Abstract
Biotechnology and nanotechnology are the key technologies of the twenty-first century, having enormous potential for innovation and growth. The academic and industrial goals for these technologies are the development of nanoscale biomolecular substances and analytical instruments for investigating cell biology at the cellular and molecular levels. Developments in nanotechnology will provide opportunities for cosmetic dermatology to develop new biocompatible and biodegradable therapeutics, delivery systems and more active compounds. Cosmetics have the primary function of keeping up a good appearance, changing the appearance, or correcting body odors, while maintaining the skin and its surroundings in good conditions. Thus cosmetic dermatology, recognizing the new realities of skin care products, has to emphasize the functional aspects of cosmetics through an understanding of their efficacy and safety in promoting good health. Nanoscience may help the scientific community to find more innovative and efficacious cosmetics. Understanding the physical model of the cell as a machine is essential to understand how all the cell components work together to accomplish a task. The efficacy and safety of new nanomaterials has to be deeply studied by ex vivo tests and innovative laboratory techniques. New delivery systems and natural nanocompounds, such as chitin nanofibrils for wound healing, are being used in cosmetic dermatology with good results, as are nanostructured TiO2 and ZnO sunscreens. The challenge is open.
The significance of nanotechnology and nanobiotechnology
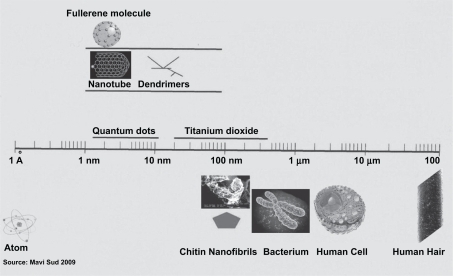
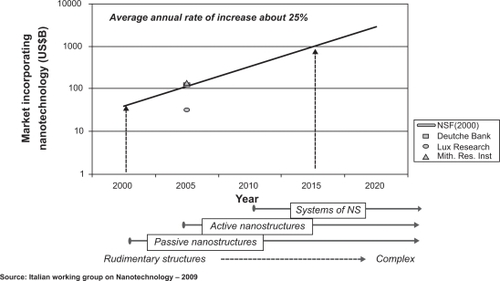
Nanotechnology is a relatively young discipline. Today nanotechnology is not only essential for marketing-oriented chemical companies, but also a tool for developing science-based solutions for innovative therapeutics and cosmetics, enhancing well-being and addressing anti-aging issues.Citation1 Nanotechnology is defined as the study of matter on an atomic or molecular scale. Generally, nanotechnology deals with structures the exact size and shape of which must be measured on a nanometric scale.Citation2–Citation4 A nanometer (nm) is one billionth of a meter (ie, 10−9) and typical atoms are about one third of a nanometer. In comparison the medial thickness of a human hair is about 10000 nm and an influenza virus has a diameter of 100 nm (see ). Nanobiotechnology, the interface between nanotechnology and biotechnology, is the branch of nanotechnology that deals with its biological and biochemical applications or uses.
Nanotechnology is thus the set of methods and techniques for processing matter on atomic and molecular scales to create products with both special and improved chemico–physico features compared to conventional products.Citation5 Therefore, the goal of nanobiotechnology is the development of nanoscale biomolecular components and analytical instruments for the investigation of cell biology at both the cellular and molecular levels.
The expectations
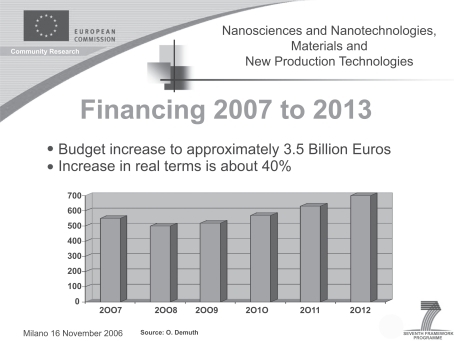
The potential uses and benefits together with the expectations of nanosciences are enormous. Accordingly, the European Commission (EU) supports the new nanotechnologies through its seventh framework program (). Thus, the use of nanomaterials in cosmetic dermatology is the subject of intense debate in the EU, and efforts to establish fundamental rules for their use and risk assessment are ongoing throughout universities, government laboratories and industry. Investment of public funds in basic nanotechnology research has increased, which has, in turn, heightened the interest of private industry in developing patented products.Citation6 The nanoscale production of a raw material may not only reduce costs, but also enhance its properties. In the biomedical and cosmetic fields nanotechnology has enormous potential, and nanobiotechnological methods are already being used in both medicine and the pharmaceutical industry.
For all these reasons, the International Organization for Standardization (ISO)Citation7 is developing uniform standards for nanotechnology and supporting innovation, research and development, including appropriate risk assessment research, in this innovative field.
However, to obtain nanoscale materials a combination of physical micro-nanoelectronic, chemical and biological technologies are necessary. Currently, the three main areas of development include the following:Citation8
nanomaterials: building specialized structures whose dimensions are controlled on a nanoscale;
nanobiotechnology: the manipulation of living systems using nanoscale engineering;
nanoelectronics: the development of microelectronics for devices such as radio frequency identification.
Nanomaterials in cosmetic dermatology
Nanomaterials and nanobiotechnology have the potential to radically change the way cosmetics and drugs deliver their benefits. Specifically, nanoparticles are being developed to encapsulate a wide range of ingredients benefical to the skin.
To obtain nanoparticles, two principles approaches are used:
the bottom-up method in which nanoparticles are assembled from the molecular dimension
the top-down approach that reduces larger particles by the use of chemico–physico methods. In cosmetics the top-down approach is more commonly used to produce different kind of structures. Examples of such structures include nanosomes, cubosomes, niosomes, and liposomes.
These labile particles with diameters from 50 to 5000 nm are used to produce micro- or nanoemulsions capable of carrying and protecting active compounds from oxidation, and also improve their penetration through the skin layersCitation9 ().
One of the major factors that determines the ability of substances to penetrate the skin is the size of the molecule. According with Johann Wiechers, the role of a delivery system is to ensure that the right concentration of the right chemical is reaching the right site in the body for the correct period of time.Citation10 However, the efficiency of an active compound depends largely on its bioavailability – it is vital that it reaches the site of action and be released for a prolonged period of time.
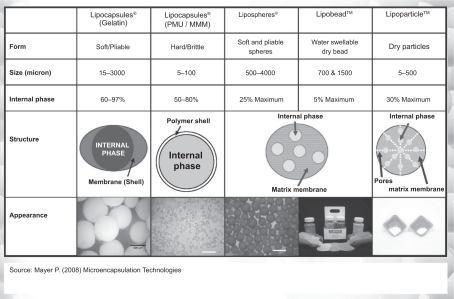
To this end, recent extensive research has developed different nanotechnology oriented, controlled delivery systems, from the simplest to the most sophisticatedCitation10 ().
Figure 4 Delivery systems: from the simplest to the most complicated. Reproduced courtesy of Pat Meyer, Lipo Chemicals.
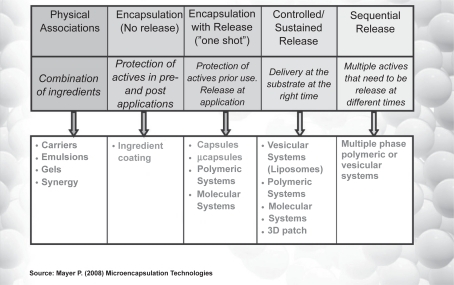
Thus nanovesicles, as a skin delivery system, and solid lipid nanoparticles (SLN) or nanostructured lipid carriers (NLC), have been developed for cosmetic and pharmaceutical applications.Citation11
Vesicles are hollow colloidal particles, consisting of amphiphilic molecules. Because of their amphiphilic properties, these molecules can, in the presence of water, form unilamellar or multilamellar vesicles. Both water-soluble and water-insoluble compounds can be entrapped in such vesicles and a wide variety of lipids and surfactants can be used to prepare them.
These vesicles might act: (a) as carriers to deliver entrapped molecules into or across the skin; (b) as penetration enhancers to modify the intercellular lipid lamellae; (c) as a depot for sustained release of active compounds; (d) as a site-limiting membrane barrier for a controlled transepidermal or transdermal delivery system.
In contrast to a vesicles-based delivery system, SLN and NLC have the great advantage of high stability. Moreover, these lipid carriers create a nanolayer lipid film on top of the skin, thereby avoiding water evaporation and thus increasing skin hydration.
Furthermore, the small size of all the nanostructured compounds ensures a closer contact with the stratum corneum, thus increasing the amount of incorporated active ingredients reaching the site of action. Wetting, spreading and penetration also may be enhanced as a result of the low surface tension of the whole system and the low interfacial tension of the oil/water (O/W) nanodroplets.
However, all the nanoparticles obtained from different emulsions have to be considered labile forms, because of the total disintegration of their components after application to the skin surface. Moreover, their mean dimension in cosmetic emulsions is about 100 nm.
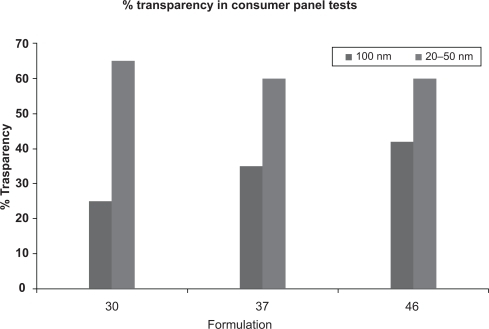
Another group of nanoparticles widely used in cosmetic dermatology and totally insoluble both in water and oil includes the inorganic physical UV filters titanium dioxide (TiO2) and zinc oxide (ZnO), considered sunscreen in the US and Japan, and in Germany only among the EU countries. These two substances are used in particles of 60 to 200 nm to obtain a transparent emulsion, thus increasing cosmetic compliance (). Of note is that the miniaturization of these minerals increases not only their transparency, but also their filtering capacity,Citation12 because of their higher reflective index.
Figure 5 The level of transparency in consumer panel tests depending on the dimension of ZnO/TiO2 particles. Courtesy Antaria Limited.
Therefore, these minerals may be considered as broad-spectrum UV blockers when used in micronized form, becoming invisible after application to the skinCitation13 (). Additionally, the surface of these nanofilters may be coated with a neutral material such as silica, polysiloxane compounds, glass, or aluminium oxide, to improve their dispersion state, photostability and efficacy, eliminating direct contact with the skin () and increasing their margin of safety.Citation14 As TiO2 is not only a UVA filter, but also an extremely efficient UVB filter, it shows by far the highest versatility of any sunscreen product.Citation15
Figure 6 A face covered with standard ZnO (on the right, ie, subject’s left side) or nano-size ZnO (on the left, ie, subject’s right side). Reproduced courtesy of Geoff Trotter, Antaria Limited.

Another important group of insoluble nanoparticles used in cosmetics are microcapsules. These are small particles containing an active agent or core material surrounded by a coating layer or shell. Their diameter may vary from 1 to 1000 μm; capsules smaller than 1 μm are called nanocapsules and capsules larger than 1000 μm are called macrocapsules.Citation16–Citation18 Microcapsules may help to solve problems in cosmetic dermatology such as incompatibility of different ingredients, and protection of substances liable to oxidation or affected by atmospheric moisture.
The general outcome of EU discussions on the safety of nanomaterials in cosmetic dermatology indicates that some risks are inevitable, although conventional toxicological methodologies are not adequate to assess their safety. At present, these risks remain hypothetical because these discussion statements are not supported by documented research results.Citation19–Citation23 Recently the EU Parliament has passed a resolution on the regulatory aspect of nanomaterials,Citation24 considering them necessary for EU citizens, if made and distributed in a responsible manner.
The EU Parliament has stressed the need for legislation that includes a more comprehensive science-based definition of nanomaterials, together with a clear assignment of liability to producers and employers arising from the use of nanomaterials, through all routes of exposure (such as inhalation and the skin).
Recent studies on the safety of nanomaterials for human health have led, for example, to false-positive or false-negative results and conclusions because of shortcomings in applied protocols ().Citation25–Citation36 Thus the Commission on Nanoscience has provided a “Code of Conduct for all individuals and civil society organizations involved or interested in nanosciences and nanotechnologies (N&N) research”.Citation37 This Code of Conduct aims to promote integrated, safe and responsible nanoscience and nanotechnology research in Europe for the benefit of society as a whole. Moreover the code must be considered complementary to existing regulations. Thus far nanobiotechnology seems to pose no special risks for medicine and pharmacology.Citation38,Citation39
Table 1 A review of the results of TiO2 and ZnO percutaneous absorption studies
However, nanotechnology is a very broad term, and most fears about human health arise as a result of the specific properties of nanomaterials and nanoparticles, whether natural or man-made. In fact, natural nanoparticles do occur in the environment, for example in waste matter, some of which are are not materials engineered by scientists.Citation39,Citation40
One type of natural nanoparticle, chitin nanofibrils, are currently used by our research group as an active compound in cosmetic dermatology. Research results obtained to date suggest this particle poses no risks to consumers but offers numerous benefits.Citation41,Citation42
Chitin nanofibril
A chitin nanofibril is a nanocrystal of a natural polysaccharide obtained from the crustacean exoskeleton after elimination of the carbonate and protein portions. Having a backbone like hyaluronic acid, chitin nanofibrils are easily metabolized by the body’s endogenous enzymes and thus is used in cosmetic dermatology and biotextiles. The crystal is so named because its average size is 240 × 7 × 5 nm (nano), and because it is shaped like a thin needle (fibril). Moreover, because it occurs naturally and is considered a safe raw material, it is safe to use. As it is easily metabolized by enzymes, it is both bio- and eco-compatible. As the nanofibril has an average size one-quarter that of a bacterium, 1 g of the product covers a surface area of 400 m2.Citation43,Citation44 Many studies have shown that chitin nanofibrils can activate the proliferation of keratinocytes as well as fibroblasts, regulating not only collagen synthesis but also cytokine secretion and macrophage activity.Citation45,Citation46
Our group has obtained interesting results showing how chitin nanofibrils can ameliorate not only the appearance of photoaged skinCitation47–Citation51 but also promote wound healing by reducing hypertrophic scar formationCitation52,Citation53 (). In vitro studies have shown how chitin nanofibrils can increase the reproduction of fibroblasts with a subsequent increase in collagen synthesis and in adenosine triphosphate production. In an in vivo double-blind study, skin hydration and superficial skin lipids were improved, with a simultaneous reduction in lipid peroxides and transepidermal water loss.Citation49–Citation51 By using different types of emulsions, chitin nanofibrils have an interesting wound healing activity.Citation52,Citation53 Healthy biotextiles have also been produced.Citation54
Research efforts in cosmetic dermatology are currently aimed at developing new environmentally friendly nanosized chemicals and new nanoparticulate systems for the skin. Therefore, during recent years several delivery systems have been developed (visible beads, microcapsules, nanocapsules and liposomes, or lipid nanoparticles, SLN) for the cosmetic and dermatological industry, and successively introduced into many marketed products. Many different active components can be encapsulated in these delivery systems, such as vitamins fragrances, botanical extracts, and drugs, which have a wide range of cosmetic or dermatological properties.
Some of these delivery systems, such as SLN, are innovative drug carrier systems first designed for intravenous administration and more recently investigated for peroral and transdermal applications in cosmetic dermatology. Thus, chemically labile agents should be protected from degradation and the release profile modulated. The much higher surface-to-mass ratio of nanomaterials should increase the efficacy of many active compounds.
In conclusion it is necessary to understand better the potential of these new technologies, so that the potential negative effects of their chemistry on human health and the environment may be minimized or avoided.
Nanoscience and nanoengineering could significantly improve our understanding of nanoscale processes at the molecular level that occur in the environment. Consequently it should be possible to develop new green technologies that minimize the production of undesirable byproducts released into the environment, into waste sites and streams for example.Citation55 Nevertheless, to speed up the development of both nanotechnology and nanobiotechnology, multidisciplinary teams of highly trained people with backgrounds in biology, medicine, applied and computational mathematics, physics, chemistry, and electrical, chemical and mechanical engineering will be needed. Despite the current international financial crisis, nanotechnology and innovative cosmetic dermatology, may have the potential to help reinvigorate a nation’s economy (). However, apart from helping to overcome some new challenges in molecular imaging techniques, quantitative analytical tools, physical models of the cell as a machine, better in vivo tests and better drug/cosmetic delivery systems, nanotechnology could play an important role in the sustainability of not only cosmetic dermatology but also of agriculture, water filtering, energy materials, and a clean environment.
In conclusion, the most complex and highly functional nanoscale systems have been invented by nature. We have to find ways of how to best use them for ameliorating our well-being and the environment. Thus, nano-products should be designed and sold in a way that fully respects the health of consumers and the environment. Both nanotechnology and biotechnology have the potential to revolutionize both our industries and way of life. They represent the key technologies of the twenty-first century, offering excellent opportunities for both research and business.
Disclosure
The author declares no conflicts of interest.
References
- MorgantiPMorgantiGYuanhongLiNanostructured products: technology and futureJ Appl Cosmetol2007254161178
- MorgantiPMorgantiGNanotechnology and wellnessSÖFW Journal200713352227
- MorgantiPMorgantiGMuzzarelliRChitin nanofibrils: a natural compound for innovative CosmeceuticalsCosmetics and Toiletries200712248188
- MorgantiPMorgantiGFabricating a good treatmentSoap Perfumery and Cosmetics20078586
- MorgantiPApplied Nanotechnology in cosmetic and functional foodEucocosmetics200721215
- MorgantiPChenHDGaoXHLiYNanoscience the challenging cosmetics healthy food and biotextilesSÖFW Journal2009135427
- Nanotechnology Commission ISO/TC 229, 2009, 24 March.
- SchuellerRRomanowskiPEmerging Technologies and the future of cosmetic scienceSchuellerRRomanowskiPBeginning Cosmetic Chemistry3rd edCoral Stream IL, USAAllured Publ. Co2009427434
- MayerPMicroencapsulation technologiesPCHI, ProceedingsShanghaiMarch 17–19, 20081522
- WiechersJWScience and Applications of Skin delivery SystemsCoral Stream, IL, USAAllured Publ. Co2008
- SoutoEBMüllerRHChallenging Cosmetics-Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC)WiechersJWScience and Application of Skin Delivery SystemsAllured Publ. CoIL, USA, Coral Stream2008227250
- MorgantiPMeccanismo d’azione dei prodotti cosmetici: miti e realta’. Presented at Giornata di studio: Prodotti Cosmetici. Approfondimenti e proposte su regolamentazione, sicurezza e mercatoMilano2009 29-Gennaio.
- HidesLTrotterGA new paradigm in mineral UVA and UVB protectionPresented at PCHI Shanghai meeting2008, March 17–19
- NasuAOtsuboYRheology and UV protection properties of suspension of fine titanium dioxide in silicon oilJ Cabloid Interfacial Sci2006296558564
- PflückerFBungerJHitzelSVitteJComplete Photoprotection-going beyond visible endpointsSÖFW Journal200513172030
- VinetskyYMagdassiSMicrocapsules in cosmeticsMagdassiSTouitouENovel Cosmetic Delivery SystemsNew YorkMarcel Dekker Inc1999295313
- Royal Society and Royal Academy of Englneering nanoscience and nanotechnologies, opportunities and uncertainties2004 URL www.royalsoc.uk/policy/.2004
- DreylerKThe future of nanotechnology: molecular manufacturing2003 URL www.eurekartet.org/context.p4p2.context=nano&slow=essays.
- SCCP Scientific Committee on Consumer ProductsPreliminary opinion on safety of nanomaterials in cosmetic products2007
- BallPNanotechnology in the firing line2003 URL http://nanotechweb.org/cws/article/indepht/18804.
- DreherKHealth risk assessment of manufactured nanomaterials: more than just sizeNanotechnology for Remediation Technical Workshop, National Health and Environmental Effects Laboratory, US Environmental Protection Agency2005Washington, USA
- OberdoesterGOberdoesterEOberdoesterJNanotoxicology: an emerging discipline evolving from studies of ultrafine particlesEnviron Health Perspect2005113782383916002369
- ColvinVPhysical properties of nanomaterialsTowards predictive assessments of risk, international nanomaterial environmental health and safety research needs assessment-Workshop 1: US national institute of health2007USA URL http://cohesion.rice.edu/CentersAndlnst//CON/emplibrary/ColvinRNADJan2007.pdf
- European Parliament resolution of 24 April 2009 on regulatory aspect of nanomaterials Available from: URL http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//TEXT+TA+P6-TA-2009-0328+0+DOC+XML+V0//EN
- NohynekGJLademannJRibaudCGrey goo on the skin? Nanotechnology, cosmetic and sunscreen safetyCrit Rev Toxicol200737325127717453934
- SternSTMcNeilSENanotechnology safety concerns revisitedReview Toxicol Sci2008101421
- DussertASGoorisECharacterisation of the mineral content of a physical sunscreen emulsion and its distribution onto human stratum corneumInt J Cosmet Sci19971911912918507639
- LademannJWeigmannHJRickmeierCHPenetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orificeSkin Pharmacol Appl Skin Physiol1999224725610461093
- SCCNFPOpinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning Titanium Dioxide2000Brussels, BelgiumEuropean Commission URL http://ec.europa.eu/health/ph_risk/committees/04_scp/04_sccp_en.htm.
- PlueckerFWendelVHohenbergHThe human stratum corneum layer: An effective barrier against dermal uptake of different forms of topically applied micronized titanium dioxideSkin Pharmacol Appl Skin Physiol200114Suppl 1929711509913
- SchulzJHohenbergFPlueckerFDistribution of sunscreens on skinAdv Drug Deliv Rev200254Suppl 1S157S16312460721
- ButzTDermal penetration of nanoparticles: what we know and what we don’t. Cosmetic. Science Conference Proceedings, MunichSÖFW Journal20091354810
- MenzelFReinetTVogtJButzTInvestigations of percutaneous uptake of ultrafine TiO2 particles at the high energy ion nanoprobe LIPSIONNucl Instrum Methods Phys Res B2004219220
- CrossSEInnesBRobertsMSTsuzukiTRobertsonTAMcCormickPHuman skin penetration of sunscreen nanoparticles: In vitro assessment of a novel micronized zinc oxide formulationSkin Pharmacol Physiol20072014815417230054
- GamerALeiboldEvan RavenzwaayBThe in vitro absorption of microfine ZnO and TiO2 through porcine skinToxicol In Vitro200620330130716182508
- MavonAMiquelCLejuneOPayreBMorettoPIn vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreenSkin Pharmacol Physiol200720102017035717
- Commission of the European CommunitiesCommission Recommendation on a Code of Conduct for responsible nanosciences and nanotechnologies researchBrussels 07/02/2008 C, 2008, 424 final.
- EMEA Reflection paper on nanotechnology-based products for human use2006 EMEA/CHMP/79769/2006.
- NohynekGJNano-cosmetics. No health riskCossma200731011
- StarzykEFrydrychASolygaANanotechnology: does it have a future in cosmetics?SÖFW Journal200813464252
- MorgantiPMuzzarelliRAAMuzzarelliCMultifunctional use of innovative chitin nanofibrils for skin careJ Appl Cosmetol200624105114
- MorgantiPMorgantiGChitin nanofibrils for advanced cosmeceuticalClin Dermatol20082633434018691512
- MuzzarelliRAAChitin and its derivates: new trends off applied researchCarbohydr Polym199335375
- PercotAVitonCDomardAOptimization of chitin extraction from shrimp shellBiomacromolecules20034818
- MuzzarelliRAAMattioli-BelmonteMPugnaloniABiochemistry, histology and clinical uses of chitins and chitosans in wound healingJollésPMuzzarelliRAAChitin and ChitinasesBasel, SwizterlandBirkhaüser Verlag1999251264
- MuzzarelliRAAMuzzarelliCChitin nanofibrilsDuttaPKChitin and Chitosan: Research Opportunities and ChallengesContai, IndiaSSM International Publication2005129146
- MorgantiPFabriziGBrunoCProtective effects of oral antioxadants on skin and eye functionSkinmed20043631031615538079
- BiaginiGZizziAGiantomassiFCutaneous absorption of nanostructured chitin associated with natural synergistic molecules (lutein)J Appl Cosmetol2008266980
- MorgantiPFabriziGPalomboPChitin-nanofibrils: a new active cosmetic carrierJ Appl Cosmetol200826105120
- MorgantiPMorgantiGFabriziGA new sun to rejuvenate the skinJ Appl Cosmetol200826159166
- MorgantiPFabriziGRuoccoEChitin nanofibrils improved photoprotectionCosmetics and Toiletries200912496673
- BiaginiGZizziATucciGChitin nanofibrils linked to chitosan glycolate as spray, gel and gauze preparations for wound repairJ Bioact Compat Polym200722525538
- MezzanaPClinical efficacy of a new chitin-nanofibrils based gel in wound healingActa Chirurgiae Plasticae2008503818419263641
- MorgantiPLeather and textile chemicals: chitin nanofibrils in textilesSpeciality Chemicals Magazine200828926
- YzerCVenkateshMNVFA and FBAE position papers on nanobiotechnology2009 URL www.FBAE.org and www.VFA.de