Abstract
The trace element selenium (Se) occurs in the form of the amino acid selenocysteine in selenoproteins. Selenoproteins exerts multiple physiological effects in human health, many of which are related with regulation of reduction-oxidation processes. In fact, the selenoenzyme families of glutathione peroxidase (GPx) and thioredoxin reductase (TRx) display the ability to act as antioxidants, protecting cells from oxidative damage. Furthermore, another class of selenoproteins are the iodothyronine deiodinase enzymes (DIO), which catalyze the conversion of thyroxine (T4) in triiodothyronine (T3), then exerting a fine tuned control on thyroid hormones metabolism. Several studies have investigated the potential positive effects of Se supplementation in thyroid diseases, characterized by increased levels of hydrogen peroxide and free radicals, like autoimmune chronic thyroiditis. These studies have supplied evidences indicating that Se supplementation, maximizing the antioxidant enzymes activity, may reduce the thyroid inflammatory status. Then, it may be postulated that Se could play a therapeutical role in thyroid autoimmune diseases. Despite the fact that recent studies seem to be concordant about Se beneficial effects in decreasing thyroid peroxidase antibodies (TPOAb) titers and ameliorating the ultrasound echogenicity pattern, several doubts have to be still clarified, before advising Se supplementation in chronic autoimmune thyroiditis.
Keywords:
Introduction
The trace element selenium (Se) was discovered in 1817 by the Swedish chemist Berzelius. Two hundred years later, Se is well-known as an essential mineral of pivotal importance for the human health. It is established that Se displays antioxidant activity, antiinflammatory chemopreventive, and antiviral characteristics (CitationRayman 2000). Its effect on human health is due to its presence within some proteins (CitationKryukov et al 2003). Unlike other metal elements that interact with proteins in form of cofactors, Se becomes cotranslationally incorporated into polypeptide chain as part of the amino acid selenocysteine (Sec), also known as the 21th aminoacid (CitationGromer et al 2005). The group of proteins that contain Sec as an integral part of their polypeptide chain are defined as selenoproteins. Between the domains, 25 selenoprotein families have been identified in humans (CitationCastellano et al 2005). The human selenoproteome is composed of 17 selenoprotein families, some with multiple genes having similar functions. These comprise glutathione peroxidase (GPx) (five genes), thioredoxin reductase (TrxR) (three genes), and iodothyronine deiodinases (DIO) (three genes), which have been functionally characterized as having oxidoreductase functions (CitationKryukov et al 2003). The entry point of Se in animals is via plants, which absorb the element in its organic form from the soil, where its content varies between different areas. In plants, Se becomes converted to organic forms such as methylated low-molecular-weight Se compounds and the amino acid selenomethionine (SeMet) and Sec (CitationWhanger 2002). Importantly, Se compounds have the capacity to redox cycle and are metabolized to more reduced states, which are thought to account for Se compounds being effective antioxidants. In fact, Sec is part of the catalytic group within selenoenzymes and is directly involved in redox reactions (CitationZhong and Holmgren 2000). The effects of Se on the organism are concentration-dependent, ranging from an antioxidant activity in the nanomolar–micromolar range to potentially prooxidant at concentrations above what is required for maximal selenoprotein synthesis. At even higher concentrations, Se compounds may accumulate and redox cycle with intracellular thiols, inducing oxidative stress and cellular damage (CitationVinceti et al 2001). Se is essential for life, and there is no doubt that adequate amounts of this element are required for optimal human health. Many of its physiologic roles may be ascribable to its presence within selenoproteins. For example, one of the most important cellular processes, DNA synthesis, depends on the presence of Se within the catalytic site of TrxR (CitationArner and Holmgren 2000). Moderate Se deficiency has been associated to many conditions, such as increased risk of cancer and infections, male infertility, increased thyreocytes damage, and serious neurological diseases, including Alzheimer’s and Parkinson’s (CitationRayman 2000). However, for some of these conditions, the evidence is rather scant, conflicting data have been published, and need further confirmations. On the contrary, a pathological condition that is certainly associated with Se deficiency is Keshan disease, a potentially fatal form of cardiomyopathy; this disease is prevalent in children and is endemic in some areas of China characterized by extremely low levels of Se in the soil (CitationLevander and Beck 1997). It is supposed that infection by Coxsackie B virus may trigger the onset of this disease. Notably, the condition is prevented or completely reversed by Se supplementation (CitationXia et al 2005). Another disease due to a combined deficiency of Se and iodine is myxedematous cretinism which is characterized by mental and growth retardation (CitationVanderpas et al 1990). It is ascertained that Se deficiency causes a reduction in GPx and DIO enzymes activity, accumulation of hydrogen peroxide (H2O2) causing damage to the thyroid gland, and impaired thyroid hormone metabolism. Se supplementation in this condition must be administered after iodide levels have been restored, as Se increases the activity of DIO, leading to a further loss of iodide from the damaged thyroid (CitationZimmermann and Kohrle 2002).
In the United States, where dietary intakes are higher than in many other countries, the current recommendation is 55 μg/day. In the United Kingdom, however, Se intake is considerably lower, and the recommended dose is 75 μg/day for men and 60 μg/day for women (CitationSurai 2006). These recommendations were based on the plasma GPx optimal enzyme activity, however, a recent study indicates that higher Se intake is required to obtain full expression of selenoprotein P (SelP) (CitationXia et al 2005). Then, it is suggested that SelP may be a better indicator of Se nutritional status than GPx, and that the recommended dietary intake may need to be revised.
Even if a large body of studies has made available a vast information about Se, the precise molecular mechanisms behind its effects in physiologic and pathologic conditions remain unknown.
The best known and most important human selenoproteins
The most common form of Se in human proteins is Sec, which becomes cotranslationally incorporated within the growing polypeptide chain. However, selenium-containing proteins exists also in other forms, such as proteins that nonspecifically incorporate Se during translation, and selenium-binding proteins that bind Se as a cofactor. Only a few of the 25 identified mammalian selenoproteins have so far been functionally characterized. Most of these selenoproteins exhibit enzymatic redox function via Sec, which confers their catalytic and antioxidant activities (CitationCastellano et al 2005).
Thioredoxin reductase
Thioredoxin reductase (TrxR) and NADPH constitute the thioredoxin system, a major cellular redox system present in all living organisms (CitationArner and Holmgren 2000). Three mammalian TrxR selenoenzymes have been identified: the cytosolic enzyme (TrxR1), the mitochondrial enzyme (TrxR2), and a testis-specific enzyme thioredoxin-gluthatione reductase (TrxR3) (CitationSun et al 1999, Citation2001). The Trx system plays a pivotal role in embryo development. Knockout mouse model for Trx gene (Txn) are not vital and homozygous mutants for TrxR1 die shortly after implantation as a result of failure to proliferate, suggesting that TrxR is essential for early differentiation and morphogenesis (CitationMatsui et al 1996; CitationJakupoglu et al 2005). The similar Txn knockout and TrxR1 homozygous mutants phenotypes are mostly likely explained by impaired DNA synthesis due to accumulation of oxidized, nonfunctional ribonucleotide reductase. These results confirm that the TrxR system is absolutely required for development, and probably cell proliferation in vivo, and also emphasize the importance of the Sec residue for catalytic activity. As observed for the TrxR1, complete removal of mitochondrial TrxR2 causes embryonic death and homozygous mutant embryos show decreased hematopoiesis, increased apoptosis in the liver, and cardiac defects (CitationNonn et al 2003; CitationConrad et al 2004).
Glutathione peroxidases (GPx)
Glutathione peroxidases (GPx) was the first mammalian protein shown to incorporate Se in the form of Sec into catalytic site and was supposed to be associated with the anti-oxidant activity of Se. GPxs are recognized for catalyzing the reduction of hydrogen peroxide and organic hydroperoxides, thus protecting cells from oxidative damage. The importance of glutathione peroxidases and their potentially beneficial role in critically ill patients concerns the mechanism of detoxification of peroxides to their respective alcohols at the expense of glutathione. It seems that all of the glutathione peroxidase isoforms share the same catalytic mechanism, with a highly conserved sequence of selenocysteine, tryptophan and glutamine (CitationAumann et al 1997; CitationBrigelius-Flohe 1999). The role of plasma GPx as an antioxidant enzyme is not fully understood. Although its preferred substrate, glutathione, usually has a low plasma concentration, increased cellular oxidative stress can export oxidized glutathione to the plasma compartment, where a combination of glutathione reductase and GPx can restore redox balance and either return reduced glutathione to the cell, or use it as a substrate for plasma GPx. Thus, Se can act as antioxidant when incorporated as selenoenzymes in the extracellualr space, the cell cytosol, in association with cell membranes, having the potential role to influence the immune processes (CitationArthur 2000). Localization and main function of the seven GPx isoenzymes are resumed in .
Table 1 Localization and function of glutathione peroxidase (CitationStawicki et al 2007)
Iodothyronine deiodinases
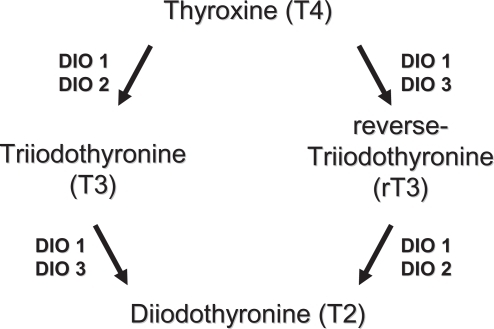
Three differentially distributed Sec-containing oxidoreductases (DIO1, DIO2, DIO3) constitute the family of iodothyronine deiodinases (DIO). They catalyze the activation (DIO1 and DIO2) and inactivation (DIO3) of the thyroid hormones thyroxine (T4), 3,5,3’-triodothyronine (T3), and reverse-3,5,3’-triiodothyronine (rT3) by removing distinct iodine moieties, as indicated in (CitationBianco and Kim 2006). Among their actions, thyroid hormones contribute to regulate the function of heart, metabolism, growth, are pivotal for the development of the fetal brain, and display multiple interactions with other hormonal systems. DIOs exhibit different localizations and function in human tissues (CitationSt Germain et al 2005). DIO1 is expressed mostly in the liver, kidney, thyroid, and pituitary; DIO2 in the thyroid, central nervous system, pituitary, and skeletal muscle; DIO3 is most prominently expressed in the pregnant uterus, placenta, embryonic liver, embryonic and neonatal brain, and neonatal skin. The three DIOs exert different actions as DIO1 plays a role in T3 production in the thyroid gland and controls the circulating T3 levels, whereas DIO2 and DIO3 are proposed to function in the local deiodination processes. Recent evidence indicates that DIO2 represents a major source of circulating T3 in euthyroid humans, whereas DIO1 contributes more substantially at high, thyrotoxic T4 levels, as seen in hyperthyroid patients. The presence of Sec appears to be absolutely required for DIO catalytic activity and thus it is not surprising that Se levels have a direct regulatory effect on the expression of DIOs (CitationBates et al 2000; CitationKohrle et al 2005). Among various selenoproteins, DIOs rank high in the hierarchy of Se supply during Se deficiency, DIO1 expression being maintained or slightly increased in the thyroid gland. In the brain and placenta, DIO1 and DIO3 expression is maintained during Se restriction, whereas DIO1 expression decreases in other tissues. The mechanisms of DIOs regulation by Se availability is not fully understood, nevertheless, it appears to be tissue- or organ-specific. Additional regulators of DIOs expression are T3 and thyrotropin (TSH) (which act in a feedback loop), and cyclic adenosine monophosphate (cAMP) (CitationKoenig 2005). Experimental models with knockout transgenic mouse, provided evidence of the crucial roles exerted by DIOs. In particular, knockout mice for DIO1 display abnormal concentrations of thyroid hormones and their metabolities, suggesting a role in preserving iodine store within the organism; DIO2 deficient mice have alterations in auditory function, thermogenesis, and brain development; DIO3 knockout models exhibit reduced viability, growth retardation, impaired fertility, reduced T3 and increased T4 levels (CitationSt Germain et al 2005). There are not well documented diseases derived from primary DIOs deficiency in humans. However, in 2005, mutations of Sec binding protein 2 have been identified. The authors reported an inherited selenocysteine defect, caused by a homozygous misense mutation of SBP2, which is an indispensable protein for selenoprotein synthesis, resulting in abnormalities in the deiodinases and defective thyroid metabolism (CitationDumitrescu et al 2005). Several diseases with disturbed thyroid hormone metabolism and expression of DIOs are known. In Graves’ hyperthyroidism the increased levels of T3 are known to induce DIO1 expression at the transcriptional level, which further contributes to the progression of the disease; on the other hand the use of propylthiouracil (PTU) inhibits DIO1 by binding to Sec residue, which represents a benefit in the treatment of hyperthyroidism (CitationBianco et al 2002; CitationLeonard and Rosenberg 2003). In hypothyroidism, decreased DIO1 levels with a concomitant increased DIO2 activity may be observed, potentially to serve as a rescue mechanism to provide local levels of T3 (CitationBianco et al 2002). Combined Se and iodine deficiency leads to the condition of myxedematous cretinism. Reduced DIOs and GPx activity are both supposed to play a central role for the development of this disease. The decreased GPx activity increase oxidative damage in the thyroid, while the reduced DIO concentration profoundly alters thyroid hormone metabolism (CitationKohrle et al 2005). The involvement of Se and selenoproteins in autoimmune thyroid diseases are discussed in a separate paragraph.
Other selenoproteins
Several others selenoproteins have been identified, and their localizations and functions have been quite clearly defined. They are involved in multiple processes that concern transport and delivery of Se, apoptosis, proliferation and cancer, muscle cells function, inflammation, and oxidation (see ). Furthermore, there are some others selenoproteins that have not been characterized (SelH, SelO, SelT, and SelV), and whose functions are thought to be related to redox processes (CitationCastellano et al 2005).
Table 2 Localizations and functions of selenoproteins other than GPx, DIO, and TrxR
Selenium and thyroid metabolism
The importance of Se for the thyroid function has been increasingly recognized and multiple laboratory experiments, clinical trials, and epidemiologic data have progressively revealed the relationships between Se, iodine, and thyroid hormones metabolism. Of interest, for example, is the condition of iodine deficiency. This situation, which is associated with high TSH values, produce increased levels of H2O2 (CitationSmyth 2003). The cytotoxic action on thyroid cells depends on both the formation of free radicals and the defence ability of antioxidative enzymes, the latter being impaired in a state of Se deficiency and altered selenoproteins activity (CitationEkholm and Bjorkman 1997). In order to accept electrons derived from oxidative reactions, the thyroid cells physiologically generate a large amount of H2O2 at the cell surface. The thyroid is thus a source of H2O2, a necessary substrate for thyroperoxidase (TPO) activity. The generation of H2O2 is the rate-limiting step in the thyroid hormone synthesis and is regulated by TSH action on an interacting second-messenger system (CitationKimura et al 1995). The production of H2O2 leads to the iodination of tyrosine residues and the coupling of iodinated tyrosines to thyroglobulin (Tg). It is known that the Tg iodination in vivo takes place on the external surface of the apical plasma membrane, where TPO is integrated and H2O2 is generated (CitationBjorkman and Erkhold 1995). H2O2 may easily cross the apical membrane to the luminal site where it reacts with TPO for the iodination of Tg. Then, when H2O2 is available, iodination may be catalyzed by TPO and controlled by GPx, which degrades H2O2. Excess H2O2 may diffuse into the cell, where it will promptly be attacked by GPx, TRx, and catalase, which is present in the peroxisomes (CitationCorvilain et al 2000). Increased GPx activity reduces H2O2 availability, whereas in conditions of Se deficiency decreased GPx activity results in higher generation of H2O2 and increased TPO activity. Therefore, the expected activity of the GPx system plays a pivotal function in the iodination process, while, the intrathyroidal concentration of Se is important for GPx activity. The GPx and catalase antioxidant systems are protected by the antioxidant protein thiol-specific anti-oxidant (TSA), which modulates H2O2-mediated responses. TSA is TSH-dependent regulated, although the effect of TSH on TSA gene expression at the transcriptional level remains unclear (CitationKim et al 2001).
Selenium and thyroid autoimmunity
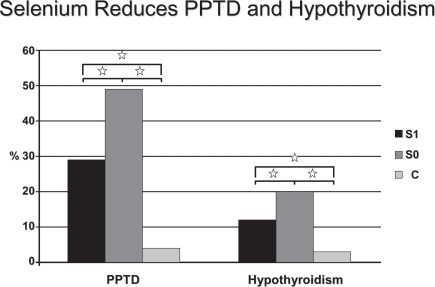
Some interventional studies have tested the hypothesis that Se administration may have a beneficial effect on autoimmune thyroiditis (AIT). It has recently been reported that in patients with AIT from an area in South Germany with borderline Se intake, 200 μg 3-month Se supplementation, in the form of selenite, significantly reduced anti-TPOAb concentration and improved ultrasound echomorphology (CitationGärtner et al 2002). In a 6-month follow-up crossover study, the anti-TPO concentrations continued to decrease significantly in the group that received Se, whereas the anti-TPOAb titers significantly increased in the group that stopped taking Se (CitationGärtner and Gasnier 2003). These findings are supported by another study conducted in patients with AIT in a non-Se-deficient area in Greece, in which anti-TPOAb concentrations also significantly decreased with a combined treatment over 6 months of 200 μg of selenomethionine and LT4 (CitationDuntas et al 2003). Anti-TPOAb reduction was prominent in the first 3 months of treatment, which may be the result of elevated intrathyroidal Se levels achieved during the study with consequent enhancement of the scavenging activity of both the GPx and TRx systems. In this respect, supplemented Se protected against goiter and thyroid tissue damage in a study from France that included 792 men and 1108 women from the SU.VIMAX study (CitationDerumeaux et al 2003). The authors also found a relationship to thyroid echostructure and concluded that Se may protect against autoimmune thyroid disease. Another recent study from Greece, evaluated the effects of Se supplementation (200 μg/day) in patients suffering from AIT. After 6 months of treatment a 9.9% reduction of TPOAb titers was observed; afterwards, while in one group the Se supplementation was continued, and a further decrement of TPOAb was observed, in the other group which stopped supplementation, a 4.8% increase took place (CitationMazopakis et al 2007). It has also been suggested that Se administration may have a protective role in pregnant women with AIT who are at a higher risk of miscarriage, as women with pregnancy loss had significantly lower Se hair content than controls (CitationAl Kunani et al 2001; CitationPrummel and Wiersinga 2004). A particularly interesting patients in the field of AIT, is represented by pregnant women. These patients are characterized by an increased risk of miscarriage, preterm delivery, and development of thyroid dysfunction after delivery (CitationNegro et al 2006). One study, involving 2143 euthyroid pregnant women (7.9% of them with TPOAb), evaluated the effects of 200 μg of selenomethionine supplementation during and after pregnancy. Results showed that, in women with autoimmune chronic thyroiditis, post-partum thyroid dysfunction and permanent hypothyroidism were lower in the treated group in respect to the untreated group (28.6 vs. 48.6%, P < 0.01; and 11.7 vs. 20.3%, P < 0.01, respectively) (CitationNegro et al 2007) (). The same study demonstrated that the Se supplemented women displayed lower TPOAb titers during the postpartum period and better thyroid ultrasound patterns when compared with the untreated ones. Finally, CitationMoncayo and Moncayo (2005) reported in a paper published in 2005 few cases of patients with autoimmune hypothyroidism who benefited from a daily Se supplementation. The cited patients, having serum Se concentrations below the lower limit, had an extraordinary recovery of thyroid function after Se treatment, with a condition of restored euthyroidism and an ameliorated ultrasound echogenicity pattern (CitationMoncayo and Moncayo 2005).
Figure 2 Percentage of patients who had PPTD (left) and hypothyroidism (right) develop in TPOAb(+) women who received Se (group S1) or placebo (group S0), and in TPOAb(−) women (group C)., P < 0.01 copyright © 2007. The Endocrine Society. Reproduced with permission from Negro R, Greco G, Mangieri T, et al. 2007. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab, 92:1263–8.
Despite their different phenotypes, Hashimoto’s thyroiditis and Graves’ disease (GD), share the production of organ-specific antibodies, and a common genetic background (CitationDayan and Daniels 1996; CitationStrieder et al 2003; CitationCaturegli et al 2007). GD is characterized by a condition of increased oxidative stress, not only in the acute phase of the disease, but also in the state of euthyroidism, induced by anti-thyroid medications (CitationAdemoğlu et al 2006). In fact, several studies outlined the association of GD with impaired antioxidant activity. Urinary malondialdehyde levels, a marker of oxidative stress, is higher in hyperthyroid patients compared to euthyroid controls (CitationGuerra et al 2005). In patients suffering from GD, the use of antithyroid drugs, either methimazole or propylthouracil, have been proven to be effective in lowering oxidant generation and improving the imbalance of the antioxidant/oxidant status (CitationSeven et al 2001; CitationAbalovich et al 2003). A recent study by CitationWertenbruch and colleagues (2007) compared serum Se levels in patients with remission and relapse of GD. The authors found that highest serum Se levels (>120 microg/l) were seen in the remission group, indicating a positive effect of Se levels on the outcome of GD. In addition, the authors showed that TSH-receptor antibodies levels and serum Se values were positively correlated in the relapse group, whereas a negative correlation of both parameters were seen in the remission group, supporting the idea of a positive effect of Se on thyroidal autoimmune process (CitationWertenbruch et al 2007). One study from Croatia, evaluated the effects of supplementation with a fixed combination of antioxidants (vitamins C and E, beta-carotene and selenium) on superoxide dismutase activity, copper and zinc concentrations, and total antioxidant status in erythrocytes derived from a group of patients with GD treated with methimazole, with respect to the rate of achieving euthyroidism (CitationBacic-Vrca et al 2005). Results showed that patients receiving antioxidant supplementation along with methimazole therapy achieved euthyroidism at a faster rate than those treated with methimazole alone. The activity of superoxide dismutase similarly decreased in both groups, while the total antioxidant status was mostly improved in the supplemented group. Taking together, the abovementioned studies’ results, may lead to think that Se supplementation may exert a beneficial effect on the course of GD.
Conclusion
Se is a mineral of pivotal importance for human health. It is an integral part of selenoproteins, many of which are involved in redox processes and are effective as antioxidants. The most frequent disease involving the thyroid gland is the autoimmune chronic thyroiditis, an inflammatory process which progressively destroy the gland. Several studies have examined the potential benefits of Se in this disease to assess if its supplementation may be effective in increasing the antioxidant defences. These studies have by consent demonstrated that Se supplementation, probably maximizing the Se-dependent enzymes activity, is able to reduce the inflammatory state, exerting a decline of TPOAb titers and an amelioration of the ultrasound pattern. Furthermore, there are some preliminary data which indicate a possible role of Se supplementation in ameliorating the course of GD, even if specifically and well-designed randomized controlled trials are necessary to address this issue.
However, several points have to be still clarified. Firstly, we have not fully understood functions and actions of the already identified selenoproteins; then, the first question is whether the effect of Se on thyroid autoimmunity depends on the maximization of GPx activity alone or also of other selenoproteins. Secondly, it has still to be defined the exact amount of Se to be given to maximize the enzymatic antioxidant activity. Thirdly, a cost/benefit evaluation is mandatory; in fact, the final result of autoimmune chronic thyroiditis is represented by hypothyroidism; Se supplementation should delay the time of Levothyroxine initiation, but obliges in any case the patient to take a tablet a day, and even when hypothyroidism develops, substitutive treatment with Levo-thyroxine has no side effects and it is a very cheap drug. One viable application of Se, may be its supplementation during pregnancy (possibly in conjunction with iodine supplementation). In fact, by now, screening for thyroid function during pregnancy is not warranted (CitationAbalovich et al 2007). Considering that up to ~10% of pregnant women are positive for TPOAb, and that about 8% of all pregnant women develop postpartum thyroiditis, it is plausible a positive effect exerted by Se in this particular kind of patients (CitationPoppe and Glinoer 2003; CitationStagnaro-Green 2004). Finally, it has to be reminded that Se have had a putative role in preventing atherosclerosis and carcinogenesis, and a possible action in improving glucose metabolism. All these hypothesis have been proven to be wrong, and in addition, a recently published study even suspected Se to be responsible for increased risk of developing diabetes (CitationHunter et al 1990; CitationGarland et al 1995; CitationBleys et al 2006; CitationStranges et al 2007).
Disclosure
Dr. Negro reports no conflicts of interest.
References
- AbalovichMLlesuySGutierrezS2003Peripheral parameters of oxidative stress in Graves’ disease: the effects of methimazole and 131 iodine treatmentsClin Endocrinol593217
- AbalovichMAminoMBarbourLA2007Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guidelineJ Clin Endocrinol Metab92S1S4717948378
- AdemoğluEOzbeyNErbilY2006Determination of oxidative stress in thyroid tissue and plasma of patients with Graves’ diseaseEur J Intern Med175455017142172
- Al KunaniASKnightRHaswellSJ2001The selenium status of women with a history of recurrent miscarriageBr J Obstet Gynaecol10810947
- ArnerESHolmgrenA2000Physiological functions of thioredoxin and thioredoxin reductaseEur J Biochem2676102911012661
- ArthurJR2000The glutathione peroxidasesCell Mol Life Sci5518253511215509
- AumannKDBedorfNBrigeluis-FloheR1997Glutathione peroxidase revisited–simulation of the catalytic cycle by computer-assisted molecular modellingBiomed Environ Sci10136559315305
- Bacic-VrcaVSkrebFCepelakI2005The effect of antioxidant supplementation on superoxide dismutase activity, Cu and Zn levels, and total antioxidant status in erythrocytes of patients with Graves’ diseaseClin Chem Lab Med43383815899653
- BatesJMSpateVLMorrisJS2000Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during developmentEndocrinology141249050010875250
- BiancoACKimBW2006Deiodinases: implications of the local control of the thyroid hormone actionJ Clin Invest1162571917016550
- BiancoACSalvatoreDGerebenB2002Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinasesEndocr Rev23388911844744
- BjorkmanUEkholmR1995Hydrogen peroxide degradation and glutathione peroxidase activity in cultures of thyroidal cellsMol Cell Endocrinol111991077649359
- BleysJMillerER3rdPastor-BarriusoR2006Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trialsAm J Clin Nutr84880717023716
- Brigelius-FloheR1999Tissue-specific functions of individual glutathione peroxidasesFree Radic Biol Med279516510569628
- CastellanoSLobanovAVCappleC2005Diversity and functional plasticity of eukaryotic selenoproteins: identification and characterization of the Sel familyProc Natl Acad Sci USA102161889316260744
- CaturegliPKimuraHRocchiR2007Autoimmune thyroid diseasesCurr Opin Rheumatol1944817143095
- ConradMJakupogluCMorenoSG2004Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart functionMol Cell Biol2494142315485910
- CorvilainBCollynLvan SandeJ2000Stimulation by iodide of H2O2 generation in thyroid slices from several speciesAm J Physiol Endocrinol Metab2786929
- DayanCMDanielsGH1996Chronic autoimmune thyroiditisN Engl J Med335991078649497
- DerumeauxHValeixPCastetbonK2003Association of selenium with thyroid volume and echostructure in 3-to 60-year-old French adultsEur J Endocrinol1483091512611611
- DumitrescuAMLiauXHAbdullahMS2005Mutations in SECISBP2 result in abnormal thyroid hormone metabolismNat Genet3712475216228000
- DuntasLHMantzouEKoutrasDA2003Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditisEur J Endocrinol1483899312656658
- EkholmRBjorkmanU1997Glutathione peroxidase degrades intracellular hydrogen peroxide and thereby inhibits intracellular protein iodination in thyroid epitheliumEndocrinology138287189202230
- GarlandMMorrisJSStampferMJ1995Prospective study of toenail selenium levels and cancer among womenJ Natl Cancer Inst874975057707436
- GärtnerRGasnierBCDietrichJW2002Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrationsJ Clin Endocrinol Metab8716879111932302
- GärtnerRGasnierBC2003Selenium in the treatment of autoimmune thyroiditisBiofactors191657014757967
- GromerSEubelJKLeeBL2005Human selenoproteins at a glanceCell Mol Life Sci6224143716231092
- GuerraLNRíos de Molina MdelCMilerEA2005Antioxidants and methimazole in the treatment of Graves’ disease: effect on urinary malondialdehyde levelsClin Chim Acta3521152015653105
- HunterDJMorrisJSStampferMJ1990A prospective study of selenium status and breast cancer riskJAMA2641128312384937
- JakupogluCPrzemeckGKSchneiderM2005Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac developmentMol Cell Biol251980815713651
- KimHParkSSuhJM2001Thyroid-stimulating hormone transcriptionally regulates the thiol-specific antioxidant geneCell Physiol Biochem112475211684813
- KimuraTOkajimaFShoK1995Thyrotropin-induced hydrogen peroxide producrion in FRTL-5 thyroid cells is mediated not by adenosine 3’,5’-monophosphate, but by Ca2+ signalling followed by phospholipase-A2 activation and potentiated by an adenosine derivativeEndocrinology136116237828520
- KoenigRJ2005Regulation of type 1 iodothyronine deiodinase in health and diseaseThyroid158354016131326
- KohrleJJakobFContempreB2005Selenium, the thyroid, and the endocrine systemEndocr Rev269448416174820
- KryukovGVCastellanoSNovoselovSV2003Characterization of mammalian selenoproteomesScience30014394312775843
- LeonardJLRosenbergIN2003Thyroxine 5’-deiodinase activity of rat kidney. Observations on activation by thiols and inhibition by propylthiouracilEndocrinology103213744748037
- LevanderOABeckMA1997Interacting nutritional and infectious etiologies of Keshan disease: insights from Coxackie virus B-induced myocarditis in mice deficient in selenium or vitamin EBiol Trace Elem Res565219152508
- MatsuiMOshimaMOshimaH1996Early embryonic lethality caused by targeted disruption of the mouse thioredoxin geneDev Biol178179858812119
- MazopakisEEPapadakisJAPapadomanolakiMG2007Effects of 12 months treatment with L-selenomethionine on serum anti-TPO levels in patients with Hashimoto’s thyroiditisThyroid176091217696828
- MoncayoRMoncayoH2005Nutritional treatment of incipient thyroid autoimmune disease. Influence of selenium supplementation on thyroid function and morphology in children and young adultsClin Nutr24530115998552
- NegroRFormosoGMangieriT2006Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complicationsJ Clin Endocrinol Metab9125879116621910
- NegroRGrecoGMangieriT2007The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodiesJ Clin Endocrinol Metab921263817284630
- NonnLWilliamsRREricksonRP2003The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous miceMol Cell Biol239162212529397
- PoppeKGlinoerD2003Thyroid autoimmunity and hypothyroidism before and during pregnancyHum Reprod Update91496112751777
- PrummelMFWiersingaWM2004Thyroid autoimmunity and miscarriageEur J Endocrinol150751515191343
- RaymanMP2000The importance of selenium to human healthLancet3562334110963212
- SevenRGelişgenRSevenA2001Influence of propylthiouracil treatment on oxidative stress and nitric oxide in Basedow disease patientsJ Toxicol Environ Health A6249550311289700
- SmythPP2003Role of iodine in antioxidant defence in thyroid and breast diseaseBiofactors1912113014757962
- St GermainDLHernandezASchneiderMJ2005Insights into the role of deiodinases from studies of genetically modified animalsThyroid159051616131333
- Stagnaro-GreenA2004Postpartum thyroiditisBest Pract Res Clin Endocrinol Metab183031615157842
- StawickiSPLyonsMAloupisM2007Current evidence from phase III clinical trials of selenium supplementation in critically ill patients: why should we bother?Mini Rev Med Chem7693917627581
- StrangesSMarshallJRNatarajanR2007Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trialAnn Intern Med1472172317620655
- StriederTGPrummelMFTijssenJG2003Risk factors for and prevalence of thyroid disorders in a cross-sectional study among healthy female relatives of patients with autoimmune thyroid diseaseClin Endocrinol59396401
- SunQAKirnarskyLShermanS2001Selenoprotein oxidoreductase with specificity for thioredoxin and glutathione systemsProc Natl Acad Sci USA983673811259642
- SunQAWuYZappacostaFJeangKT1999Redox regulation of cell signalling by selenocysteine in mammalian thioredoxin reductasesJ Biol Chem274245223010455115
- SuraiP2006Selenium in nutrition and healthNottingham, UKNottingham University Press
- VanderpasJBContempreBDualeNL1990Iodine and selenium deficiency associated with cretinism in northern ZaireAm J Clin Nutr521087932239787
- VincetiMWeiETMalagoliC2001Adverse health effects of selenium in humansRev Environ Health162335112041880
- WertenbruchTWillenbergHSSagertC2007Serum selenium levels in patients with remission and relapse of Graves’ diseaseMed Chem3281417504200
- WhangerPD2002Selenocompounds in plants and animals and their biological significanceJ Am Coll Nutr212233212074249
- XiaYHillKEByrneDW2005Effectiveness of selenium supplements in a low-selenium area of ChinaAm J Clin Nutr818293415817859
- ZhongLHolmgrenA2000Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutationsJ Biol Chem27518121810849437
- ZimmermannMBKohrleJ2002The impact of iron and selenium deficiencies on iodine and thyroid metabolism. Biochemistry and relevance to public healthThyroid128677812487769