Abstract
Psoriasis is a chronic, genetically determined, immune-mediated, inflammatory skin disease affecting approximately 2% to 3% of Caucasian population. Given the well-established role of the immuno-mediated inflammation in the pathogenesis of psoriasis, in the past few years several key steps in the pathogenesis of this disease have been elucidated and the increased knowledge led to the development of specific drugs, commonly defined as “biologics” targeting one or more of these steps. At present an anti-CD11a antibody (efalizumab), an anti-LFA3/CD2 receptor (alefacept) and 3 antitumor necrosis factor alpha agents (adalimumab, etanercept, infliximab) are now commercially available for the treatment of both psoriasis and psoriatic arthritis. Recent studies have demonstrated that interleukins (IL) 12 and 23 play an important role in the pathophysiology of psoriasis. In fact members of the IL-12 family of cytokines have the potential to act as the next major cytokine(s) in pathogenesis and the treatment of psoriasis. Ustekinumab (CNTO 1275, Centocor Inc, Malvern, PA, USA) is a human monoclonal antibody that binds to the shared p40 protein subunit of human interleukins 12 and 23 with high affinity and specificity, thereby preventing interaction with their surface IL-12Rβ1 receptor. Different clinical studies have been conducted to date. In particular a phase II study and two phase III studies, PHOENIX 1 together with PHOENIX 2, show very encouraging results. This review reports on the latest progress made in the clinical use of biologic drugs for psoriasis focusing on the new human IL-12/23 monoclonal antibody, ustekinumab, for psoriasis.
Introduction
Psoriasis is a chronic, genetically determined, immuno-mediated, inflammatory skin diseaseCitation1 affecting approximately 2% to 3% of Caucasian population.Citation2 Plaque type is the most common form,Citation3,Citation4 prevalence ranging between 5% and 42%, and depending on the population studied, psoriatic arthritis may occur.Citation5 In the majority of patients (70%) psoriasis is present for several years before arthritis onset; in 10% to 15% arthritis precedes psoriasis, while in 15% the initial presentation includes arthritis and psoriasis together.Citation6,Citation7 Psoriasis can be associated with significant psychological morbidity, comparable with that of major medical illnesses such as rheumatoid arthritis, hypertension, heart disease and depression.Citation8,Citation9 Furthermore, an alarmingly higher percentage of depression and suicidal ideation has been observed in psoriatic populations compared to other severe diseases in the absence of a correct philosophy counseling.Citation10–Citation12 Numerous co-morbidities, such as dyslipidemia, heart disease, hypertension, diabetes and emphysema have been reported.Citation13
This review reports on the latest progress made in the clinical use of biologic drugs for psoriasis, focusing on the new human IL 12/23 monoclonal antibody, ustekinumab, for psoriasis.
Overview of antipsoriatic agents
The choice of treatment for psoriasis depends on the severity of the disease. However, the evaluation of its severity may be difficult in some cases, as not only objective parameters (eg, extension, erythema, infiltration) but also the impact on the quality of life of the patients must be taken into consideration. Indeed smaller lesions may cause higher psychological or physical discomfort for the patient, depending on their localization.
Given the well-established role of the immuno-mediated inflammation in the pathogenesis of psoriasis, the immune system is the main target of almost all systemic treatments available for this disease.
Systemic agents, including ciclosporin, methotrexate and acitretine, or phototherapy are usually prescribed in patients affected by moderate to severe plaque-type psoriasis or psoriatic arthritis. However, various factors may limit their long-term use, in particular the risk of serious cumulative organ toxicityCitation14 and the lack of efficacy over time.Citation15 Furthermore, underlying diseases, such as hypertension as well as renal and/or hepatic alterations, may represent contraindications to the above-mentioned systemic treatments. Thus, effective drugs with less long-term toxicity are needed.
An excellent therapeutic improvement has been recently obtained by the introduction of the “biological response modifiers”, or more commonly defined as “biologics”. Biologics are a heterogeneous group of monoclonal antibodies, fusion proteins and recombinant cytokines, designed to modify and regulate pivotal and specific mechanisms involved in psoriasis immunopathogenesis. To date, biologics have been suggested to have a more favorable side effect profile than conventional treatments.Citation16
Biologic agents for the treatment of psoriasis
Psoriasis can be considered as the prototype chronic inflammatory disorder. In the past few years several key steps in the pathogenesis of this disease have been elucidated and the increased knowledge has led to the development of specific drugs targeting one or more of these steps.
Recent literatureCitation17 indicates the activation of the innate immune system as the initial factor triggering psoriasis. Genetically determined defects in the skin barrier (eg, PSORS4 and PSORS6) lead to the activation of skin resident plasmocytoid dendritic cells by antimicrobial peptides such as cathelicidin (LL37) or beta-defensin 2 induced by saprophytic flora or trauma. These cells, belonging to the innate branch of the immune system, overproduce inflammatory cytokines such as IFNα, LTα and IL1β which in turn activate CD11c expressing myeloid dendritic cells.Citation18
The activation of these cells appears to be a key step in psoriasis plaque initiation. Indeed CD11c positive myeloid dendritic cells (also called dermal dendritic cells) are capable of activating naïve-T lymphocytes in lymph nodes and to induce their polarization to Th1 or Th17 cells.Citation18 Production of two specific heterodimeric cytokines (interleukins 12 and 23, IL-12 and IL-23) which share a common subunit (p40) are necessary to direct T-cells toward the Th1 (IL-12) or Th17 (IL-23) phenotype.
Activated T lymphocytes are then subjected to a cell trafficking/homing step, involving the interaction between leucocyte function-assisted antigen-1 (LFA1) and ICAM1 molecules, which directs them towards inflamed skin. Once in the inflamed site T-cells are reactivated by dermal dendritic cells and produce a specific set of cytokines including antitumor necrosis factor alfa (TNFα), IL-22, interferon gamma (IFNγ), IL-6, IL-2 and IL-8 in order to propagate inflammation and induce keratinocyte hyperproliferation, ultimately leading to the development of mature psoriasis plaque.
Each of these pathogenetic mechanisms represent optimal target for highly specific therapeutical intervention being upstream events much better targets than downstream steps.
describes the different specific drugs targeting specific pathogenetic events.
Figure 1 An overview of different pathogenetic steps of psoriasis and the specific drugs targeting each of these steps.
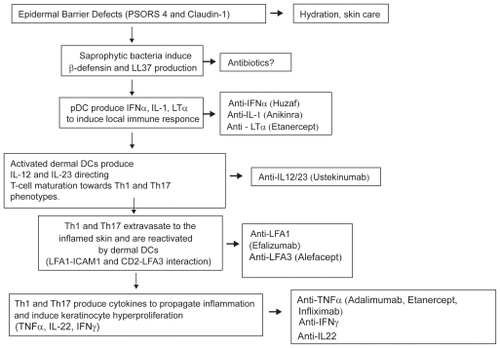
Numerous biologics are approved by FDA or EMEA for the treatment of psoriasis, including an anti-CD11a antibody, namely efalizumab, and an anti-LFA3/CD2 receptor, alefacept. In addition 3 TNFα agents (adalimumab, etanercept, infliximab) are now commercially available for the treatment of both psoriasis and psoriatic arthritis.
Alefacept
Alefacept (Amevive®, Biogen Inc.) is a fully humanized fusion protein composed from the first extracellular domain of LFA3 and the constant (CH2 and CH3) and hinge domain of IgG1. It binds to the natural ligand of LFA3, the CD2 receptor (present on T lymphocytes), thus blocking the normal interaction between LFA3 and CD2 and, consequently, between antigen presenting cells and T lymphocytes. Moreover, because of its molecular structure, alefacept mediates cognate interaction between cells expressing CD2 and cells expressing Fc-gamma receptor type IIIA, like natural killer (NK) cells: this leads to the recruitment of NK cells on alefacept binding CD2 positive T-cells leading to their apoptosis.Citation19 Alefacept can be administered intramuscularly or intravenously, at the dosage of 15 mg per week and 7.5 mg per week, respectively
Alefacept was the first biologic agent approved by the US Food and Drug Administration for the treatment of moderate to severe plaque psoriasisCitation20 and it is estimated that it has been used in more than 10,000 patients. Efficacy was firstly evaluated in two randomized, double-blind, placebo-controlled phase III trials,Citation21,Citation22 which used intravenous and intramuscular administration, respectively.
Efalizumab
Efalizumab is a recombinant, humanized monoclonal antibody that targets the alpha-subunit (CD11a) of the LFA1. By blocking the interaction of CD11a and of the intercellular adhesion molecule-1 (ICAM-1), efalizumab interferes at multiple levels with the pathogenesis of psoriasis, inhibiting leukocyte trafficking into the skin as well as the continuous activation of T-lymphocytes by antigen-presenting cells in the dermis and epidermis.Citation23 Efalizumab is indicated for use in adults with moderate-to severe plaque psoriasis who are eligible for systemic therapy and considered as “high need” and is self-administrated by the patient once weekly by subcutaneous injection at the recommended dose is 1 mg/kg/week.Citation24,Citation25 Several clinical trials have been performed with efalizumab, which validated its efficacy in short-term (12 weeks), mid-term (24 weeks) or long-term (36 or more months) treatment.Citation26,Citation27
Anti-TNFα agents
Etanercept
Etanercept is a dimer of a chimeric protein genetically engineered by fusing the extracellular, ligand binding domain of the human TNF receptor 2 and the Fc domain of human IgG1 antibody (including the hinge, CH2 and CH3 regions but not the CH1 region).Citation28 Because of its structure, etanercept binds and neutralizes the inflammatory cytokines TNF and lymphotoxin-alpha (TNFβ).Citation29 Etanercept has been approved for the control of signs and symptoms, inhibiting radiographic progression and improving function in adults with psoriatic arthritis; it is also approved for moderate to severe plaque-type psoriasis. Etanercept is self-administrated subcutaneously, at a dosage regimen of 25 mg twice weekly or 50 mg once weekly (50 mg twice weekly can be used for the initial 12 weeks). Its efficacy and safety for the different dosings have been demonstrated in several randomized clinical trials.Citation30–Citation33 Pharmacokinetic modeling and simulation, as well as clinical studies, suggest that the systemic exposure is equivalent for the 25 mg twice weekly and the 50 mg once weekly regimens.Citation34,Citation35 However the 50 mg twice weekly dose seems to have stronger clinical activity. Current regulation allows using etanercept in 24-week courses.
Infliximab
Infliximab is a chimeric (25% mouse, 75% human) monoclonal IgG1 antibody able to bind TNFα (transmembrane and soluble form) and, consequently, to inhibit TNFα activity.Citation36 Infliximab was the first anti-TNFα therapy successfully used in psoriasis, in a woman affected also by refractory inflammatory bowel disease and therefore treated with infliximab 5 mg/kg.Citation37 Several studies show that infliximab acts on multiple steps of the pathogenetic process of psoriasis. Infliximab is approved for the treatment of psoriatic arthritis and plaque type psoriasis. Infliximab is administered intravenously, usually at the dose regimen of 5 mg/kg at weeks 0, 2, 6, and successively every 8 weeks. The efficacy and safety of infliximab has been evaluated in several clinical trials.Citation36,Citation38,Citation39 Infliximab shows a fast efficacy in the treatment of plaque and arthritic psoriasis.
Adalimumab
Adalimumab is a fully human monoclonal antibody, genetically engineered using phage display technology, and it is structurally and functionally analogous to naturally occurring human immunoglobulins G1 (IgG1). This monoclonal antibody demonstrates a high specificity and affinity for TNFα but not other cytokines, such as lymphotoxin (LTβ).Citation40 The recommended dosing of adalimumab for the treatment of plaque-type psoriasis and psoriatic arthritis is an initial dose of 80 mg, followed by 40 mg given every other week starting 1 week after the initial dose.Citation41 The efficacy and safety of adalimumab has been evaluated in several clinical trials.Citation42–Citation45 Moreover adalimumab was the first biologic drug for which a comparative study with a conventional drug (methotrexate) has been performed.Citation46
Role of interleukins 12 and 23 in pathogenesis of psoriasis
Recent studies have demonstrated that IL-12 and IL-23 play an important role in the pathophysiology of psoriasis. Genetic polymorphisms in the genes that encode the shared p40 subunit of these cytokines (IL-12B), and one of the IL-23 receptor subunits that confer protection against Crohn’s diseaseCitation47 has also been shown to confer protection against psoriasis.Citation48 The p40 subunit of IL-12 and IL-23 is overexpressed in psoriasis plaques,Citation49 and preclinical studies implicate p40-containing cytokines in the pathogenesis of psoriasis.Citation50
These cytokines participate in immune function through activation of natural killer cells and CD4+ T-cell differentiation. IL-12 induces differentiation towards the T helper 1 (Th1) response and expression of type 1 cytokines, such as IFNγ and TNFα, which are important in the pathophysiology of psoriasis.Citation51–Citation53 IL-23 induces differentiation towards a T helper 17 (Th17) phenotype,Citation54 expression of interleukin 17, and increased keratinocyte expression of inducible nitric oxide synthase, which has also been implicated in the pathophysiology of psoriasis.Citation55,Citation56 Cytokines produced by Th17 cells, including interleukin 20 and interleukin 22, have been implicated in the keratinocyte hyperproliferative response in psoriasis.Citation57,Citation58 Although the mechanisms by which IL-12 and IL-23 contribute to the pathophysiology of psoriasis are incompletely understood, the shared p40 protein might have a key role in the inflammatory cascade.Citation59
Many current therapies used in the treatment of psoriasis modulate levels of IL-12 and IL-23, which are speculated to contribute to their efficacy,Citation50 and agents that specifically target these cytokines improved psoriasis in early clinical trials.Citation60–Citation62
Ustekinumab
Ustekinumab (CNTO 1275, Centocor Inc, Malvern, PA, USA) is a human monoclonal antibody that binds to the shared p40 protein subunit of human IL-12 and IL-23 with high affinity and specificity, thereby preventing interaction with their surface IL-12Rβ1 receptor.Citation63
Clinical studies
Different clinical studies have been conducted to date, in particular a phase I study, a phase II study and two phase III studies (PHOENIX 1 together with PHOENIX 2).
Phase I study
This was the first-in-human, non-randomized open-label study. The primary objective of this study was to evaluate the short-term efficacy, assess the pharmacokinetics, and determine the clinical response of single, ascending, intravenous (iv) administrations of anti-IL-12p40 in subjects with moderate to severe psoriasis vulgaris. Eighteen subjects with at least 3% body surface area involvement were enrolled in 4 dose groups (0.1, 0.3, 1.0, and 5.0 mg/kg). Safety, pharmacokinetics, and clinical response (eg, Psoriasis Area and Severity Index [PASI]) were monitored at baseline and at specific time points over a 16-week follow-up period. In this study noticeable clearing of psoriatic plaques was observed as early as 2 weeks following the study agent administration, with maximal therapeutic benefit achieved at 12 weeks for the majority of the subjects. In particular 12 of the 18 subjects (67%) achieved at least a 75% improvement in the PASI score between 8 and 16 weeks after the study agent administration. Both the rate and extent of the clinical response across the four dose groups was dose dependent. Moreover the drug was well tolerated and no treatment-related serious adverse events or adverse events requiring discontinuation or dose reduction were reported in this study.
In conclusion, this clinical study has provided the first information on the safety, pharmacokinetics, and clinical response of a single iv administration of anti-IL-12p40 in human subjects with moderate-to-severe plaque psoriasis.Citation60
Phase II study
In this double-blind, parallel group, placebo-controlled trial the safety and efficacy of single and multiple doses of the IL-12/23 monoclonal antibody (CNTO 1275) for the treatment of psoriasis was evaluated. A total number of 320 patients with moderate to severe plaque psoriasis have been enrolled. With the use of adaptive treatment allocation based on a biased-coin minimization algorithm, patients were randomly assigned to one 45-mg subcutaneous dose of the IL-12/23 monoclonal antibody, one 90 mg dose, 4-weekly 45 mg doses, 4-weekly 90 mg doses, or placebo. Randomization was stratified by investigational site and by the weight of the patient relative to 95 kg. At week 16, patients with a physician’s global assessment (termed PGA) of less than excellent (≥3 on a scale on which 1 is best and 6 is worst) received one additional injection of their originally assigned dose. At week 20, patients in the placebo group crossed over to receive one 90-mg injection of the IL-12/23 monoclonal antibody. Efficacy evaluations were performed through week 32. The primary end point was the proportion of patients achieving PASI 75 at week 12 and the analysis was performed in an intention to treat basis to include data from all patients who had undergone randomization.
At week 12 (primary end point), PASI 75 was achieved in 52% of the patients who received one 45 mg dose of the interleukin-12/23 monoclonal antibody, in 59% of those who received one 90 mg dose, in 67% of those who received 4-weekly 45 mg doses and in 81% of those who received 4-weekly 90 mg doses, as compared with 2% of those who received (p < 0.001 for each comparison), moreover PASI 90 was achieved in in 23%, 30%, 44% and 52%, respectively, of patients who received the monoclonal antibody as compared with 2% of patients who received placebo (p < 0.001 for each comparison).
Adverse events occurred in 79% of the patients treated with the IL-12/23 monoclonal antibody as compared with 72% of the patients in the placebo group. Serious adverse events occurred in 4% of patients who received the monoclonal antibody and in 1% of those who received placebo.
This study has demonstrated the therapeutic efficacy of an IL-12/23 monoclonal antibody in psoriasis, providing further evidence of a role of the IL-12/23 p40 cytokines in the pathophysiology of psoriasis. The authors suggested that larger studies are needed to determine whether serious adverse events might limit the clinical usefulness of this new therapeutical target.Citation61
PHOENIX 1
This was a phase III, double-blind, placebo-controlled, multicenter trial in which a total of 766 patients with moderate to severe psoriasis were enrolled. These patients were at least 18 years old, with a diagnosis of plaque psoriasis for at least 6 months, candidates for phototherapy or systemic therapy, with a PASI score of 12 or higher at baseline and with at least 10% body surface area involvement. Patients with a history or symptoms of active tuberculosis were excluded from the study; however, those with latent tuberculosis (a positive Mantoux tuberculin skin test without radiological evidence of tuberculosis) could be enrolled if treatment for latent tuberculosis according to local country guidelines for immunocompromised patients was initiated either before or simultaneous to the first administration of study agent.
This study was divided in 3 phases: a placebo-controlled phase (weeks 0–12), a placebo-crossover and active treatment phase (weeks 12–40), and a randomized withdrawal phase (weeks 40–76). The safety and efficacy of ustekinumab were assessed over these three periods. At baseline, patients were randomly assigned in equal proportions to receive subcutaneous injections of ustekinumab 45 or 90 mg at weeks 0 and 4 and every 12 weeks thereafter or placebo at weeks 0 and 4, with half randomized to crossover to ustekinumab 45 mg and half to ustekinumab 90 mg at week 12. At week 40, patients who had initially been randomized to receive ustekinumab who achieved long-term response (at least 75% improvement from baseline in PASI score [PASI 75] at weeks 28 and 40) were re-randomized to continue maintenance treatment with ustekinumab or were withdrawn from active treatment (placebo). Patients withdrawn from treatment at week 40 were re-treated when they lost at least 50% of PASI improvement. Patients not achieving PASI 75 at week 28 or 40 were not re-randomized, and their dosing was discontinued or modified. Baseline randomization was stratified by investigational site, weight (≤90 kg or >90 kg), and the number of conventional systemic therapies to which patients had an inadequate response, intolerance, or contraindication (<3 or ≥3). Week 40 randomization was stratified by investigational site and baseline weight (≤90 kg or >90 kg).
The primary efficacy endpoint was the proportion of patients achieving PASI 75 at week 12. Major secondary endpoints included: the proportion of patients with a physician’s global assessment score of cleared or minimal at week 12; the change in dermatology life quality index from baseline at week 12; and, in the randomized withdrawal phase, time to loss of PASI 75 response in the group receiving maintenance ustekinumab compared with the group withdrawn from treatment at week 40. About two-thirds of patients in each group were men. On average, patients had a 20-year history of psoriasis and about a quarter of their body surface area affected by psoriasis. Over 90% of patients had used topical treatments previously, and at least 50% each had previously used conventional systemic or biological therapies.
All randomized patients were included in the efficacy analysis. 171 (67.1%) patients receiving ustekinumab 45 mg, 170 (66.4%) receiving ustekinumab 90 mg, and 8 (3.1%) receiving placebo achieved PASI 75 at week 12 (difference in response rate vs placebo 63.9%, 95% CI 57.8–70.1, p < 0.0001 for 45 mg and 63.3%, 95% CI 57.1–69.4, p < 0.0001 for 90 mg). Moreover PASI 90 score was achieved in 42%, 37% and 2% of patients treated with ustekinumab 45 mg, 90 mg and placebo respectively. At week 40, long-term response had been achieved by 150 patients in the 45 mg group and 172 patients in the 90 mg group. Of these, 162 patients were randomly assigned to maintenance ustekinumab and 160 to withdrawal. PASI 75 response was better maintained to at least 1 year in those receiving maintenance ustekinumab than in those withdrawn from treatment at week 40 (p < 0.0001 by log-rank test). During the placebo-controlled phase, adverse events occurred in 278 (54.5%) of the 510 patients receiving ustekinumab and 23 (48.2%) of the 255 receiving placebo. Serious adverse events occurred in 6 (1.2%) of 510 patients receiving ustekinumab and in 2 (0.8%) of 255 receiving placebo in this phase. The pattern of adverse events was much the same in the placebo crossover and randomized withdrawal phases as it was in the placebo-controlled phase.
This study has concluded that ustekinumab seems to be efficacious for the treatment of moderate to severe psoriasis and that dosing every 12 weeks maintains efficacy for at least a year in most patients.Citation63
PHOENIX 2
In this multicenter, phase III, double-blind, placebo-controlled study a total number of 1230 patients with moderate-to-severe psoriasis were enrolled. The inclusion criteria were the same as in the PHOENIX 1 study. The study was divided into a placebo-controlled phase (weeks 0–12), a placebo crossover and active treatment phase (weeks 12–28), and a randomized dose intensification phase (weeks 28–52). The patients were randomly assigned to receive ustekinumab 45 mg (n = 409) or 90 mg (n = 411) at weeks 0 and 4, then every 12 weeks, or placebo. Partial responders (ie, patients achieving ≥50% but <75% improvement from baseline in PASI) were re-randomized at week 28 to continue dosing every 12 weeks or escalate to dosing every 8 weeks. The baseline randomization was stratified by investigational site, weight (≤90 kg or >90 kg), and whether the patient had an inadequate response, intolerance, or contraindication to less than 3 or more than 3 conventional systemic therapies. The primary efficacy endpoint was the proportion of PASI 75 responders at week 12. Major secondary endpoints were the proportion of patients with a physician’s global assessment score of cleared or minimal at week 12, change in dermatology life quality index from baseline at week 12, and, in the re-randomized partial responders, the number of visits with PASI 75 response between weeks 40 and 52 in the group receiving study drug every 8 weeks compared with the group receiving the drug every 12 weeks. Analyses were by intention to treat.
All randomized patients were included in the efficacy analysis. 273 (66.7%) patients receiving ustekinumab 45 mg, 311 (75.7%) receiving ustekinumab 90 mg, and 15 (3.7%) receiving placebo achieved the primary endpoint (difference in response rate 63.1%, 95% CI 58.2–68.0, p < 0.0001 for the 45 mg group vs placebo and 72.0%, 67.5–76.5, p < 0.0001 for the 90 mg group vs placebo). More partial responders at week 28 who received ustekinumab 90 mg every 8 weeks achieved PASI 75 at week 52 than did those who continued to receive the same dose every 12 weeks (22 [68.8%] vs 11 [33.3%]; difference in response rate 35.4%, 95% CI 12.7–58.1, p = 0.004). There was no such response to changes in dosing intensity in partial responders treated with ustekinumab 45 mg. During the placebo-controlled phase, 217 (53.1%) patients in the 45 mg group, 197 (47.9%) in the 90 mg group, and 204 (49.8%) in the placebo group experienced adverse events; serious adverse events were seen in 8 (2.0%) patients in the 45 mg group, 5 (1.2%) in the 90 mg group, and 8 (2.0%) in the placebo group.
This study has concluded that although treatment with ustekinumab every 12 weeks is effective for most patients with moderate-to-severe psoriasis, intensification of dosing to once every 8 weeks with ustekinumab 90 mg might be necessary to elicit a full response in patients who only partially respond to the initial regimen.Citation64
Discussion
The use of a single cytokine as a potential target of antipsoriatic treatment has been widely validated with the success of the anti-TNFα therapies. In fact fusion proteins (etanercept) or antibodies (inliximab, adalimumab) blocking this cytokine have been shown to exert a potent antipsoriasic activity. Based on this fact and taking into consideration the different pathogenetic steps of psoriasis, other master cytokines with therapeutic potential for psoriasis may be used. One such candidate is a member of the IL-12 family of heterodimeric proteins sharing the p40 chain, as the blocking of the ILp40/IL-12Rβ1 interaction prevents the biological activity of IL-12 and IL-23, so interfering with the pathogenetic Th1 and Th17 environment in psoriasis. In fact members of the IL-12 family of cytokines have the potential to act as the next major cytokine(s) in pathogenesis and the treatment of psoriasis.Citation59
The encouraging results presented in the above-mentioned studies regarding the use of ustekinumab for the treatment of psoriasis support the notion that IL-12 and IL-23 have a role in the immunopathophysiology of psoriasis.
Treatment with two 45 or 90 mg injections of ustekinumab at week 0 and week 4, followed by dosing every 12 weeks, led to PASI 75 response in more than three quarters of patients with moderate to severe psoriasis in both PHOENIX 1 and PHOENIX 2. In fact more than 90% of patients experienced clinically meaningful improvements, while the PHOENIX 2 study further characterized determinants of treatment resistance as well as potential therapeutic approaches for the treatment-resistant subpopulation.Citation63,Citation64 Clinical improvements were also associated to improvements in patient-reported outcomes, measured by dermatology life quality index.
Onset of response was seen within 2 weeks of the first dose of ustekinumab, and psoriasis response was generally maintained for at least a year and a half in patients who received the drug every 12 weeks. The maintenance of efficacy between longer dosing intervals may be due to the fact that the pharmacodynamic effects of ustekinumab are longer than its pharmacokinetic effects. In patients withdrawn from ustekinumab, psoriasis gradually recurred – showing that temporary blockade of IL-12 and IL-23 did not reverse the underlying causal mechanisms of psoriasis – and quality-of-life improvements were lost. Combined, these observations suggest that maintaining response and quality-of-life improvements could require continuous maintenance dosing. Among patients withdrawn from ustekinumab, response was generally restored within 12 weeks of reinitiating treatment.
In conclusion the results of different clinical studies suggest that ustekinumab could be an important therapeutic agent for treating patients with psoriasis. The high level of efficacy of ustekinumab was generally maintained with dosing every 12 weeks, a schedule that could offer a novel level of convenience for patients and physicians. These attributes might be particularly important for treatment compliance, which is low in patients with psoriasis and could, at least in part, result from dissatisfaction with effectiveness or convenience of available treatments.Citation65,Citation66
Nevertheless more studies are needed in order to evaluate the efficacy of ustekinumab also for the treatment of psoriatic arthritis, as joint involvement may be associated with the skin condition (in 5% to 42% of the patients).Citation5
Disclosures
MP serves as a consultant to Schering-Plough and as a speaker for Abbott and Schering-Plough; AC serves as a speaker for Serono; SC serves as consultant to Centocor, Schering-Plough, Serono, and Wyeth.
References
- PrinzJThe role of T cells in psoriasisJ Eur Acad Dermatol Venereol20031725727012702062
- ChristophersEPsoriasis – epidemiology and clinical spectrumClin Exp Dermatol20012631432011422182
- LebwohlMPsoriasisLancet20033611197120412686053
- SchonMPBoehnckeWHPsoriasisN Engl J Med20053521899191215872205
- GladmanDDBrockbankJPsoriatic arthritisExpert Opin Investig Drugs2000915111522
- CroffordLJKlippelJHPsoriatic ArthritisPrimer on the rheumatic diseases12th editionAtlanta (GA)Arthritis Foundation2001234238
- GladmanDDShuckettRRusselMLPsoriatic arthritis (PSA): an analysis of 220 patientsQ J Med1987621271413659255
- de ArrudaLHDe MoracsAPThe impact of psoriasis in quality of lifeBr J Dermatol2001144 Suppl 58333611501512
- RappSRFeldmanSRExumMLPsoriasis causes as much disability as other major medical diseasesJ Am Acad Dermatol19994140140710459113
- EspositoMSaracenoRGiuntaAAn Italian study on psoriasis and depressionDermatology200621212312716484818
- GuptaMAGuptaAKDepression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasisBr J Dermatol19981398468509892952
- GuptaMASchorkNJGuptaAKSuicidal ideation in psoriasisInt J Dermatol1993321881908444530
- PearceDJMorrisonAEHigginsKBThe comorbid state of psoriasis patients in a university dermatology practiceJ Dermatolog Treat2005165–631932316428152
- Tristani-FirouziPKruegerGGEfficacy and safety of treatment modalities for psoriasisCutis19986111219787987
- AshcroftDMLi Wan PoAGriffithsCETherapeutic strategies for psoriasisJ Clin Pharm Ther20002511010771459
- SterryWBarkerJBoehnckeWHBiologicals in psoriasis consensusBr J Dermatol2004151 Suppl 6931715265063
- LandeRGregorioJFacchinettiVPlasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptideNature2007449716256456917873860
- LowesMAKikuchiTFuentes-DuculanJPsoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cellsJ Invest Dermatol200812851207121118200064
- da SilvaAJBrickelmaierMMajeauGRAlefacept, an immunomodulatory recombinant LFA-3/IgG1 fusion protein, induces CD16 signaling and CD2/CD16-dependent apoptosis of CD2(+) cellsJ Immunol20021684462447111970990
- PiascikPAlefacept, first biologic agent approved for treatment of psoriasisJ Am Pharm Assoc200343649650
- KruegerGGPappKAStoughDBA randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasisJ Am Acad Dermatol20024782183312451365
- LebwohlMChristophersELangleyRAn international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasisArch Dermatol200313971972712810502
- JullienDPrinzJCLangleyRGT-cell modulation for the treatment of chronic plaque psoriasis with efalizumab (Raptiva): mechanisms of actionDermatology2004208429730615178911
- RaptivaProduct information Available at: http//www.kompendium.ch
- Raptiva EMEA approved product information Available at: http://www.emea.com
- GuptaAKChermanAMEfalizumab in the treatment of psoriasisJ Cutan Med Surg200610576817241576
- WoolacottNHawkinsNMasonAEtanercept and efalizumab for the treatment of psoriasis: a systematic reviewHealth Technol Assess200610125217083854
- European Medicines Agency (EMEA)European Public Assessment Report on Enbrel Available at http://www.emea.europa.eu/humandocs/PDFs/EPAR/Enbrel/H-262-PI-en.pdfAccessed July 31, 2007
- MeasePPsoriatic arthritis: the role of TNF inhibition and the effect of its inhibition with etanerceptClin Exp Rheumatol200220 6 Suppl 2811612111892698
- LeonardiCLPowersJLMathesonRTEtanercept as monotherapy in patients with psoriasisN Engl J Med20033492014202214627786
- GottliebABMathesonRTLoweNA randomized trial of etanercept as monotherapy for psoriasisArch Dermatol20031391627163214676082
- PappKATyringSLahfaMA global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reductionBr J Dermatol20051521304131215948997
- TyringSGottliebAPappKEtanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomized phase III trialLancet2006367293516399150
- ZhouHClinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion proteinJ Clin Pharmacol20054549040715831771
- NestorovIZitnikRLuddenTPopulation pharmacokinetic modeling of subcutaneously administered etanercept in patients with psoriasisJ Pharmacokinet Pharmacodyn20043146349016222785
- GottliebABEvansRLiSInfliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trialJ Am Acad Dermatol20045153454215389187
- OhCJDasKMGottliebABTreatment with anti-tumor necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesionsJ Am Acad Dermatol200042 5 Pt 182983010775863
- ReichKNestleFOPappKInfliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trialLancet20053661367137416226614
- MenterAFeldmanSRWeinsteinGDA randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasisJ Am Acad Dermatol200756311517097378
- SalfeldJKaymakcalanZTraccyDGeneration of fully human anti- TNF antibody D2E7 [abstract]Arthritis Rheum199841Suppl 57
- Humira [package insert]. Abbott Park, Ill. Abbott
- MeasePGladmanDRitchlinCAdalimumab for the treatment of patients with moderately to severely active psoriatic arthritisArthritis Rheum200552103279328916200601
- GladmanDMeasePRitchlinCAdalimumab for long-term treatment of psoriatic arthritis. Forty-eight week data from the adalimumab effectiveness in psoriatic arthritis trialsArthritis Rheum200756247648817265483
- GordonKBLangleyRGLeonardiCClinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension studyJ Am Acad Dermatol20065559860617010738
- MenterATyringSGordonKAdalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trialJ Am Acad Dermatol200858110611517936411
- SauratJHStinglGDubertretLEfficacy and safety results from the comparative study of adalimumab (Humira) versus methotrexate versus placebo in psoriasis patients (CHAMPION)Br J Dermatol2008158355856618047523
- DuerrRHTaylorKDBrantSRA genome-wide association study identifies IL23R as an inflammatory bowel disease geneScience20063141461146317068223
- CaponFDi MeglioPSzaubJSequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasisHum Genet20071222010617587057
- PiskinGSylva-SteenlandRMRBosJDTeunissenMBMIn vitro and in situ expression of IL-23 by psoriasis lesions: enhanced expression in psoriatic skinJ Immunol20061761908191516424222
- TortiDCFeldmanSRInterleukin-12, interleukin-23, and psoriasis: current prospectsJ Am Acad Dermatol2007571059106817706835
- TrinchieriGInterleukin-12 and the regulation of innate resistance and adaptive immunityNat Rev Immunol2003313314612563297
- KruegerJGThe immunologic basis for the treatment of psoriasis with new biologic agentsJ Am Acad Dermatol20024612311756941
- ToichiETorresGMcCormicSAn anti-IL-12p40 antibody down-regulates Type 1 cytokines, chemokines and IL-12/IL23 in psoriasisJ Immunol200617774917492616982934
- WilsonNJBonifaceKChanJRDevelopment, cytokine profile and function of human interleukin 17-producing helper T cellsNat Immunol200789505717676044
- Bruch-GerharzDFehselKSuschekCMichelGRuzickaTKolb-BachofenVA proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytesJ Exp Med19961842007128920887
- NickoloffBJCracking the cytokine code in psoriasisNat Med20071324224417342112
- Vanden EijndenSGorielySDe WitDWillemsFGoldmanMIL-23 upregulates IL-10 and induces IL-17 synthesis by polyclonally activated naïve T cells in humanEur J Immunol2005354697515682457
- ZhengYDanilenkoDMValdezPInterleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosisNature20074456485117187052
- NestleFOConradCThe IL-12 family member p40 chain as a master switch and novel therapeutic target in psoriasisJ Invest Dermatol2004123XIVXV15610500
- KauffmanCLAriaNToichiEA phase I study evaluating the safety, pharmacokinetics, and clinical response of a human IL-12 p40 antibody in subjects with plaque psoriasisJ Invest Dermatol200412361037104415610511
- KruegerGGLangleyRGLeonardiCA human interleukin-12/23 monoclonal antibody for the treatment of psoriasisN Engl J Med20073565809217287478
- KimballAGordonKBLangleyRGSafety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: results of a randomized, placebo-controlled, phase 2 trialArch Dermatol200814420020718283176
- LeonardiCKimballABPappKEfficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomized, double-blind, placebo-controlled trial (PHOENIX 1)Lancet20083711165117418395577
- PappKALangleyRGLebwohlMEfficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomized, double-blind, placebo-controlled trial (PHOENIX 2)Lancet20083711675168418486740
- SternRSNijstenTFeldmanSRMargolisDJRolstadTPsoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfactionJ Invest Dermatol Symp Proc2004913639
- RichardsHLFortuneDGGriffithsCEAdherence to treatment in patients with psoriasisJ Eur Acad Dermatol Venereol20062037037916643132