Abstract
Aim:
To assess serum myeloperoxidase (MPO) levels in autistic children with severe gastrointestinal (GI) disease and to test the hypothesis that there is an association between serum MPO concentration and inflammatory GI disease, including antineutrophil cytoplasmic antibodies (ANCA), previously seen in a subgroup of autistic children.
Subjects and methods:
Serum from 40 autistic children with chronic digestive disease (most with ileo-colonic lymphoid nodular hyperplasia (LNH) and inflammation of the colorectum, small bowel and/or stomach), and 48 controls (12 age-matched autistic children with no GI disease, 20 age-matched children without autism or GI disease, and 16 nonautistic individuals with no family history of autism) were tested using enzyme-linked immunosorbent assays designed to quantitate serum MPO levels. MPO serum concentration of autistic children with GI disease was compared to GI disease severity (including LNH and erythema) and presence of ANCA.
Results:
We found that a significant number of autistic children with chronic digestive disease had low serum levels of MPO. However, there was no significant relationship between these levels and severity of GI disease, including the presence of ANCA.
Discussion:
These results suggest a relationship between low MPO levels and GI disease seen in a subpopulation of autism spectrum disorders individuals. MPO concentration may therefore be a useful biomarker for GI disease in this group of autistic children.
Introduction
Oxidants are thought to be key components of the neutrophil host defense system. Upon contact with a pathogen, particularly bacteria and fungi, neutrophils produce a respiratory burst characterized by intense uptake of oxygen. The resulting superoxide dismutates into hydrogen peroxide (H2O2). The toxicity of H2O2 to microbe pathogens is greatly enhanced by the heme enzyme myeloperoxidase (MPO), found in the azurophilic granules of neutrophils, which uses H2O2 to convert chloride (Cl−) into hypochlorous acid (HOCl). Although the exact mechanism is not completely understood, MPO also kills by directly chlorinating phagocytosed bacteria.Citation1
In addition to killing bacteria, the products of the MPO-hydrogen peroxide-Cl system are believed to play a role in killing fungi, parasites, protozoa, viruses, tumor cells, natural killer (NK) cells, red cells, and platelets, and they may be involved in terminating the respiratory burst, because individuals with MPO deficiency have a prolonged reaction.Citation2
Other functions of MPO include tyrosyl radical production, generation of tyrosine peroxide, mediation of the adhesion of myeloid cells via b2-integrins, and oxidation of serum lipoproteins.Citation2
Autistic spectrum disorder (ASD) is a neurodevelopmental syndrome with onset prior to the age of 36 months. Diagnostic criteria consist of impairments in socialization and communication plus repetitive and stereotypic behaviors.Citation3 Traits strongly associated with autism include movement disorders and sensory dysfunctions.Citation4 Although autism may be apparent soon after birth, many autistic children experience at least several months, up to a year or more in some cases, of normal development followed by regression, defined as loss of function or failure to progress.Citation4–Citation6
Children with ASD frequently have accompanying gastrointestinal (GI) symptomsCitation7–Citation9 and pathology, which includes inflammation of the GI tract.Citation10–Citation13 Many autistic children, particularly those with GI disease, have a higher propensity and related incidence of fungal infections.Citation14,Citation15
We previously reported that a significant number of autistic children with GI disease have antineutrophil cytoplasmic antibodies (ANCA; both antiproteinase 3 [anti-PR3] and anti-MPO), and that there is a relationship between individuals with ANCA and severity of intestinal disease.Citation16 We have also reported that a significant number of autistic children with chronic digestive disease have anti-PR3 ANCA, low serum alpha-1 antitrypsin (AAT), and high serum PR3, which correlate with high severity of GI disease, suggesting that high PR3 levels may be causing ANCA in autistic children with severe GI problems.Citation17 Because of this, and the observation that a significant number of autistic children with GI disease have anti-MPO ANCA, we hypothesized that altered MPO levels might be associated with the production of ANCA to MPO.
The data presented here suggests that a significant number of autistic children with GI disease are MPO deficient, and, although no correlation was found between GI disease, ANCA, and MPO levels, this data suggests that MPO deficiency might be a biomarker for this subgroup of autistic children.
Materials and methods
ELISA to measure serum MPO levels (Kit - I.C.L. Inc, Newberg, OR 97132)
All reagents and specimens were equilibrated to room temperature before the assay was performed. One hundred microliters of a 1:10 dilution of the patient samples, calibrators (15–1.875 ng/ml of affinity purified MPO), negative control of serum diluent alone, were added to the appropriate microwells of a microculture plate (each well contained anti-MPO). Wells were incubated for 60 minutes (±5 min) at room temperature, then washed 4× with wash buffer. One hundred microliters of anti-human MPO conjugated with hydrogen peroxidase (HPO) was added to all microwells, incubated for 60 minutes (±5 min) at room temperature, then washed 4× with wash buffer. One hundred microliters of HPO substrate was then added to each microwell. After approximately 30 minutes at room temperature, the wells were read at 450 nm with an enzyme-linked immunosorbent assay (ELISA) reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Subjects and scoring of severity of GI disease
Serum from autistic individuals with GI disease was obtained from the Thoughtful House, Austin, Texas.Footnotea All 40 children in this study with ASD (median age 6 years; range 2–16 years; 34 male) having GI symptoms, were investigated by ileo-colonoscopy. Esophagogastroduodenoscopy was performed on these patients, but only those with GI symptoms were part of this study.
A reproducible scoring system, similar to the Crohn’s Disease Endoscopic Index of Severity (CDEIS) was developed and used to evaluate this unique type of observed enterocolitis.
Macroscopic and histologic features for both upper and lower GI tract (such as ulcerations, erosions, lymphoid nodular hyperplasia [LNH], and erythema) were each scored out of 3: mild (1 point), moderate (2 points), or marked (3 points) for a total score out of 12. A point system was also developed for severity of LNH alone. Patients were scored according to mild (1 point), moderate (2 points), or marked (3 points) LNH in each area (upper and lower GI) for a maximum of 6 points. And finally, a point system was also developed for severity of erythema alone. Patients were scored according to mild (1 point), moderate (2 points), or marked (3 points) erythema in each area (upper and lower GI) for a maximum of 6 points.
Controls
Three control groups (total n = 48) were studied, including 12 age- (mean 68 months), gender-(80% male), and diagnosis- (61% regressive onset) matched autistic children with no GI disease; 20 age- (mean 71 months) and gender- (75% male) matched children without autism or GI disease; 16 nonautistic individuals with no family history of autism. Serum and medical history of age- and gender-matched controls were obtained from the Autism Genetic Resource Exchange (AGRE).Footnoteb Serum from nonautistic individuals with no family history of autism was obtained from National Disease Research Interchange.Footnotec
Serums
Experimental (Thoughtful House) and control (AGRE) serums were frozen at −70 °C immediately after collection and cell/serum separation, then stored at −70 °C until thawed for use in ELISAs.
Statistics
Inferential statistics were derived from analysis of variance (ANOVA), Student’s t-test and odds ratios with 95% confidence intervals.
Results
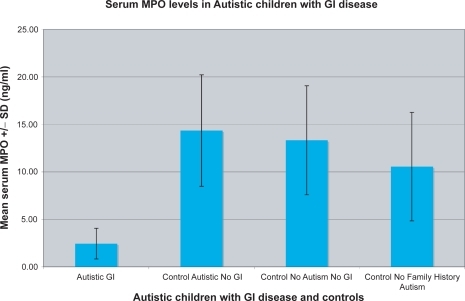
Using the ELISA described above, autistic children with chronic digestive disease were tested for serum MPO levels. Results of a typical assay are shown in . In each assay, MPO concentrations were determined by comparing experimental and control serum levels with MPO standards and negative controls (sample diluent alone) (). For each assay, there were three or four replicate samples tested in each group (control and experimental), and each assay was repeated at least twice. We found that the MPO concentration of autistic children with GI disease (n = 40; m = 2.45 ng/ml ± 1.62) was significantly lower than three control groups; age/diagnosis-matched autistic children with no GI disease (n = 12; m = 14.35 ± 5.87; p < 0.01); age/diagnosis-matched nonautistic children with no GI disease (n = 20; m = 13.33 ± 5.74; p <0.01) and individuals with no family history of autism (n = 16; m = 10.55 ± 5.71; p < 0.01) ().
Figure 1 Serum MPO concentration was measured in a typical ELISA. Five autistic children (A) with GI disease, two autistic children with no GI disease controls (C*), and three nonautistic children with no GI disease controls (C**) were tested. Four replicate samples were tested for each individual.
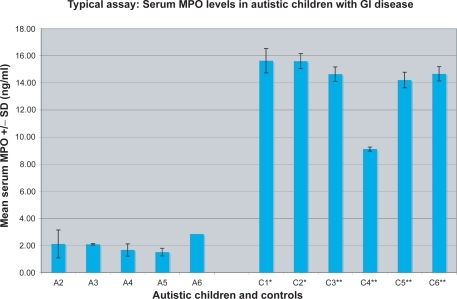
Figure 2 MPO serum concentration was established for each individual by testing and correlating to known standards of various concentrations of MPO (15–1.875 ng/ml) as well as a negative control (serum diluent alone).
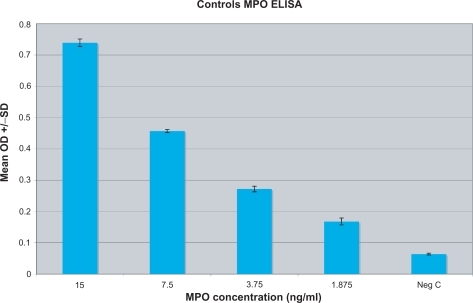
Figure 3 The mean ± SD MPO concentration (ng/ml) of 40 autistic children with chronic digestive disease (most with ileo-colonic LNH and inflammation of the colorectum, small bowel and/or stomach) (autistic GI), and 48 controls (12 age matched autistic children with no GI disease; control autistic, no GI), 20 age-matched siblings of autistic children, without autism or GI disease (control, no autism, no GI), and 16 nonautistic individuals with no family history of autism (control no family history autism).
Fifteen of the 40 autistic children with GI disease were categorized as having severe disease (score equal to or greater than 7 on total GI severity score criteria described above), six of 40 had severe LNH (score equal or greater than 4), and five of 40 had severe erythema (). Although most of the autistic children with GI disease had low MPO serum levels, there was no significant difference between MPO levels of these individuals and severity of GI disease, GI LNH (p = 0.4674) or erythema (p = 0.7806) (). Although those individuals with severe GI disease did have higher MPO levels, the difference was still not significant (p = 0.058). We also did not find a significant difference between presence of ANCA and MPO serum concentration (p = 0.3718) ().
Table 1 The total data from two assays of experimental and control groups is represented. The mean, standard deviation and standard error of the mean of MPO concentration (ng/ml) of 40 autistic children with chronic digestive disease (most with ileo-colonic LNH and inflammation of the colorectum, small bowel and/or stomach) (autistic GI), and 48 controls (12 age-matched autistic children with no GI disease; autistic, no GI), 20 age-matched siblings of autistic children, without autism or GI disease (nonautistic, no GI), and 16 nonautistic individuals with no family history of autism (no family history autism)
Table 2 MPO concentration of autistic children with GI disease with ANCA (anti-MPO or anti-MPO/anti-PR3; bold) and without ANCA are compared (p = 0.3718)
Table 3 MPO concentration of autistic children with GI disease with (bold) and without LNH, with (bold) and without erythema and with (bold) and without severe total GI disease are compared
Discussion
Our results show that autistic children with severe GI disease have low serum levels of MPO, and, although our data does not demonstrate a relationship between these low levels and type or level of severity of GI disease or the presence of ANCA, the data suggests that low serum MPO may be a biomarker for this subgroup of autistic children.
In addition to facilitating the destruction of pathogens, MPO may play a role in the etiology of atherosclerosis. Researchers have demonstrated that patients with stable coronary artery disease have an increased cardiovascular risk if plasma MPO levels are elevated.Citation18 These high MPO levels result in high oxidative stress associated with an increase in lipid peroxidation. There is evidence to suggest that MPO deficiency may protect against cardiovascular disease.Citation19
MPO-deficient neutrophils produce superoxide and H2O2 properly, but are unable to convert H2O2 to HOCl. As a consequence, neutrophil killing of some organisms is diminished early, but is normal late, as demonstrated by killing assays.Citation20
MPO-deficient neutrophils are normally able to phagocytize most microbes. However, the ability of these cells to kill bacteria seems impaired. For organisms such as Staphylococcus aureus, Serratia species, and Escherichia coli, killing is initially impaired, but then reaches normal levels after a period of time. This suggests that an apparently slower, alternative mechanism of killing by these MPO-deficient cells is functioning.Citation21
In contrast, the capacity to kill certain fungi seems completely absent in MPO-deficient neutrophils. In vitro studies have shown that Candida albicans, C. krusei, C. stellatoidea, and C. tropicalis cannot be killed by MPO-deficient polymorphonuclear leucocytes (PMNs). In contrast, an MPO-independent mechanism can kill C. glabrata, C. parapsilosis, and C. pseudotropicalis. This leads to the conclusion that bacterial killing may not necessarily be a problem for patients with MPO deficiency, but the killing of certain fungi may be difficult, depending on the severity of the deficiency.Citation22–Citation24 Despite this, there are few case reports of serious infectious complications associated with MPO deficiency.Citation25–Citation27
There is quite a bit of evidence suggesting that oxidative stressCitation28–Citation32 and inflammationCitation10–Citation13 (particularly associated with the GI tract) are associated with autism. Studies have shown that MPO may serve as a biomarker for oxidative stress,Citation33 and MPO deficiency may also be associated with an increase in incidence of inflammation.Citation34
Interestingly, MPO may also play a role in the downregulation of the inflammatory response by regulating NK cells, decreasing peptide binding to chemotactic receptors, and auto-oxidizing and inactivating products of PMNs, such as alpha1-proteinase inhibitor and chemotaxins.Citation2
MPO generates numerous reactive oxidants and diffusible radical speciesCitation35 that are capable of both initiating lipid peroxidationCitation36,Citation37 and promoting an array of post-translational modifications to target proteins, including halogenation, nitration, and oxidative cross-linking.Citation38,Citation39 Lipid peroxidation has also been suggested to be a potential biomarker for autism.Citation40–Citation42
Some physicians, who have prescribed antifungals for autistic children with GI disease, have reported improvement in behaviors. We suggest that these improvements may be, at least in part, the result of MPO deficiency, which is acquired from antifungal administration and may result in reduced lipid peroxidation.
This report suggests that a subgroup of autistic children with GI disease have MPO deficiency. It is unclear whether this deficiency is acquired or inherited. Since MPO deficiency is associated with oxidative stress, increased inflammation, and propensity for fungal infections, all of which are mechanisms also associated with autism, one could hypothesize that MPO deficiency may be directly linked with GI pathology seen in this subgroup of autistic children.
Although we did not see a relationship between MPO levels in the autistic children with GI disease and severity of GI disease, or presence of ANCA, this could be explained by the fact that such a large number of individuals in this group had very low levels of MPO, making small differences insignificant.
One possible reason for this MPO deficiency in this subgroup is that children with GI disease, such as inflammatory bowel disease, as well as autistic children with GI disease, are often given anti-inflammatory drugs, which have been found to cause MPO deficiency.Citation43–Citation46 We did not have data available to investigate the possible relationship between antifungal therapy and MPO deficiency in this group of children with GI disease, but this area needs to be explored. If MPO deficiency is temporarily acquired because of antifungal therapy, and many autistic children receiving antifungal therapy are improving with respect to behavior, then is that improvement, at least in part, do to less oxidative stress and/or reduction in inflammation resulting from MPO reduction?
In summary, we have discovered that a group of autistic children with GI disease have MPO deficiency. Since the production of MPO is a major way that neutrophils are involved in antifungal defense, this deficiency may associate with increased fungal infection seen in many of these children. It is also possible, however, that anti-inflammatory and anti-fungal drugs given to this group of autistic children may be causing or exasperating the deficiency and that these very low levels of MPO result in reduced oxidative stress and improved behavior.
Disclosures
The authors report no conflicts of interest in this work.
Notes
a The Thoughtful House Center for Children, founded in 2005 and located in Austin, Texas, is a collaboration between medical professionals and scientists seeking means to help children with autism spectrum disorders through a combination of medical care, education, and research.
b The Autism Genetic Resource Exchange (AGRE) is the first collaborative gene bank for the study of autism spectrum disorders and one of the world’s largest shared resources for the study of autism and related disorders, with a collection of over 900 well characterized multiplex and simplex families made available to the greater scientific community. Founded by Cure Autism Now (CAN) in 1997, AGRE is currently funded by the National Institute of Mental Health (NIMH) and Autism Speaks (AS), which merged with CAN in 2006.
c National Disease Research Interchange, Philadelphia, PA, USA.
References
- DaleDCBoxerLLilesWCThe phagocytes: neutrophils and monocytesBlood200811293594518684880
- NauseefWMInsights into myeloperoxidase biosynthesis from its inherited deficiencyJ Mol Med199876106616689766843
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders4th editionWashington DCAmerican Psychiatric Association1994
- GillbergCColemanMThe Biology of the Autistic Syndromes2nd editionLondon, UKMac Keith Press1992
- FilipekPAccardoPBaranekGThe screening and diagnosis of autistic spectrum disordersJ Autism Dev Disord199929643948410638459
- BaileyAPhillipsWRutterMAutism: towards an integration of clinical, genetic, neuro-psychological, and neurobiological perspectivesJ Child Psychol Psychiatry199637891268655659
- HorvathKPermanJAutistic disorder and gastrointestinal diseaseCurr Opin Pediat200214583587
- MolloyCManning-CourtneyPPrevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disordersAutism2003716517112846385
- Valicenti-McDermottMMcVicarKRapinIFrequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune diseaseJ Dev Behav Pediatr200627Suppl 2S128S13616685179
- AshwoodPAnthonyAPellicerAATorrenteFWalker-SmithJAWakefieldAJIntestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathologyJ Clin Immunol200323650451715031638
- BalzolaFDanielaCRepiciAAutistic enterocolitis: confirmation of a new inflammatory bowel disease in an Italian cohort of patientsGastroenterology2005128Suppl 2A-303
- WakefieldAJPulestonJMMontgomerySMAnthonyAO’LearyJJMurchSHReview article: the concept of entero-colonic encephalopathy, autism and opioid receptor ligandsAliment Pharmacol Ther20021666367411929383
- WakefieldAJAnthonyAMurchSHEnterocolitis in children with developmental disordersAm J Gastroenterol2000952285229511007230
- ShawWKassenEChavesEIncreased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic featuresClin Chem199541109411047628083
- ShawWKassenEChavesEAssessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometryClin Pract Alternat Med200011526
- RussoAJKrigsmanAJepsonBWakefieldAAnti-PR3 and anti-MPO IgG ANCA in autistic children with chronic GI diseaseImmunology and Immunogenetics Insights200922128
- RussoAJKrigsmanAJepsonBWakefieldALow serum alpha-1 antitrypsin associated with anti-PR-3 ANCA in autistic children with GI diseasePediatrics and Genomics2009In press
- StefanescuABraunSNdrepepaGPrognostic value of plasma myeloperoxidase concentration in patients with stable coronary artery diseaseAm Heart J200815535636018215608
- KutterDDevaquetPVanderstockenGConsequences of total and subtotal myeloperoxidase deficiency: risk or benefit?Acta Haematol2000104101511111115
- CramerRSoranzoMRDriPIncidence of myeloperoxidase deficiency in an area of northern Italy: histochemical, biochemical and functional studiesBr J Haematol19825181876280744
- NauseefWMLessons from MPO deficiency about functionally important structural featuresJpn J Infect Dis2004575S4S515507769
- LanzaFClinical manifestation of myeloperoxidase deficiencyJ Mol Med1998766766819766845
- ParryMFRootRKMetcalfJADelaneyKKKaplowLSRicharWJMyeloperoxidase deficiency: prevalence and clinical significanceAnn Intern Med1981952933016267975
- LehrerRIClineMJLeukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infectionJ Clin Investig196948147814885796360
- KutterDPrevalence of myeloperoxidase deficiency: population studies using Bayer-Technicon automated hematologyJ Mol Med199876106696759766844
- NunoiHKohiFKajiwaraHSuzukiKPrevalence of inherited myeloperoxidase deficiency in JapanMicrobiol Immunol200347752753112953846
- TaioliEBenhamouSBouchardyCMyeloperoxidase G463A polymorphism and lung cancer: a HuGE genetic susceptibility to environmental carcinogens pooled analysisGenet Med20079677317304047
- ChauhanAChauhanVOxidative stress in autismPathophysiology200613317118116766163
- DethRMuratoreCBenzecryJPower-CharnitskyVAWalyMHow environmental and genetic factors combine to cause autism: A redox/methylation hypothesisNeurotoxicology200829119020118031821
- MacFabeDFRodríguez-CapoteKHoffmanJEThe Kilee Patchell-Evans Autism Research Group. A novel rodent model of autism: intraventricular infusions of propionic acid increase locomotor activity and induce neuroinflammation and oxidative stress in discrete regions of adult rat brainAm J Biochem Biotechnol200842146166
- McGinnisWRCould oxidative stress from psychosocial stress affect neurodevelopment in autism?J Autism Dev Disord200737599399417404828
- YaoYWalshWJMcGinnisWRPraticòDAltered vascular phenotype in autism: correlation with oxidative stressArch Neurol20066381161116416908745
- HondaHUedaMKojimaSAssessment of myeloperoxidase and oxidative {alpha}1-antitrypsin in patients on hemodialysisClin J Am Soc Nephrol20094114215119129321
- MillaCYangSCornfieldDNMyeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantationAm J Physiol Lung Cell Mol Physiol2004287L706L71415020295
- KlebanoffSJOxygen metabolism and the toxic properties of phagocytesAnn Intern Med1980934804896254418
- ZhangRBrennanMLShenZMyeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammationJ Biol Chem2002277461164612212359714
- ZhangRShenZNauseefWMHazenSLDefects in leukocyte-mediated initiation of lipid peroxidation in plasma as studied in myeloperoxidase-deficient subjects: systematic identification of multiple endogenous diffusible substrates for myeloperoxidase in plasmaBlood2002991802181011861298
- PodrezEAAbu-SoudHMHazenSLMyeloperoxidase-generated oxidants and atherosclerosisFree Radic Biol Med2000281717172510946213
- HeineckeJWOxidative stress: new approaches to diagnosis and prognosis in atherosclerosisAm J Cardiol20039112A16A12505564
- MingXSteinTPBrimacombeMJohnsonWGLambertGHWagnerGCIncreased excretion of a lipid peroxidation biomarker in autismProstaglandins Leukot Essent Fatty Acids200573537938416081262
- JoryJMcGinnisWRed-cell trace minerals in children with autismAmerican Journal of Biochemistry and Biotechnology20084101104
- MingXChenMYochumCHallidayAWagnerGEvidence of oxidative stress in autism derived from animal modelsAmerican Journal of Biochemistry and Biotechnology20084218225
- RuzickaTBauerAGlückSBornMEffects of dapsone on passive Arthus reaction and chemotaxis and phagocytosis of polymorphonuclear leukocytesArch Dermatol Res19812703473516455972
- StendahlOMolinLDahigrenCThe inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone. A possible mechanism in the treatment of dermatitis herpetiformisJ Clin Invest197862214220207742
- LanzaFClinical manifestation of myeloperoxidase deficiencyJ Mol Med199876106766819766845
- SimmondsNMillarABlakeDRamptonDAntioxidant effects of aminosalicylates and potential new drugs for inflammatory bowel disease: assessment in cell-free systems and inflamed human colorectal biopsiesAliment Pharmacol Ther19991336337210102970