients with poor prognosis RA identified by Persistent Inflammatory Symmetrical Arthritis (PISA) score system and who had symptoms less than 1 year demonstrated a beneficial effect of infliximab and MTX introducing the concept of remission induction and maintenance therapy borrowed by oncology treatments.Citation7 Furthermore, this study successfully demonstrated an arrest of the inflammatory bone loss in this patient population.Citation8
Etanercept (Enbrel®)
Etanercept is a fully human, soluble fusion protein created by the linkage of two ligand binding regions of p75 TNF-α receptor and the Fc portion of human IgG1, and possess the shortest half life (3 to 4 days) of all the TNF-α inhibitors. Etanercept has unique properties that distinguish it from infliximab and adalimumab. In contrast to other anti-TNF agents, it also binds lymphotoxin-alpha (otherwise known as TNF-β) which has been associated with tumor growth control independent of TNF activity.Citation9 In addition it does not lyse the cells expressing transmembrane TNF in the presence or absence of complement. Etanercept was approved by the FDA for use in the treatment of adult RA in 1998. Although frequently administered along with MTX in the clinical practice, etanercept has been approved as monotherapy and does not have to be co-administered with MTX.Citation10
Moreland et al first evaluated the efficacy of etanercept monotherapy in a phase II RCT, recruiting 180 patients with refractory RA for 3 months. A dose dependent reduction in disease activity was seen, with 75% of the high dose group achieving ACR20 responses as compared to 14% in the placebo group.Citation11 Subsequently, the results were confirmed by a phase III trial comparing two doses of etanercept (10 and 25 mg sc, twice weekly). Both doses proved to be more effective than placebo and the 25 mg dose was more effective than the 10 mg dose.Citation12
Combination of MTX and etanercept in active early RA (COMET) trial compared remission and radiographic non-progression in patients treated with MTX monotherapy or with MTX plus etanercept. The observed analysis suggested 50% of the patients on combination therapy with etanercept and MTX successfully achieved clinical remission of the primary endpoint (DAS28 score) as compared to 28% taking MTX alone. Furthermore, the halting of radiographic progression was seen in 80% patients on combination therapy, compared to 59% patients taking MTX monotherapy.Citation13
In one of the major biologic trials (TEMPO), a double blind, parallel-group, global study, 686 subjects were randomized to etanercept (25 mg twice weekly), MTX (up to 20 mg/week) or a combination of etanercept and MTX. Primary radiographic end point was change in the van der Heijde-modified total Sharp Score (TSS) at 52 weeks. Secondary radiographic endpoints were: changes in total erosions, changes in total joint space narrowing, number of eroded joints, non-progression (TSS change ≤0.5 and ≤3.0) and progression greater than the smallest detectable difference. Two observers, blinded for the sequence of the films, treatment mode and patient identity scored each X-ray.
Results of the TEMPO trial demonstrated that 74.2% RA patients treated with etanercept plus MTX experienced no progression of joint damage. In comparison, only 65.5% and 59.2% of etanercept monotherapy and MTX monotherapy-treated patients respectively, had no radiographic progression of joint damage at 2 years.Citation14
Adalimumab (Humira®)
Adalimumab is a recombinant human IgG1 monoclonal antibody specific for human TNF-α. It not only inhibits the binding of TNF-α to its receptors, but also lyses cells expressing membrane bound TNF-α in the presence of complement. Adalimumab has an estimated half life of 6 to 14 days and can be used as monotherapy or in combination with MTX for RA.Citation10
These results are also supported by a prospective single-arm intervention study, wherein 59 patients with established RA treated with fortnightly injections of subcutaneous 40 mg adalimumab for 6 months reported significant improvements in the following: perceived work ability [Work Ability Index (WAI)], quality of life [Rheumatoid Arthritis Quality of Life instrument (RAQoL)], and fatigue [Checklist Individual Strength (CIS)].Citation15
A 2-year double-blind RCT (PREMIER) evaluated the efficacy of combination therapy of adalimumab plus MTX against MTX or adalimumab monotherapy in 799 patients with early active RA. Results after 1 year of therapy demonstrated achievement of ACR50 responses in 62% of patients treated with combination therapy as compared to 46% and 41% of patients receiving MTX or adalimumab monotherapy respectively (P < 0.001). Furthermore, after 2 years of treatment, 49% of patients receiving combination therapy achieved disease remission (DAS28 < 2.6). Adalimumab in combination with MTX was also found to be more effective than either monotherapy in slowing the radiographic disease progression.Citation16 The superior efficacy of adalimumab plus MTX over MTX monotherapy has also been demonstrated in a recent double-blind RCT in Taiwanese patients with active RA.Citation17
The ARMADA trial, a 6 month placebo controlled, phase II/III study with 271 enrollees, demonstrated significant reductions in the signs and symptoms of RA, improvement in physical function, and the safety of adalimumab plus MTX vs placebo plus MTX. At 24 weeks, the combination treatment arm (adalimumab plus MTX) had significant higher ACR responses (ACR20: 67%, ACR50: 55%, and ACR70: 27%) compared with 15%, 8%, and 5%, respectively, in patients who had received placebo + MTX (P < 0.001).Citation18
In conclusion, adalimumab demonstrated significant and sustained reduction in signs and symptoms, inhibition of radiographic progression, but and also improved functional status, quality of life and work productivity in patients with RA.
Newly approved TNF-α inhibitors
Certolizumab pegol (Cimzia®)
Certolizumab is the first and only pegylated Fc-free anti-TNF agent which possesses a unique structure that does not include a crystallizable fragment (Fc) portion present in the other anti-TNFα agents, and have a unique way of signaling through the membrane TNF. Unlike other TNF-a inhibitors (infliximab, adalimumab, etanercept), which contain an Fc region, certolizumab is not capable of mediating antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).Citation19
Efficacy in RA has been shown, when used as an add-on therapy to MTX, providing long-term improvement in physical function, HRQoL, and pain relief. It has been evaluated by two phase 3, double-blind RCTs.Citation20,Citation21 Smolen et al followed 619 patients for a period of 24 weeks and noticed that the patients in certolizumab pegol 200- and 400-mg groups achieved ACR20 response rates of 57.3% and 57.6%, respectively vs 8.7% in placebo.Citation20 Another double-blind RCT by Keystone et al recruited 982 patients for 52 weeks, and showed that ACR20 response rates in groups receiving 200 mg and 400 mg of certolizumab pegol were 58.8% and 60.8%, respectively, compared with 13.6% for placebo in patients who had previously failed to respond to MTX. The trial also showed that the drug had slowed mean radiographic progression from baseline by week 52, and improved physical function as early as week 1.Citation21
Recently, the FAST4WARD study demonstrated the efficacy and safety of 400 mg certolizumab monotherapy given every 4 weeks, in 220 patients previously failing ≥1 DMARD therapy. The ACR20 response rate achieved after 24 weeks was 45.5% in certolizumab group as compared to 9.3% in the placebo group (P < 0.001). Other significant outcomes achieved during the study include ACR50, DAS28(ESR)3 scores.Citation22
Although the efficacy profile of certolizumab appears to be at par with other TNF inhibitors, serious adverse events are not unusual, infections being the most frequent. Among the most frequent serious infectious adverse events were lower respiratory infection, gastroenteritis, urinary tract infections, and reactivation of tuberculosis.Citation21
Golimumab (Simponi®)
Golimumab is similar in structure to infliximab except that it has been engineered to be fully human and is given in the dose of 50 mg as once-monthly subcutaneous injection.
The efficacy of golimumab has been evaluated through phase III clinical trials in the treatment of patients with active RA despite MTX therapy. Investigators observed; the patients receiving 100 mg golimumab + MTX or 50 mg golimumab + MTX achieved 56.2% and 55.1% ACR20 response rates, respectively as compared to 44.4% in patients receiving 100 mg golimumab plus placebo.Citation23 The addition of golimumab to MTX significantly reduced the signs and symptoms of RA and improved physical function.
The efficacy of golimumab plus MTX was confirmed in a multicenter, double-blind, placebo-controlled, dose-ranging RCT involving 172 patients who were randomly assigned to receive placebo plus MTX or 50 mg or 100 mg golimumab every 2 or 4 weeks plus MTX through week 48. At week 16, 61% of patients in the combined golimumab plus MTX groups achieved ACR20 response as compared to 37% of patients in the placebo plus MTX group (P = 0.010) and 79% of patients in the group receiving 100 mg golimumab every 2 weeks achieved ACR20 response (P = 0.001).Citation24
Findings from 2 phase III clinical trials presented at the EULAR Annual Conference of Rheumatology provide data for the efficacy of golimumab in RA. The GO-AFTER trial evaluated the efficacy of golimumab in 461 patients with active RA, who were previously treated with TNF inhibitors, but where TNF inhibitors were discontinued due to lack of efficacy, intolerance or other reasons. At week 14, 35% and 38% of patients receiving 50 mg and 100 mg golimumab, respectively, achieved the primary endpoint of ACR 20 improvement compared with 18% of patients receiving placebo (P < 0.001). At week 24, 52% of golimumab-treated patients experienced clinically relevant improvement (increase in HAQ score of at least 0.25 from baseline) compared with 34% of placebo-treated patients (P < 0.001). Also at week 24, patients receiving golimumab experienced a mean improvement in HAQ of 0.27 ± 0.51, compared with an improvement of 0.05 ± 0.51 among patients receiving placebo (P < 0.001). Importantly, among patients whose prior anti-TNF therapy was discontinued due to lack of efficacy, golimumab-treated patients experienced a mean improvement of 0.23 ± 0.50 in HAQ, compared with an average improvement of 0.06 ± 0.51 for patients receiving placebo (P < 0.05).Citation25
Table 1 TNF-α inhibitors in rheumatoid arthritis (RA)
In the GO-FORWARD trial, involving 444 patients, 50 mg and 100 mg monthly doses of golimumab were evaluated in patients who had active RA and were previously treated with MTX. Patients in the active treatment group achieved higher ACR 20 response at week 14 and significantly higher improvement of physical function at week 24 (HAQ 0.46 ± 0.53 vs 0.13 ± 0.58 [P < 0.001]).Citation25
The combination of golimumab and MTX appears to be generally well tolerated, with most adverse events ranging mild to moderate in severity. The most common adverse effects of golimumab therapy include nausea, headache and injection site reactions. Pneumonia was the most common serious adverse event observed in the patients receiving golimumab. Skin malignancy risk is elevated in golimumab-treated patients, as is with other TNF inhibitors.Citation24
The current data show that treatment with golimumab may induce an important depth of response, improving multiple aspects of RA and leading to significant decreases in disease activity. However, the efficacy of golimumab has not been tested against other TNF inhibitors in the existing studies. Thus, the appeal of golimumab in an already crowded arena will probably be as a self-injectable, fully humanized molecule given monthly. Notably, the currently approved dose in the US is 50 mg and, therefore, efficacy and safety data extrapolated from the clinical trials should correspond to this particular dose.
Safety and tolerability of TNF inhibitors
TNF inhibitors have been indicated for the treatment of RA for more than 10 years and, as a result, they have a well established safety profile.
There is growing evidence that TNF inhibition is associated with serious infections, and clearly an impairment of host defense mechanism to fight gram-positive, gram-negative bacteria, and less common pathogens causing opportunistic infections. Of particular concern are multiple reports of reactivation of Mycobacterium tuberculosis latent infections. Serious bacterial infections have been reported, including 2 fatal cases of pneumococcal sepsis and necrotizing fasciitis, and multiple cases of listeriosis (mostly with infliximab therapy).Citation10
A number of studies have attempted to determine the incidence of tuberculosis in RA. As investigated in Korean population, the risk of tuberculosis was 8.9-fold higher for patients with RA and 30.1-fold higher for patients with RA treated with infliximab; etanercept use was not associated with any increased risk of tuberculosis above that seen in the general RA population.Citation26 In lieu of these safety concerns, many experts recommend vigilant monitoring for the development of tuberculosis while on anti-TNF therapy and preventive measures. Evidence for an increased risk for serious infections and a dose dependent increase in malignancies was identified in systematic review and meta-analysis of nine randomized controlled trials of infliximab and adalimumab in patients with RA.Citation27 Reports have also shown an increased mortality rate in RA patients with congestive heart failure NYHA III/IV treated with TNF inhibitors (especially infliximab).Citation10,Citation28
Evidence of invasive fungal infections (IFIs) also exists in association with the use of TNF inhibitors. As reported by Tsiodras et al, 80% of cases of IFIs were associated with infliximab, 16% with etanercept, and 4% with adalimumab; and the most prevalent IFIs identified were histoplasmosis (30%), candidiasis (23%), and aspergillosis (23%).Citation29 The increased susceptibility to IFIs was thought to be attributed to the inhibition of IFN-γ production, decreased expression of pattern-recognition receptors, and leukocyte apoptosis.Citation30 Therefore a high index of suspicion and increased surveillance of IFIs complicating TNF blockade is recommended because the course of such infections can be serious or fulminant, and rapid access to health care should be provided.
Recently Carter et al evaluated the safety profile of TNF inhibitors in pregnancy.Citation31 Their report suggested that 59% of children born to mothers taking TNF inhibitors had one or more congenital anomalies that are part of vertebral abnormalities, anal atresia, cardiac defect, tracheoesophageal, renal, and limp abnormalities (VACTERL) association and the most common reported congenital anomaly was some form of heart defect. However, in an abstract presented at the 2008 ACR scientific meeting, none of the fetuses exposed to infliximab in utero presented with VATER (VACTERL) malformations.Citation32
Similarly, in another two 2008 ACR presentations on the pregnancy outcomes in women exposed to adalimumab (119 women) and etanercept (154 women) respectively, no concerns were raised regarding increased risks for specific pregnancy related outcomes.Citation33,Citation34
Ongoing registries, such as the one kept by the Organization of Teratology Information Specialists (OTIS), will be able to address the issue of safety in pregnancy more definitively in the near future. Certolizumab, one of the newer biologics, has the theoretical advantage of lacking an Fc portion, which is necessary for transport through the placenta, and it remains to be proven whether it may be safer for women trying to conceive.
TNF inhibitors have also been associated with the development of autoimmune diseases like systemic lupus erythematosis, lupus-like syndrome, cutaneous vasculitis, interstitial lung disease, and Behcet’s disease.Citation35,Citation36 Possible mechanisms that may induce antibody-production by TNF-α inhibitors include dysregulation of apoptosis with release of autoimmungenic plasma nucleosomes from apoptotic cells that trigger formation of autoantibodies against cytoplasmic and nuclear compounds or inhibition of a cytotoxic T lymphocyte response that normally suppresses autoreactive B cells.Citation37 However, greater concentrations and frequencies of antinuclear, anticardiolipin and anti-dsDNA antibodies described with infliximab compared with etanercept or adalimumab suggest that factors other than TNF blockade may be responsible for induction of these autoimmune diseases.Citation38,Citation39
Because there may be a class effect, patients who fail treatment with a TNF inhibitor because of a tolerability or safety issue may be at increased risk for a similar safety problem on an alternative TNF inhibitor. A UK-based study reported; the reasons for discontinuation of the switch-over (second) anti-TNF-α agent were related to the reasons for stopping the first anti-TNF-α agent. Furthermore, the risk for developing an adverse event with a second TNF inhibitor increased 2-fold in patients switched because of an adverse event.Citation40
Interleukin-1 receptor blocker
Anakinra (Kineret®)
Anakinra is recombinant, non-glycosylated form of IL-1 receptor antagonist with half life of 4 to 6 hours and is administered as 100 mg subcutaneous daily injection. Presently, anakinra is the only IL-1 antagonist marketed and approved for the treatment of RA, alone or in combination with MTX. In Europe, this agent has been approved for the treatment of RA only in combination with MTX.
Table 2 Interleukin-1 (IL-1) receptor blockers in rheumatoid arthritis (RA)
The efficacy of anakinra was evaluated as an add-on therapy in RA patients with inadequate response to monotherapy with non-biological DMARDS. Anakinra (100 mg, daily sc) was administered along with topical corticosteroid cream, despite patients on MTX (n = 48), leflunomide (n = 42), or cyclosporine-A (n = 32) treatment. At 48 weeks, the percentage of patients achieving ACR20, ACR50, and ACR70 responses were 73%, 41%, and 23% respectively.Citation41 Another pooled analysis of 5 clinical trials involving 2846 patients reported significant improvement of ACR20 response in the participants treated with anakinra 50 to 150 mg daily vs placebo after 24 weeks (38% vs 23%). Significant improvements were also observed with anakinra vs placebo in the following parameters: ACR50 (18% vs 7%), ACR70 (7% vs 2%), HAQ score, visual analogue score for pain, Larsen radiographic scores, and ESR.Citation42
Anakinra is fairly well tolerated, with injection-site reactions being the most common side effects, occurring in up to 70% of patients in dose-dependent manner.Citation42–Citation44 These reactions do not require treatment and can diminish with continued use. An increased incidence of serious infections was also noticed in anakinra treated patients, and the agent should be discontinued in the face of an active infection.Citation45
The drug has been successfully used for many auto-inflammatory syndromes.Citation46 However, the use of anakinra for the treatment of RA has been limited worldwide due to its modest efficacy, especially when compared with the TNF inhibitors and the newer biologics. Although head-to-head comparison trials have not been carried out, the absolute improvement was less pronounced when compared to studies using other biological therapies. However, the recent development of long-acting IL-1 inhibitors (rilonacept, canakinumab) for the auto-inflammatory syndromes, may provide us with new tools, if appropriate trials are ever conducted, to answer the question whether the lack of efficacy of anakinra lies within its mechanism of action (IL-1 inhibition) vs specific attributes of the molecule, especially its pharmacokinetic properties.
Interleukin-6 receptor blocker
Tocilizumab (Actemra®)
Tocilizumab, a new humanized, antihuman IL-6 receptor antibody with a number of randomized controlled trials evaluating its efficacy in RA, is now approved for the treatment of RA in Japan.Citation47 Elevated levels of IL-6 in the serum and synovial fluid of RA patients contribute to the chronic inflammatory process characterizing RA and correlate positively with disease activity. Tocilizumab binds selectively and competitively to soluble and membrane-expressed IL-6 receptors, blocking IL-6 signal transduction.Citation48 A number of trials in patients with early or long standing RA, have demonstrated the efficacy of intravenous tocilizumab 8 mg/kg every 4 weeks in improving disease activity, structural joint damage and/or HRQoL.Citation49
Tocilizumab as monotherapy has shown efficacy in patients with an inadequate response to MTX therapy.Citation50,Citation51 The AMBITION study randomized 673 patients with active RA to receive either tocilizumab or MTX for a period of 24 weeks. Results demonstrated statistically significant improvement of the primary endpoint (ACR20) in patients treated with tocilizumab compared to MTX treatment (69.9% vs 52.5%, P < 0.0001).Citation50 The SATORI (Study of Active controlled TOcilizumab monotherapy for RA patients with an Inadequate response to MTX) study also demonstrated superior efficacy among 80.3% of patients treated with 8 mg/kg of tocilizumab monotherapy every 4 weeks, achieving the primary endpoint (ACR20) at 24 weeks in comparison to 25% patients treated with 8 mg/week MTX therapy.Citation51
Table 3 IL-6 receptor blocker (tocilizumab) in rheumatoid arthritis (RA)Table Footnotea
The combination therapy of tocilizumab plus MTX was found to be more efficacious than tocilizumab monotherapy by various investigators. In a European study, researchers observed ACR20 response in 74% of patients receiving combination of 8 mg/kg tocilizumab plus MTX in comparison to 63% in patients receiving 8 mg/kg tocilizumab monotherapy. Additionally, statistically significant ACR50 and ACR70 responses (P < 0.05) were found in patients receiving the combination therapy.Citation52
A study conducted by Emery et al demonstrated convincingly efficacy of tocilizumab plus MTX in RA patients with inadequate response to TNF inhibitors, a growing subpopulation of RA patients.Citation53
As of safety profile, tocilizumab was well tolerated by adult patients with early and long-standing RA. Most frequently reported treatment-emergent adverse effects were mild to moderate in intensity, included upper respiratory tract infections, nasopharyngitis, headache, hypertension, and total cholesterol and ALT elevations.Citation49 However, recently a study also identified marked suppression in the number of neutrophils in the peripheral blood of RA patients 1 day after the administration of tocilizumab, which may predispose to the development of infections, by adding an additional risk factor to an already underlying immunosuppressed state.Citation54 Further studies are being conducted to better define the safety profile of this agent.
In conclusion, intravenous tocilizumab is effective and generally well tolerated when administered either as monotherapy or in combination with conventional DMARDs in adult patients with moderate to severe active RA, regardless of disease duration or prior therapy.
B-cell depletion therapy
Rituximab (Rituxan®)
B-cell depletion using anti-CD20 antibodies is becoming a widely recognized therapeutic option for patients with severe RA, and currently rituximab is FDA approved in the United States for the treatment of RA in patients who have exhibited an inadequate response to or were intolerant to one or more TNF inhibitors in combination with MTX, on the basis of several randomized placebo-controlled studies.Citation55–Citation57
Rituximab is chimeric human/mouse anti-CD20 antibody, with plasma half life of 40 to 400 hours, and is administered in dose of two 1000 mg intravenous infusions separated by 2 weeks. Rituximab induces a rapid depletion of normal CD20-expressing B-cells in the peripheral blood, and levels remain low or undetectable for 2 to 6 months before returning to pretreatment levels, generally within 12 months. Serum immunoglobulin levels remain largely stable, although a reduction in IgM has been described.
Table 4 B-cell depleting agents (anti-CD20) in rheumatoid arthritis (RA)
The Randomised Evaluation oF Long-term Efficacy of rituXimab in RA (REFLEX) phase 3 study on 517 RA patients showed that rituximab plus MTX treatment significantly reduced joint damage progression compared with placebo plus MTX. Significant reductions were observed from baseline to 56 weeks for rituximab plus MTX compared with placebo plus MTX in the following measures: Genant modified Sharp score (1.00 vs 2.31 in placebo; P = 0.005), erosion score (0.59 versus 1.32; P = 0.011) and joint space narrowing score (0.41 vs 0.99; P < 0.001).Citation58 This difference is remarkable, given that the majority of patients in the placebo group (81%) received at least one course of rituximab, because from weeks 16 to 24 patients who had failed to respond to treatment (< 20% improvement in swollen joint counts) could receive rescue therapy. In addition, rituximab has been shown to inhibit the radiographic progression independent of clinical response in such patient population.Citation1
Results of an observational study in a population of patients with inadequate response to one or more TNF inhibitors indicated that rituximab may be more effective at controlling disease activity than switching to an alternative TNF inhibitor. Significant decrease in DAS 28 was reported in patients treated with rituximab (−1.61, 95% CI = −1.97 to −1.25) than in those treated with an alternative TNF inhibitor (−0.98, 95% CI = −1.33 to −0.62) after 6 months of therapy.Citation59 However, when the motive for interrupting TNF-α therapy was something other than ineffectiveness, both rituximab and alternative TNF-α agents appear to offer similar levels of effectiveness, as shown in previous studies.Citation40
Rituximab is by far the only agent that has formally demonstrated significant slowing of structural joint damage in RA patients with an inadequate response to or who are intolerant to TNF inhibitors. Although well known for its efficacy, rituximab can result in serious, including fatal infusion reactions, and rare progressive multifocal leukoencephalopathy (PML). PML has been well reported infectious complication occurring in patients with systemic lupus erythematosus (SLE). As reported by Molloy et al, nearly two thirds of cases of PML in patients with rheumatic diseases reported in the medical literature occurred in SLE patients.Citation60 Twenty-three rituximab-treated oncology patients were reported by the FDA to have developed PML as of December 6, 2007. Most of these patients received rituximab in combination with chemotherapy or stem cell transplantation.Citation61 However the occurrence of PML in RA patients has not been reported till date. Although the risk due to rituximab is difficult to assess given the multiple confounders, continued vigilance is warranted. Therefore the rheumatologists need to be vigilant and pursue the diagnosis of PML in all patients with unexplained neurological signs or symptoms with clinical and MRI findings compatible with the diagnosis. Once established, rituximab therapy should be discontinued; dose reductions and discontinuation should be considered for any concomitantly administered immunosuppressants. Additional concern that remains unaddressed is the number of rituximab infusions that can be safely administered.
Ocrelizumab
Ocrelizumab (OCR) is a novel anti-CD20 humanized monoclonal antibody, currently in clinical trials for the treatment of RA. In comparison with rituximab, ocrelizumab binds to a different, but overlapping, epitope of the extracellular domain of CD20 receptor. In vitro characterization of ocrelizumab demonstrated enhanced ADCC and reduced CDC compared with rituximab.Citation62
The ACTION trial (a randomized, placebo-controlled, blinded, phase I/II study of escalating doses of ocrelizumab in patients with moderate to severe RA on stable doses of concomitant MTX) investigated ocrelizumab across a wide range of doses in patients with moderate to severe RA receiving concomitant MTX therapy.Citation62 A single course of ocrelizumab (2 infusions on days 1 and 15) at doses ranging from 10 to 1000 mg was administered. Clinical response was evaluated at 24 weeks and safety profile at 72 weeks of follow-up. A higher proportion of patients in all of the ocrelizumab groups achieved an ACR20, ACR50, or ACR70 response at week 24 as compared with patients in the placebo group. The ACR20 response rates at week 24 were 42%, 35%, 45% and 50% in those receiving 10 mg, 50 mg, 200 mg, and 500/1000 mg of OCR respectively. The ACR20 response rate in the placebo group was 22%.
The safety profile of ocrelizumab in this study was consistent across dosing groups and suggested only slight differences compared with placebo. The incidence of serious adverse events in the ocrelizumab-treated patients was 17.9% as compared with 14.6% in placebo group. The incidence of serious infections was 2.0% in all ocrelizumab treated patients and 4.9% in placebo-treated patients. All serious infections that were observed, resolved without sequelae.
Although ocrelizumab was well tolerated and appeared to be safe, additional experience with multiple trials will be required to validate the outcomes and further understand the clinical significance of human anti-human antibodies and potential advantages of this therapeutic approach over treatment with chimeric antibodies.
T-cell targeted therapy
Abatacept (Orencia®)
Abatacept is a fusion protein consisting of the human cytotoxic T-lymphocyte-associated antigen-4 molecule (CTLA-4) and immunoglobulin G1, both of which occur naturally in the body. By mimicking the actions of CTLA-4, abatacept inhibits one of the key costimulatory pathways (CD28:CD80/CD86) required for full T-cell activation.Citation63 The drug has been approved for the treatment of RA patients who have exhibited an inadequate response to or were intolerant of one or more DMARDs or TNF inhibitors in the USA, or one or more TNF inhibitors only in Europe.Citation1 Abatacept may be used either as a monotherapy or concomitantly only with DMARDs. As reported in the ASSURE (Abatacept Study of Safety in Use with other Rheumatoid arthritis therapies) trial, abatacept in combination with biologic background therapies was associated with an increased rate of serious adverse events.Citation64 Therefore, abatacept is contra-indicated for concomitant use with TNF-α inhibitors, anakinra, and/or other biological therapies.
Table 5 T-cell costimulation (abatacept)in rheumatoid arthritis (RA)
Several phase III trials have shown abatacept to be an effective option in patients who are refractory to TNF-α inhibition with impressive quality of life improvements. Two clinical trials evaluated the efficacy of abatacept for difficult-to-treat patients: the AIM for MTX-resistant cases and the ATTAIN for patients who are resistant to TNF-α inhibitors.Citation65,Citation66
A more recent randomized, double-blind placebo- and active (infliximab)-controlled, 12-month global trial known as ATTEST (for Abatacept or infliximab vs placebo, a Trial for Tolerability, Efficacy and Safety in Treating rheumatoid arthritis) suggested that standard weight-based abatacept might have comparable efficacy with a more favorable safety profile than infliximab 3 mg/kg.Citation67 Trial was designed to obtain data on the magnitude of the treatment effect in RA of abatacept or infliximab (an established inhibitor of TNF for RA) vs placebo, and to obtain relative efficacy and safety data on these two biological treatments in a single study. The study utilized a double-blind, randomized, placebo-controlled design for the first 6 months to validate efficacy responses, and the study duration allowed for the opportunity to directly compare the safety profile of the active biologic treatment groups over 1 year.Citation67
In this study, abatacept and infliximab (3 mg/kg every 8 weeks) demonstrated similar efficacy. But overall, abatacept had a relatively more acceptable safety and tolerability profile, with fewer serious adverse events, serious infections, acute infusional events and discontinuations due to adverse events than the infliximab group. Limitations of the study were its short duration and the fact that the comparator (infliximab) dose was 3 mg/kg, the only approved dose at the time in the European Union.
The efficacy of combined therapy, abatacept plus MTX have been confirmed by a 2-year follow-up study, where 80.3% of patients taking abatacept showed ACR20 improvement, 30.9% achieved remission (CRP-DAS28 < 2.6) and 66.8% enhanced their physical function (as measured by the HAQ disability index). The mean changes in the physical and mental components summary scores of the Short-Form-36 also confirmed a good improvement of HRQoL.Citation68
The efficacy and safety of abatacept has also been evaluated in RA patients receiving etanercept. However, the percentage improvements in ACR20 response after 6 months of abatacept therapy were disappointing. Furthermore, after 1 year of association of these two biologics, no notable changes in ACR responses were observed.Citation69 Moreover this combination was shown to be linked to an increase in serious adverse events rate compared with patients receiving placebo and etanercept (16.5 vs 2.8%).
Unlike previous clinical trials enrolling patients with long-standing RA, Westhovens et al recently evaluated the efficacy of abatacept in MTX-naïve patients with early RA.Citation70 Abatacept study to Gauge Remission and joint damage progression in MTX-naïve patients with Early Erosive RA (AGREE) was a 2-year, double-blind trial that enrolled patients with less than 2 years of disease without MTX exposure (≤10 mg/week for ≤3). The study population of 509 patients was randomized to receive placebo plus MTX (increased up to 20 mg/week) or abatacept 10 mg/kg plus MTX for 12-months before an open-label 12 month extension. The co-primary endpoints of the study were remission (DAS28 < 2.6) and Genant-modified Sharp total score. At year 1, 41.4% of abatacept-treated patients achieved remission as compared to 23.3% patients of placebo group. The proportion of patients with no radiographic progression was 61.2% and 52.9% in the abatacept and placebo-treated patients, respectively (difference of 8.3, 95% CI − 1.0, 17.5). The change from baseline in total Genant-modified Sharp scores and erosion scores were significantly lower for abatacept, while minimal joint space narrowing progression was noted in both groups. Furthermore, 71.9% of abatacept-treated patients had clinically important improvement in the HAQ-DI compared to 62.1% in the placebo group (P = 0.024).
The safety profile of abatacept is comparable to that of other biologics. Severe infections were more common in abatacept-treated patients than in placebo-treated patients.Citation71 Opportunistic infections are rare in patients with abatacept and the frequency of malignancies, based on post-marketing surveillance and international patient cohorts, is not higher than expected in RA patients treated with DMARDS.Citation72
Upcoming biological targets and therapies
Since substantial cross-talk between pro-inflammatory cytokines IL-1β, TNF-α, IL-6, and IL-17 is essential to induce joint destruction in RA,Citation1 IL-1β and TNF-α promote IL-6 and tumor growth factor-beta (TGF-β) driven process of Th17 cell commitment and IL-17 production.Citation73 Th17 cell polarization further induces IL-1β, TNF-α, IL-6, IL-8, and IL-17 production ().Citation74–Citation77 Numerous studies have also demonstrated the critical role of B-cells in RA pathogenesis. A range of activated leukocyte cell types produce the TNF family B-cell pro-survival factors BLys (B-Lymphocyte stimulator) or BAFF (B-cell activation factor belonging to the TNF family) and APRIL (a proliferation inducing ligand).Citation78,Citation79 Levels of BAFF and APRIL are elevated in RA patients, with significantly higher levels in synovial fluid than in the serum.Citation80,Citation81 As illustrated in , BLyS/BAFF binds 3 receptors: BLyS receptor 3 (BR3, also termed BAFFR), transmembrane activator and calcium-signaling modulating and cyclophilin ligand (CAML) interactor (TACI), and B cell maturation antigen (BCMA) in contrast to APRIL, which selectively promote TACI and BCMA receptor-mediated NF-kβ signaling mechanisms.Citation82
Figure 1 Molecular targets of drugs in clinical trials for rheumatoid arthritis.
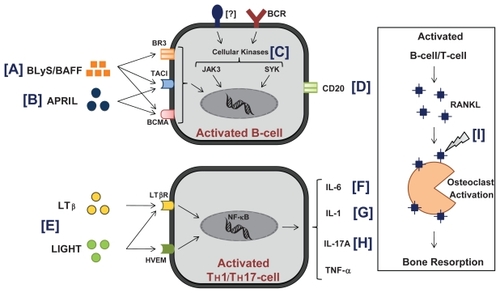
The following biological agents are being evaluated in ongoing trials ( and ):
Figure 2 Inflammatory cascade and molecular targets of current biologics in rheumatoid arthritis.
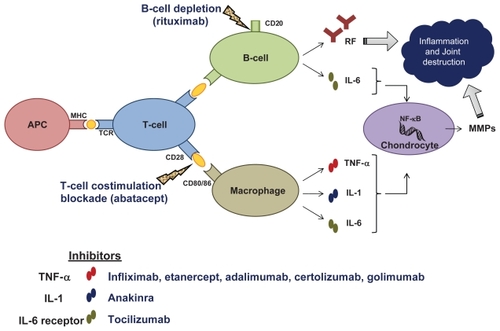
Table 6 Biological agents in development for rheumatoid arthritisTable Footnotea
B-cell agents targeting CD20 (ocrelizumab, ofatumumab, and TRU-015), agents targeting cytokines important in the later stages of B cell maturation: BLyS or BAFF (belimumab, briobacept) and APRIL (atacicept), as well as other compounds targeting intracellular kinases Jak3 (CP 690,550) and Syk.
T-cell agents targeting lymphotoxin beta (LTβ) and LIGHT (baminercept)
Cytokine targeting agents: IL-1 (AMG 108), IL-6 (tocilizumab), and IL-17A (AIN 457)
Agents targeting osteoclasts via RANKL inhibition (denosumab)
Discussion
The following are unanswered questions on biological therapy in RA:
Initiation of biologics: early or established RA?
Can biomarkers guide initiation of biological therapy?
Inter-class comparison of existing and upcoming biologics: which and when to start?
Need for a standard step-up/step-down therapy protocol for biologics: establishing a therapeutic algorithm for RA?
Safety issues: Unique to an individual biologic agent or representative of a class effect?
Ongoing vigilance: Is there a better way to monitor and record potential side effects of biologics identified after approval?
Is it possible to discontinue biological therapy in patients achieving remission and, if so, when is the optimal timing?
Perioperative infection risks while a patient on biological therapy: what is the timing of discontinuation of treatment/procedure/reinitiation of therapy? Need to readdress for every new biologic.
Biologics, usually in combination with traditional DMARDs such as MTX, have revolutionized the treatment of RA, producing significant improvement in clinical, radiographic and functional outcomes not seen previously. However, even with the availability of these medications, a significant proportion of patients either do not respond at all, or may respond initially and subsequently efficacy fades. Therefore, new agents, with different mechanisms of action, ie, targeting molecules involved in cellular interaction and/or signaling in immuno competent cells are being investigated through various clinical trials.
Results from existing RCTs are difficult to compare, because they involve different patient populations, study designs, and treatment strategies. Moreover there may be specific factors involving the different mechanisms and onset of action which may further complicate the comparison of different biological agents.
The key question of how to select the first/one particular biological agent to be given to a patient has been elegantly answered by Scott et al.Citation83 The probable deciding factors explained include patient’s preference, relative efficacy, toxicity, and cost-effectiveness of different biologics. Different mechanisms of action might provide a theoretical rationale for the preference of one agent over another.
Targeting individual cytokines is a tried and successful approach in RA. However, careful consideration must be given not only to the cytokine targeted but also to the stage of the disease process being targeted. Furthermore, there is a lack of data, both on efficacy and safety, about the applicability of multiple cytokine inhibition.
Despite their clinical promises, monoclonal antibodies are raising concern about the potential adverse effects of long-term use. Published data currently do not exclude clinically important increased risks, nor do they refute beneficial effects. As per definition, much of the currently available safety data from trials or clinical practice do not capture the impact of any effects from sustained exposure to biologics. Additional studies are warranted to understand whether all these safety issues are unique to an individual biologic agent or representative of a class effect. Therefore, the treating physician must carefully weigh the benefits of these new biologics against their risks, particularly in frail patients at risk for infection.
Although biologics have undisputed benefits in the treatment of RA, the cost issue remains unsolved. Costs are dramatically higher than for conventional medications, pharmacoeconomic concerns have been brought in the spotlight with their ever-expanding use. Across the board, the estimated yearly cost to use a biological agent is about US$20,000. The significant cost has to be balanced against the detrimental economic impact of RA on the individual patient and society as a whole. If biologics can prevent the morbidity, disability, and deterioration of the quality of life that RA often causes, then the use of a biological agent can be a cost-effective decision for societies. The development of biosimilar biologics, ie, generic medications that replicate the exact aminoacid structure of existing biologic DMARD molecules that lose their patent protection, may soon alter the landscape of biologics and its associated costs. However, biologics require a sophisticated manufacturing process, different from existing conventional medications and tight regulation will be required to avoid possibly additional safety risks and make these agents truly cost-effective.
Several new therapies, currently in the pipeline, may soon be added to our already expanded number of treatment options. Different types of RA patients will require different therapies, especially those who have failed multiple agents. These new options look promising in filling gaps in the treatment of RA patients. Comparative studies in sufficient numbers of patients should help shed more light on their exact role in RA treatment.
Disclosures
PE has received consulting and/or speakers’ fees from Bristol-Myers Squibb, Wyeth, and Centocor.
References
- Rubbert-RothAFinckhATreatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical reviewArthritis Res Ther200911 Suppl 1S119368701
- SaklatvalaJTumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilageNature1986Aug–13;32260795475493736671
- TaylorPCAnti-TNF therapy for rheumatoid arthritis and other inflammatory diseasesMol Biotechnol20011019215316811725485
- SmolenJSVan Der HeijdeDMSt ClairEWPredictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trialArthritis Rheum200654370271016508926
- BreedveldFCEmeryPKeystoneEInfliximab in active early rheumatoid arthritisAnn Rheum Dis200463214915514722203
- AllaartCFGoekoop-RuitermanYPde Vries-BouwstraJKBreedveldFCDijkmansBAAiming at low disease activity in rheumatoid arthritis with initial combination therapy or initial monotherapy strategies: the BeSt studyClin Exp Rheumatol2006246 Suppl 43S77S82
- QuinnMAConaghanPGO’ConnorPJVery early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trialArthritis Rheum2005521273515641102
- HaugebergGConaghanPGQuinnMEmeryPBone loss in active early rheumatoid arthritis patients treated with infliximab and methotrexate compared with methotrexate treatment alone. Explorative analysis from a twelve-month randomized, double blind, placebo-controlled studyAnn Rheum Dis2009421 [Epub ahead of print]
- DobrzanskiMJReomeJBHollenbaughJAHylindJCDuttonRWEffector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastasesCancer Res200464140641414729652
- EfthimiouPMarkensonJARole of biological agents in immune-mediated inflammatory diseasesSouth Med J200598219220415759950
- MorelandLWBaumgartnerSWSchiffMHTreatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion proteinN Engl J Med199733731411479219699
- MorelandLWSchiffMHBaumgartnerSWEtanercept therapy in rheumatoid arthritis. A randomized, controlled trialAnn Intern Med1999130647848610075615
- EmeryPBreedveldFCHallSComparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trialLancet2008372963637538218635256
- KlareskogLvan der HeijdeDde JagerJPTherapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trialLancet2004363941067568115001324
- HovingJLBarteldsGMSluiterJKPerceived work ability, quality of life, and fatigue in patients with rheumatoid arthritis after a 6-month course of TNF inhibitors: prospective intervention study and partial economic evaluationScand J Rheumatol20091519337948
- BreedveldFCWeismanMHKavanaughAFThe PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatmentArthritis Rheum2006541263716385520
- ChenDYChouSJHsiehTYRandomized, Double-blind, Placebo-controlled, Comparative Study of Human Anti-TNF Antibody Adalimumab in Combination with Methotrexate and Methotrexate Alone in Taiwanese Patients with Active Rheumatoid ArthritisJ Formos Med Assoc2009108431031919369178
- WeinblattMEKeystoneECFurstDEKavanaughAFChartashEKSeguradoOGLong term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended studyAnn Rheum Dis200665675375916308341
- BourneTFossatiGNesbittAA PEGylated Fab’ fragment against tumor necrosis factor for the treatment of Crohn disease: exploring a new mechanism of actionBioDrugs200822533133718778114
- SmolenJSLandeweRBMeasePJEfficacy and Safety of Certolizumab Pegol Plus Methotrexate in Active Rheumatoid Arthritis: The RAPID 2 StudyAnn Rheum Dis200968679780419015207
- KeystoneEHeijdeDMasonDJrCertolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group studyArthritis Rheum200858113319332918975346
- FleischmannRVencovskyJvan VollenhovenRFEfficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD studyAnn Rheum Dis200968680581119015206
- KeystoneECGenoveseMCKlareskogLGolimumab, a human antibody to TNF-{alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate: The GO-FORWARD StudyAnn Rheum Dis200968678978619066176
- KayJMattesonELDasguptaBGolimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging studyArthritis Rheum2008458496497518383539
- KeystoneECGenoveseMCKlareskogLGolimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD StudyAnn Rheum Dis200968678979619066176
- SeongSSChoiCBWooJHIncidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockersJ Rheumatol200734470671117309133
- BongartzTSuttonAJSweetingMJBuchanIMattesonELMontoriVAnti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trialsJAMA2006295192275228516705109
- BehnamSMBehnamSEKooJYTNF-alpha inhibitors and congestive heart failureSkinmed2005Nov–Dec4636336816276152
- TsiodrasSSamonisGBoumpasDTKontoyiannisDPFungal infections complicating tumor necrosis factor alpha blockade therapyMayo Clin Proc200883218119418241628
- van der MeerJWPopaCNeteaMGSide effects of anticytokine strategiesNeth J Med2005633788015813418
- CarterJDLadhaniARiccaLRValerianoJVaseyFBA safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration databaseJ Rheumatol200936363564119132789
- SnoeckxYGKSandersMGardinerMPregnancy Outcomes In Women Taking Infliximab: The Infliximab Safety Database2008American College of Rheumatology Annual Scientific MeetingSan Francisco, CA2008
- JohnsonDLKLJCChambers. Pregnancy Outcomes for Women Exposed to Adalimumab: OTIS Autoimmune Diseases in Pregnancy Project2008American College of Rheumatology Annual Scientific MeetingSan Francisco, CA2008
- JohnsonDLKLJChambersCPregnancy Outcomes in Women Exposed to Etanercept: The OTIS Autoimmune Diseases in Pregnancy Project2008American College of Rheumatology Annual Scientific MeetingSan Francisco, CA2008
- Ramos-CasalsMBrito-ZeronPSotoMJCuadradoMJKhamashtaMAAutoimmune diseases induced by TNF-targeted therapiesBest Pract Res Clin Rheumatol200822584786119028367
- ElezoglouAKafasiNKaklamanisPHInfliximab treatment-induced formation of autoantibodies is common in Behcet’s diseaseClin Exp Rheumatol2007254 Suppl 45S65S6917949554
- KocharlaLMongeyABIs the development of drug-related lupus a contraindication for switching from one TNF alpha inhibitor to another?Lupus200918216917119151120
- De RyckeLBaetenDKruithofEVan den BoschFVeysEMDe KeyserFInfliximab, but not etanercept, induces IgM antidouble- stranded DNA autoantibodies as main antinuclear reactivity: biologic and clinical implications in autoimmune arthritisArthritis Rheum20055272192220115986349
- TraceyDKlareskogLSassoEHSalfeldJGTakPPTumor necrosis factor antagonist mechanisms of action: a comprehensive reviewPharmacol Ther2008117224427918155297
- HyrichKLLuntMWatsonKDSymmonsDPSilmanAJOutcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort studyArthritis Rheum2007561132017195186
- KaranikolasGCharalambopoulosDVaiopoulosGAdjunctive anakinra in patients with active rheumatoid arthritis despite methotrexate, or leflunomide, or cyclosporin-A monotherapy: a 48-week, comparative, prospective studyRheumatology (Oxford)20084791384138818603660
- MertensMSinghJAAnakinra for Rheumatoid Arthritis: A Systematic ReviewJ Rheumatol2009
- DayerJMFeigeUEdwardsCK3rdBurgerDAnti-interleukin-1 therapy in rheumatic diseasesCurr Opin Rheumatol200113317017611333344
- BresnihanBAnakinra as a new therapeutic option in rheumatoid arthritis: clinical results and perspectivesClin Exp Rheumatol2002205 Suppl 27S32S3414989427
- FleischmannRMAddressing the safety of anakinra in patients with rheumatoid arthritisRheumatology (Oxford)200342Suppl 2ii29ii3512817093
- EfthimiouPFlavellRAFurlanAAutoinflammatory syndromes and infections: pathogenetic and clinical implicationsClin Exp Rheumatol2008261 Suppl 48S53S6118570755
- BinghamCO3rdEmerging therapeutics for rheumatoid arthritisBull NYU Hosp Jt Dis200866321021518937634
- NowellMARichardsPJFieldingCARegulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritisArthritis Rheum20065472084209516802343
- OldfieldVDhillonSPloskerGLTocilizumab: a review of its use in the management of rheumatoid arthritisDrugs200969560963219368420
- JonesGSebbaAGuJComparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION studyAnn Rheum Dis2009
- NishimotoNMiyasakaNYamamotoKStudy of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapyMod Rheumatol2009191121918979150
- MainiRNTaylorPCSzechinskiJDouble-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexateArthritis Rheum20065492817282916947782
- EmeryPKeystoneETonyHPIL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trialAnn Rheum Dis200867111516152318625622
- NakamuraIOmataYNaitoMItoKBlockade of interleukin 6 signaling induces marked neutropenia in patients with rheumatoid arthritisJ Rheumatol200936245946019208585
- EdwardsJCSzczepanskiLSzechinskiJEfficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritisN Engl J Med2004350252572258115201414
- CohenSBEmeryPGreenwaldMWRituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeksArthritis Rheum20065492793280616947627
- EmeryPFleischmannRFilipowicz-SosnowskaAThe efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trialArthritis Rheum200655451390140016649186
- KeystoneEEmeryPPeterfyCGRituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapiesAnn Rheum Dis200968221622118388156
- FinckhACiureaABrulhartLB cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agentsArthritis Rheum20075651417142317469098
- MolloyESCalabreseLHProgressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk?Autoimmun Rev20088214414618700172
- BorenEJCheemaGSNaguwaSMAnsariAAGershwinMEThe emergence of progressive multifocal leukoencephalopathy (PML) in rheumatic diseasesJ Autoimmun2008301–2909818191544
- GenoveseMCKaineJLLowensteinMBOcrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I/II randomized, blinded, placebo-controlled, dose-ranging studyArthritis Rheum20085892652266118759293
- CoughlinMImproving patient outlook in rheumatoid arthritis: experience with abataceptJ Am Acad Nurse Pract2008201048649519128344
- WeinblattMCombeBCovucciAArandaRBeckerJCKeystoneESafety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: A one-year randomized, placebo-controlled studyArthritis Rheum20065492807281616947384
- KremerJMGenantHKMorelandLWEffects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trialAnn Intern Med20066201441286587616785475
- GenoveseMCBeckerJCSchiffMAbatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibitionN Engl J Med2005353111114112316162882
- SchiffMKeisermanMCoddingCEfficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multicentre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexateAnn Rheum Dis20086781096110318055472
- KremerJMGenantHKMorelandLWResults of a two-year followup study of patients with rheumatoid arthritis who received a combination of abatacept and methotrexateArthritis Rheum200858495396318383390
- WeinblattMSchiffMGoldmanASelective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trialAnn Rheum Dis200766222823416935912
- WesthovensRRoblesMXimenesACClinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factorsAnn Rheum Dis200919 [Epub ahead of print]
- WesthovensRKremerJMMorelandLWSafety and efficacy of the selective costimulation modulator abatacept in patients with rheumatoid arthritis receiving background methotrexate: a 5-year extended phase IIB studyJ Rheumatol2009436473674219273451
- SimonTASmittenALFranklinJMalignancies in the rheumatoid arthritis abatacept clinical development program: An epidemiological assessmentAnn Rheum Dis2008
- BettelliECarrierYGaoWReciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cellsNature2006441709023523816648838
- HwangSYKimHYExpression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patientsMol Cells200519218018415879699
- BenderdourMTardifGPelletierJPInterleukin 17 (IL-17) induces collagenase-3 production in human osteoarthritic chondrocytes via AP-1 dependent activation: differential activation of AP-1 members by IL-17 and IL-1betaJ Rheumatol20022961262127212064845
- KoshyPJHendersonNLoganCLifePFCawstonTERowanADInterleukin 17 induces cartilage collagen breakdown: novel synergistic effects in combination with proinflammatory cytokinesAnn Rheum Dis200261870471312117676
- KoendersMIJoostenLAvan den BergWBPotential new targets in arthritis therapy: interleukin (IL)-17 and its relation to tumour necrosis factor and IL-1 in experimental arthritisAnn Rheum Dis200665Suppl 3iii29iii3317038468
- ScapiniPNardelliBNadaliGG-CSF-stimulated neutrophils are a prominent source of functional BLySJ Exp Med2003197329730212566413
- ScapiniPCarlettoANardelliBProinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseasesBlood2005105283083715358625
- TanSMXuDRoschkeVLocal production of B lymphocyte stimulator protein and APRIL in arthritic joints of patients with inflammatory arthritisArthritis Rheum200348498299212687540
- CheemaGSRoschkeVHilbertDMStohlWElevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseasesArthritis Rheum20014461313131911407690
- TremlJFHaoYStadanlickJECancroMPThe BLyS family: toward a molecular understanding of B cell homeostasisCell Biochem Biophys200953111619034695
- ScottDLCopeANew tumour necrosis factor inhibitors for rheumatoid arthritis: are there benefits from extending choice?Ann Rheum Dis200968676776919435722