Abstract
Osteoporosis is an escalating global problem. Hip fractures, the most catastrophic complication of osteoporosis, continue to cause significant mortality and morbidity despite increasing availability of effective preventative agents. Among these agents, oral bisphosphonates have been the first choice for the treatment and prevention of osteoporotic fractures. However, the use of oral bisphosphonates, especially in the older population, has been limited by their side effects and method of administration thus compromising their persistent use. The resultant low adherence by patients has undermined their full potential and has been associated with an increase in the incidence of fragility fractures. Recently, annual intravenous zoledronic acid (ZOL) has been approved for osteoporosis. Randomized controlled trials have demonstrated ZOL to be safe, have good tolerability and produce significant effect on bone mass and microarchitecture. Adherence has also been shown to be better with ZOL. Furthermore two large trials firmly demonstrated significant anti-osteoporotic effect (∼59% relative risk reduction of hip fractures) and mortality benefit (28% reduction in mortality) of ZOL in older persons with recent hip fractures. In this review, we report the current evidence on the use of ZOL for the prevention of hip fractures in the elderly. We also report the pharmacological characteristics and the advantages and disadvantages of ZOL in this particular group.
Introduction
Osteoporosis is a disease associated with low bone mass, which predisposes to fractures.Citation1,Citation2 Osteoporosis is also a major public health problem that represents a significant burden for health care budgets due to the increasing prevalence of fractures and disability.Citation3 Aging is the most important risk factor for osteoporosisCitation4,Citation5 being highly prevalent in both elderly men and women with its incidence expected to increase in the upcoming years.Citation6,Citation7
Although all fractures not associated with trauma in older adults should be interpreted as osteoporotic fractures,Citation8 hip fractures represent the most typical and catastrophic events associated with osteoporosis in older adults. In fact, hip fractures are considered a predictor of mortality in both institutionalized and ambulatory older persons. With hip fractures, most deaths occur in the first 3 to 6 months following the event, of which 20% to 30% are causally related to the fracture event itself.Citation9 Women with hip fractures have mortality rates 10% to 20% greater than expected for their age within the first yearCitation10 and this excess mortality persists for several years after the hip fracture.Citation11 In fact, a white woman when sustaining a hip fracture at age 70 has an excess mortality of 3%, 4%, 7% and 13% at 1, 2, 5 and 10 years after injury, respectively.Citation11
Unfortunately, a significant proportion of elderly patients who have suffered a hip fracture do not receive further treatment for osteoporosis.Citation12–Citation17 Considering that the occurrence of a hip fracture is a predictor of new fractures,Citation18–Citation21 mortalityCitation9,Citation22–Citation24 and disability,Citation25 a more pro-active and effective therapeutic approach should be encouraged in all elderly patients after presenting with hip fractures.
There have been additional therapeutic agents available in the management of osteoporosis in recent years.Citation26–Citation28 New medications have shown to be effective for primary and secondary prevention of osteoporotic fractures.Citation27,Citation28 In addition, less frequent dosing of bisphosphonates, a group of medications that constitute the mainstay of treatment for osteoporosis, have demonstrated their impact by improving compliance, a major limitation for effective osteoporosis treatment in the older population.Citation28
Amongst the bisphosphonates, an annual dose of zoledronic acid (ZOL) has been recently proven as an effective approach for the management of osteoporosis.Citation29,Citation30 This intravenous dose has several advantages over other bisphosphonates, compliance being the most important. In this review, we will summarize the pharmacological characteristics of ZOL and the recent evidence on annual ZOL in primary and secondary prevention of hip fractures focusing on the evidence in the older population.
Mechanisms of action of zoledronic acid
Pharmacodynamics
Bisphosphonates are pyrophosphate analogs with high affinity for hydroxyapatite and bind directly to mineralized bone.Citation26,Citation31 The bound bisphosphonate blocks the surface of bone and prevents osteoclasts from binding to the resorption surface. The bisphosphonates also inhibit osteoclastic activity, reduce the lifespan of the osteoclasts and alters the bone or bone mineral to reduce the rate of dissolution.Citation32
There are two classes of bisphosphonates: the second generation that contains nitrogen in either an alkyl chain (alendronate, pamidronate and ibandronate) or within a heterocyclic ring (risedronate and ZOL) and the earlier generation that does not (etidronate and clodronate). Those without the nitrogen side chain have low antiresorptive potency and works by being incorporated into molecules of adenosine triphosphate (ATP). Subsequent intracellular accumulation of these nonhydrolyzable ATP analogues inhibit ATP-dependent cellular processes, thus inducing osteoclast-apoptosis.Citation26,Citation33,Citation34 The more potent nitrogen containing bisphosphonates affect osteoclast activity and survival through the inhibition of the enzymes within the mevalonate pathway, the biosyn-thetic pathway for cholesterol and isoprenoid lipids such as farnesyl pyrophosphate (FPP) and geranylgeranyl diphosphate (GGPP). The key enzyme is thought to be FPP synthase.Citation36 Biochemical studiesCitation31,Citation36–Citation38 have demonstrated that ZOL and other nitrogen-containing bisphosphonates inhibit FPP synthase, thus preventing the synthesis of FPP and GGPP, which are necessary for prenylation of small guanosine triphosphate (GTP)-binding proteins in osteoclasts. The loss of these prenylated proteins is associated with decreased osteoclast activity and apoptosis.Citation39,Citation40
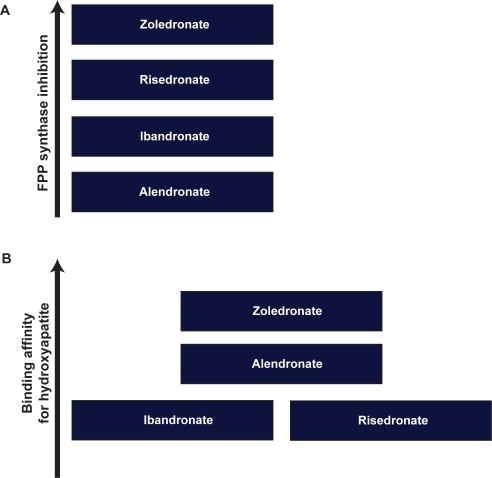
The mechanism of apoptosis in osteoclasts was further studied by Benford et al.Citation39 Osteoclast apoptosis was associated with activation of caspase-3–like proteases, loss of mitochon-drial membrane potential and classic morphologic changes. Of the bisphosphonates studied (risedronate, pamidronate, clodronate, etidronate, tiludronate, alendronate, and ZOL), ZOL was the most potent inhibitor of FPP synthaseCitation41 and caused the greatest increase in caspase-3–like activity at 48 hours after treatment. This order of potency in inhibiting FPP synthase (ZOL > risedronate > ibandronate > alendronate) () closely matched the order of anti-resorptive potency.Citation10 Ultimately the relative potency of different bisphosphonates is related to their binding affinities for hydroxyapatite and their degree of inhibition of FPP synthase. Nancollas et alCitation42 demonstrated that the different binding affinities of various bisphosphonates correlated with the differences in the R2 side chain (ZOL > alendronate > ibandronate = risedronate) ().
Figure 1 Compared with other bisphosphonates of the same generation, zoledronic acid has shown a higher inhibitory effect on farnesyl pyrophosphate synthase (FPP) (A) as well as higher affinity for hydroxyapatite in bones (B).
In addition, ZOL may have direct effects on osteoblasts. Studies examining its effects on BMD, bone architecture, bone strength and biochemical markers of bone metabolism have demonstrated favorable effects which included dose-dependent prevention of increased cortical bone porosity and decreased bone resorption.Citation43,Citation44 In vitro studies also showed that ZOL inhibits vitamin D3-induced calcium release and that at effective doses for inhibiting bone resorption, it did not inhibit bone mineralization to any greater degree compared to other bisphosphonates.Citation38
Pharmacokinetics
ZOL is administered as a once-yearly 5-mg intravenous infusion over no less than 15 minutes.Citation45 ZOL is not metabolized in humans and about 40% of an IV dose of ZOL is eliminated unchanged via the kidney.Citation46 The remaining 60% binds to bone. In vivo studies in rats showed that high levels of 14C-labeled ZOL (> 1.105 pmol/g) distributed widely throughout the axial and appendicular skeleton, mandible, skull, and kidney within 1 hour after IV injection.Citation38 Bone concentrations remained high although levels of ZOL in blood and soft tissues declined to low levels (<1 pmol/g) after a period of 8 months following administration.
A pharmacokinetic studyCitation47 showed that ZOL produced dose-proportional plasma concentrations that declined rapidly in a triphasic manner: rapid biphasic disappearance from the systemic circulation followed by a long elimination phase with a terminal elimination half-life of 146 hours. Twenty-four hours after injection, plasma concentrations declined to <1% of the peak concentration at the end of infusion.
The substance is thought to have little or no capacity as a direct acting and/or irreversible metabolism dependent inhibitor of cytochrome P450 enzymes, and thus is unlikely to reduce the metabolic clearance of substances that are metabolized via the cytochrome P450 enzyme systems. No specific drug–drug interactions have been documented with ZOL.
The renal clearance of ZOL correlated with creatinine clearance. Small observed increases in AUC (0–24 hours), by about 30% to 40% in mild to moderate renal impairment, compared to a patient with normal renal function, and lack of accumulation of drug with multiple doses irrespective of renal function, suggest that dose adjustments of ZOL in mild (Clcr = 50–80 mL/minute) and moderate (Clcr = 35–50 mL/minute) renal impairment are not necessary. In fact, a recent study by Boonen et alCitation48 looking at renal safety of annual ZOL infusions in 7714 osteoporotic postmenopausal women found that transient changes in renal function can occur following an annual ZOL infusion but, in the long term, renal function was not different from that in control patients. Nevertheless, as with other bisphosphonates, the use of ZOL in elderly patients with creatinine clearance <35 mL/minute is not recommended due to limited clinical safety data in such patients.
Safety and tolerability
Overall ZOL is safe and well tolerated when used in the treatment and prevention of osteoporosis. The most common adverse events observed were acute phase reactions,Citation29 usually characterized by flu-like symptoms, headache, bone pain, arthralgias and myalgias. Most of these symptoms occurred within the first 3 days after infusion and tended to resolve within several days after administration. They are usually easily managed with acetaminophen or ibuprofen and the incidence of these symptoms decreased with subsequent annual infusions.
Renal effects
No clinically significant long-term effects of ZOL on renal function have been observed so far. In the HORIZON-PFT, there was a significant increase in serum creatinine of more than 44 μmol/L in 1.3% of ZOL-treated patients, compared with 0.4% of placebo patients at 9 to 11 days post infusion. Within 30 days, however, the levels in more than 85% of patients had returned to within 44 μmol/L of pre-infusion values, and the remainder had returned to this level by the next annual follow-up. At 3 years, there was no significant difference in either serum creatinine levels or creatinine clearance between the placebo group and ZOL group. Boonen et alCitation48 reported these changes testing the renal-function of 5035 osteoporotic postmenopausal women from the HORIZON Pivotal Fracture Trial. They also found that two of the four patients in the ZOL group who suffered acute renal failure requiring hospitalization had baseline creatinine clearance of 31 mL per min. Overall, they reported the mean decrease in serum creatinine in these elderly patients to be similar in both groups, as well as the overall number of observed renal adverse effects.
Comparable results were reported in HORIZON-RFT;Citation30 the incidence of serum creatinine elevations greater than 44 μmol/L did not differ between the ZOL and placebo groups (6.2% and 5.6%). The result from McClung et alCitation49 trial is similar. Only 0.02% of patients in the ZOL group experienced a rise in serum creatinine or creatinine clearance. Furthermore these patients had low baseline creatinine clearance of around 30 mL/min. At the end of 1 year the renal function recovered to near baseline. Reid et alCitation50 administering various doses of ZOL also did not observe any significant renal side effects.
Atrial fibrillation (AF)
The association with AF remains uncertain, as a causal relationship has not been established. In HORIZON-PFT,Citation29 the number of patients who had arrhythmia in the ZOL group (266 patients, or 6.9%) was significantly higher than that in the placebo group (203 patients, or 5.3%; p = 0.003). The overall incidence of all AF adverse events was 2.4% of patients (94 of 3862) in the ZOL group, compared with 1.9% of patients (73 of 3852) in the placebo group. AF judged to be serious occurred in 50 patients in the ZOL group (1.3%), as compared with 17 patients (0.4%) in the placebo group. Among the 50 patients, the events occurred more than 30 days after infusion in 47 patients. Among 559 patients who underwent electrocardiography, the prevalence of ZOL (2.1% in the ZOL group and 2.8% in the placebo group) and other electrocardiographic abnormalities did not differ significantly between the study groups. Interestingly the HORIZON-RFTCitation30 did not find a difference in the incidence of AF events: 29 (2.8%) in the ZOL group versus 27 (2.6%) cases for the placebo group. In summary, data on the incidence of AF reported in clinical trials using ZOL for fracture prevention is limited to only two studies. More evidence is required to consider whether this finding is just chance or a real side effect that should be seriously considered when treating old patients.
Osteonecrosis of the jaw (ONJ)
The incidence of ONJ in patients treated for osteoporosis using bisphosphonates is estimated to be less than 1 in 100,000 patients.Citation51 Although there have been case reports of ONJ in patients on alendronateCitation51,Citation52 and risedronate,Citation53 there have been no reports of spontaneous ONJ in patents treated with ZOL. However, in HORIZON-PFTCitation29 an independent adjudication committee blinded to study treatment identified two potential cases of ONJ, one in the placebo arm and one in the ZOL group.
Other adverse effects
Comparable number of patients in HORIZON-PFTCitation29 and in HORIZON-RFTCitation30 had notable declines of serum calcium levels (to <7.5 mg/dL) after administration of ZOL; but no symptomatic cases of hypocalcemia were observed. It is possible that the loading of vitamin D and continued administration of vitamin D and calcium may mask the incidence of hypocalcemia or reduce its severity.
Another bone safety aspect that has been debated for several years is that prolonged use of bisphosphonates at high doses might be associated with micro fractures as described in animal models.Citation54,Citation55 These findings are not clear in the clinical setting as the doses used in these experiments are about 6-fold higher than that used in humans. Furthermore a study examining micro cracks in bones of dogs receiving alendronate and risedronate did not show effect on mechanical properties despite confirming increased micro-damage accumulation.Citation56 Finally, in the FLEX trial, Recker et alCitation57 did not find evidence of mineralization defect in bones of women who had been on alendronate for 10 years. Finally, no evidence associating ZOL and bone micro cracks has been reported.
Clinical trials with zoledronic acid
The effects of ZOL on bone mineral density (BMD) was investigated by Reid et al.Citation50 They conducted a multinational one year study that randomized 45–80 year old postmenopausal women with T-scores at the lumbar spine of less than −2 SD to receive either placebo or one of five ZOL regimens: 0.25 mg, 0.5 mg, or 1 mg at 3-month intervals; a single 4-mg dose; or 2 doses of 2 mg administered 6 months apart. Although, the rate of increase tended to slow in the second half of the study, there were significant improvement in the average mean lumbar spine BMD and femoral neck BMD in the ZOL group compared with the placebo group (see ). In contrast, BMD at the distal radius responded to ZOL treatment to a lesser extent. Significant decreases in bone turnover markers were also observed with the ZOL group. Markers of bone resorption reached a nadir at 1 month; however median decreases of 65% to 83% in serum C-telopeptide and 50% to 69% in the urinary N-telopeptide (Ntx):creatinine ratio were achieved. Furthermore the decrease in markers of resorption tended to be dose-dependent, particularly at 3 months of treatment. The incidence of adverse events was similar between all ZOL groups. The most commonly reported were myalgia and pyrexia.
Table 1 Summary of results from randomized controlled clinical trials of zoledronic acid
Saag et alCitation58 compared the onset of action, effects on bone resorption and tolerability of ZOL with alendronate. Postmenopausal women aged 45 to 79 years were randomized to receive either a single infusion of ZOL 5 mg plus oral placebo or weekly oral alendronate 70 mg plus a single placebo intravenous infusion. Women with either a femoral neck or lumbar spine BMD T-score of −2 or less at study initiation and no previous bisphosphonate use of 2 or more years were included.
At week 1, significantly lower mean urine NTx value was seen in the ZOL group as opposed to the alendronate group (15.2 nmol bone collagen equivalents (BCE)/mmol creatinine and 35.5 nmol BCE/mmol creatinine, respectively). After this nadir, levels gradually increased but were significantly lower than the alendronate group throughout the 24-week study and remained stable within the study reference range of premenopausal women (17.8–46.4 nmol BCE/mmol creatinine) from week 12 to study end. Despite comparable adverse events (91.3% in the ZOL group and 86.4% in the alendronate group) and similar premature discontinuation rates (8.7% in the ZOL group and 8.5% in the alendronate group), the majority of patients preferred the annual ZOL infusion to the weekly alendronate, citing convenience as the primary factor.
In another multicenter trial, McClung et alCitation49 similarly demonstrated better adherence and preference for annual infusion of ZOL over 70 mg weekly of oral alendronate. Postmenopausal women who were receiving oral alendronate for at least 1 year and had lumbar spine or femoral neck BMD T-score values ≤ −2.0 SD were randomized to one 15-min IV infusion of ZOL 5 mg plus 52 weeks of oral placebo or one IV infusion of placebo plus 52 weeks of oral alendronate 70 mg. Bone marker levels decreased steadily in both groups and at month 12 remained in the lower half of the premenopausal reference range. The overall rates of adverse events were comparable in the 2 groups (ZOL 5 mg, 86.7%; alendronate 70 mg, 80.4%). The majority (78.7%) of patients regardless of study groups expressed preference for once yearly infusion over weekly oral therapy. This result suggests that patients could be switched from oral alendronate to ZOL 5 mg infusion without affecting the therapeutic benefits of oral bisphosphonates. This evidence is useful in cases in which oral bisphosphonates are not well tolerated or when adherence is a major issue.
Whether the actions of a single dose of ZOL on bone turnover and on BMD extends beyond 12 months was recently investigated by Borba et alCitation59 This was a prospective study evaluating the changes in bone turnover at 12 months (T12) and 18 months (T18). The subjects had low BMD, had at least one other risk factor (in all but three) and had a mean age of 60.5 ± 16 years. Other metabolic bone diseases and past use of bisphosphonates were included in the exclusion criteria.
Median serum bone turnover markers levels were significantly suppressed at T12 and continued to be suppressed at T18 in comparison with baseline values. BMD at 12 months showed significant increase at the lumbar spine (LS) which was sustained at T18 (). A modest increase in total hip BMD was also evident and maintained at T18. The results of this study suggest that there may be a role of extending the time between infusions of ZOL beyond 12 months without losing efficacy and thereby possibly reducing costs and increasing adherence even further.
The long-term safety and efficacy of ZOL in women with postmenopausal osteoporosis was further evaluated by Devogelaer et al.Citation60 This extension of a previous studyCitation50 was composed of 2 consecutive open-label, 2-year periods. Women who entered the first extension study received ZOL 0.5 mg every 3 months; the others were administered ZOL 1 mg every 3 months. Patients who entered the second extension study received either calcium only or ZOL 4 mg. Patients received treatment for either 2, 3, or 5 years. The efficacy of ZOL was measured by DEXA assessment of BMD at the proximal femur, lumbar spine, distal radius, and total body. BMD was found to increase in all three subgroups by the end of the 5-year total study period.
Arthralgia was the most commonly reported adverse event and 8 patients experienced serious adverse events, with 7 of the 15 total events reported as cardiovascular related. Interpretation of this study is however relatively limited due to the open-label design, small treatment groups, and lack of clarification of cardiovascular adverse events.
Zoledronic acid and hip fractures
Hip fractures are highly prevalent in older osteoporotic patients. However, despite a myriad of literature on osteoporosis and its treatment, only a number of trials have assessed hip fractures as primary outcomes.Citation61 In the case of ZOL, very few studies have been pursued looking at its effectiveness on primary and secondary prevention of hip fractures () and just a few of them have looked specifically in older populations.
Recently, ZOL impact on re-fracture reduction was demonstrated in The HORIZON (Health Outcomes and Reduced Incidence with ZOL Once Yearly) Pivotal Fracture trial.Citation29 This is the first trial to specifically evaluate the effects of ZOL on fractures. This double-blind, placebo-controlled trial enrolled 7765 postmenopausal women (mean age, 73 years) with confirmed osteoporosis and evidence of clinical fractures. Prior to randomization, patients were stratified according to their concomitant use of osteoporosis medications (raloxifene, calcitonin and hormone replacement therapy but not other bisphosphonates). 3889 were randomly assigned to receive a single 15-minute infusion of ZOL (5 mg) and 3876 were assigned to receive placebo at baseline, at 12 months, and at 24 months. The patients were followed for 3 years. All patients received oral daily calcium (1000–1500 mg) and vitamin D (400–1200 IU). Primary end points were new vertebral fractures (in patients not taking concomitant osteoporosis medications) and hip fractures (in all patients). Secondary end points included BMD, bone turnover markers, and safety outcomes.
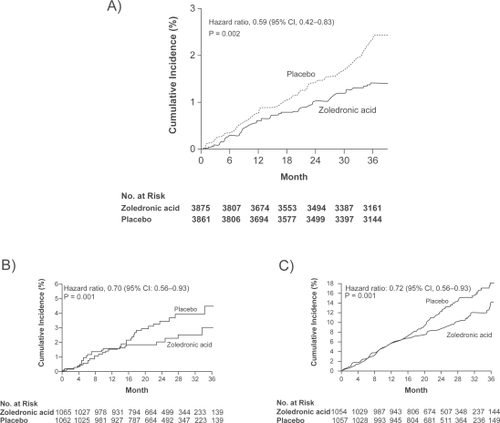
In women not receiving concomitant medications, ZOL was found to reduce the risk of morphometric vertebral fracture by 70% during the 3-year study period, as compared with placebo (3.3% in the ZOL group vs 10.9% in the placebo group; absolute risk reduction of 7.6%) and from both arms of treatment there was a reduction in the risk of hip fracture of 41% (1.4% in the ZOL group vs 2.5% in the placebo group; ARR 1.1%) ().
Figure 2 Clinical trials showing a significant reduction in the incidence of hip fractures in primary A) and secondary prevention B) In addition zoledronic acid a significant reduction in mortality after 2 years of treatment was found in the HORIZON trial. C) Adapted with permission (Part A) from Black D, Delmas P, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356(18):1809–1822;Citation29 (Parts B and C) Lyles K, Colon-Emeric C, Magaziner J, et al Zoledronic acid and clinical features and mortality after hip fracture. N Engl J Med. 2007; 357(18):1799–1809.Citation30 Copyright © 2007 Massachusetts Medical Society. All rights reserved.
Secondary outcomes also favored the ZOL group including a 33% reduction in any clinical fractures (8.4% vs 12.8%), 77% reduction in clinical vertebral fractures (0.5% vs 2.6%) and multiple vertebral fractures (0.2% vs 2.3%) and 25% in nonvertebral fractures (8.0% vs 10.7%). As compared with placebo, ZOL was also associated with a significant improvement in BMD and bone metabolism markers. BMD was increased at the total hip (6.02%), lumbar spine (6.71%) and the femoral neck (5.06%). Tolerability was acceptable with 81% of patients receiving the maximum of three infusions. Retention rates were comparable with 2.1% of the ZOL group discontinuing treatment due to adverse events compared with 1.8% in the placebo group.
Adverse events, including change in renal function were similar in the two study groups. Interestingly, there was an inexplicable statistically significant increase in serious AF in the ZOL group compared with the placebo group (50 vs 20 patients, respectively), which occurred more than 30 days after infusion in 47 women in the treatment group. This suggests that it may not be related to the acute infusions. Hypocalcemia as a cause was deemed unlikely from the regular evaluation of electrolyte changes. The author reported that there were significant differences between study groups in the change in serum calcium from the level before the first infusion of ZOL to the level 9 to 11 days after the infusion, but the magnitude of the difference was relatively small (a reduction of 0.2 ±0.5 mg/dL in the ZOL group vs an increase of 0.03±0.4 in the placebo group). This difference was not evident at 12 months, and no significant changes occurred with subsequent doses. Furthermore, the change in calcium levels after the first infusion did not differ between women with AF and those without AF. Over the 3 years of the study, mean serum calcium levels increased in both study groups, although the mean increase was slightly larger in the placebo group than in the ZOL group. Magnesium and potassium levels did not differ between the two study groups and were similar in women with and those without AF. It should be noted also that episodes of AF did not cluster in time immediately after any infusion, when serum electrolytes are most affected. Taken together, these findings suggest a possible alternative explanation.
Finally, there are some features of this study that make it unique in terms of the population studied. Although an active comparator would be of great clinical interest, the numbers required for such a trial to demonstrate equivalent or superior fracture risk reductions would be difficult to obtain and possibly prohibitive. Instead, to simulate reality, the protocol specified that patients had to be unable or unwilling to take oral bisphosphonates and all women were counseled on the risk of fracture and the availability of approved osteoporosis medications. Another allowance that mirrors clinical reality was that women who were receiving nonbisphosphonate treatments for osteoporosis (including hormone therapy, raloxifene, and calcitonin) were included (“stratum 2”), and all patients were free to begin any of these treatments during the study while continuing to receive the study treatment. Furthermore, the researchers monitored patients for excessive bone loss or multiple fractures, and women who met either criterion were again counseled about alternative treatment options.
Nevertheless, the evidence provided by this study suggests that a once-yearly infusion of ZOL during a 3-year period significantly reduced the risk of hip fractures in this particular population.
Zoledronic acid and mortality
The second major trial is the HORIZON recurrent fracture trial.Citation30 This well designed trial in a population of frail older subjects constitutes the first trial of an osteoporosis intervention to show a reduction in mortality. This 3-year, multi-center, multinational, double-blind, placebo-controlled trial of 2127 patients with a recent hip fracture assessed the effect of ZOL administered within 90 days after surgical repair. Men and women 50 years of age or older were eligible for inclusion within 90 days after surgical repair of a hip fracture sustained with minimal trauma (ie, a fall from standing height or a lower height). All patients who were enrolled in the trial had undergone repair of a hip fracture and were unable or unwilling to take an oral bisphosphonate. Additional enrollment criteria included being ambulatory before the hip fracture and having both legs. Concomitant therapy with nasal calcitonin, selective estrogen-receptor modulators, hormone replacement, tibolone, and external hip protectors was allowed at the discretion of the investigator. Previous use of bisphosphonates or parathyroid hormone was allowed after a washout period that varied according to the drug and the duration of its use. Previous use of strontium or sodium fluoride was not allowed. Any patient with a serum 25-hydroxyvitamin D level of 15 ng/mL or less, or if the level was not available, a loading dose of either vitamin D3 or D2 (at a dose of 50,000 to 125,000 IU) was given orally or intramuscularly 14 days before the first infusion of ZOL. Thereafter, all patients received daily supplementation with oral calcium (1000 to 1500 mg) and vitamin D (800 to 1200 IU). Exclusion criteria were previous hypersensitivity to a bisphosphonate, a potential for pregnancy, a calculated creatinine clearance of less than 30 mL per minute, a corrected serum calcium level of more than 11.0 mg/dL (2.8 mmol/L) or less than 8.0 mg/dL (2.0 mmol/L), active cancer, metabolic bone disease other than osteoporosis, and a life expectancy of less than 6 months in the investigator’s judgment. BMD at the hip and femoral neck and the calculated creatinine clearance were determined at baseline and annually. The primary endpoint was the incidence of further hip fractures and secondary endpoints included the change in BMD in the nonfractured hip, new vertebral fractures, and pre-specified safety endpoints, including death.
Of a total of 2127 patients, 1065 patients were randomly assigned to receive ZOL, and 1062 patients were assigned to receive placebo; 71.3% of the patients completed the trial. The median follow-up time was 1.9 years. Baseline demographic and clinical characteristics were similar in the two groups, with 41.8% of patients having a T score less than −2.5 SD at the femoral neck.
At 24 months ZOL was associated with a rate of new clinical fractures of 8.6%, as compared with 13.9% in the placebo group, an absolute risk reduction of 5.3% and a relative reduction of 35%. Among patients who had a fracture, the mean time to clinical fracture was 39.8 months in the ZOL group and 36.4 months in the placebo group. The risk reduction was very similar in the intention-to-treat and per-protocol populations. The rates of new clinical vertebral and nonvertebral fractures were significantly lower (1.7% vs 3.8% and 7.6% vs 10.7%, respectively).
However the incidence of new hip fractures was not significantly different (2.0% vs 3.5%, a nonsignificant reduction in relative risk of 30%) (). Although in a post hoc analysis, significant divergence in the fracture-free survival curves between the two groups for all clinical fractures was seen as early as 12 months (p = 0.02 by the log-rank test). BMD at the total hip increased in the ZOL group by 2.6%, 4.7%, 5.5% at 12, 24 and 36 months respectively compared with a decline BMD in the placebo group by 1.0%, 0.7%, and 0.9%, respectively. BMD at the femoral neck also increased in the ZOL group by 0.8%, 2.2% and 3.6% at 12, 24 and 36 months respectively and declined in the placebo group by 1.7%, 2.1%, and 0.7%, respectively over the same period. All increases in BMD were statistically significant compared with placebo. Finally, the difference in delayed union of fractured bone between the two study groups was not significant (3.2% in the ZOL group and vs 2.7% in the placebo group; 95% CI, 0.72 to 1.90; p = 0.61).
In the safety analysis, mortality rates were similar in the first 12 months but were significantly different at 24 months with 9.6% in the ZOL group vs 13.3% in the placebo group (a relative reduction of 28% in the risk of death) (). The causes of deaths were primarily from cardiovascular and cerebrovascular diseases. Serious adverse events occurred with similar frequency in the two groups (38.3% in the ZOL group and 41.2% in the placebo group). More patients in the ZOL group than in the placebo group reported pyrexia, myalgia, bone pain, and musculoskeletal pain. More patients in the placebo group (11.4%) reported having fallen than in the ZOL group (9.7%). The incidence of cardiovascular events was similar in the two groups. A total of 24 patients in the ZOL group (2.3%) and 39 patients in the placebo group (3.7%) had a serious adverse event of arrhythmia; rates of AF were similar in the two groups 1.1% vs 1.3%. The incidence of renal adverse events was similar in the two study groups, including events among patients with a baseline creatinine clearance of 30 to 60 mL per minute.
Although no specific case-finding effort was made, no cases of ONJ were reported. In summary, this is a well conducted trial showing that treatment efficacy is possible in the frail elderly group in whom oral bisphosphonates is less efficacious due to side effects and noncompliance. In addition, the reduction in number of deaths is almost the same as the reduction in number of fractures suggesting that fewer post fracture deaths are unlikely to account for the benefit seen.Citation62 Whether concomitant treatment with calcitonin, selective estrogen-receptor modulators, and hormone-replacement therapy had an effect on the results is also not certain. However when this study was designed, the only therapies that had been proven to reduce the risk of hip fractures were alendronate and risedronate.Citation63–Citation65 Thus, it is likely that the use of nonbisphosphonate therapies did not have an important influence on the overall outcome. In addition the study by Black et alCitation29 did not show any difference in the incidence of hip, nonvertebral, or all clinical fractures between subjects who received, and those who did not receive, concomitant therapy (10% in both study groups).
For the mortality results, it is more likely that the reason for this reduction in mortality is multifactorialCitation62 as suggested by the separation of the mortality curves occurring after a year; however, further investigation and more detailed analysis of these data may be needed to elucidate the reasons. It is also interesting that despite the reduction in hip fracture not reaching statistical significance the number needed to treat of 67 to 71 is quite comparable to other bisphosphonate trials.Citation29,Citation63 The baseline risk of death in this patient population was substantially different from that in previous ZOL trialsCitation29 and may also account for some of the variance in results. In addition, the study was not powered to show the differences between treatment groups for this end point.
Special comments should also be made regarding the incidence of AF. Although an increased rate of AF in association with this medication had been a concern in the HORIZON trial,Citation29 it is interesting that in this study, the incidence did not differ between the medication and placebo groups.
Zoledronic acid in the older population
Although the evidence supporting ZOL as a choice for the prevention of osteoporosis fractures in older patients is scarce, there are several characteristics of ZOL that make it an appealing choice in this population.
In terms of compliance, several studies have demonstrated that older patients are more prone to discontinue their treatment after 6 months of administration due to several reasons including difficulties with the strict instructions required for oral bisphosphonates, the lack of symptoms associated with osteoporosis and the poor encouragement received from their family practitioners to continue with their treatment.Citation66–Citation68 In this situation, ZOL constitutes an important choice since it only requires 1 dose every year. Nevertheless, the patients should continue taking vitamin D and calcium in a permanent manner.
A second argument in favor of ZOL in older patients is the recent evidence showing that additionally to its effect on re-fracturing, patients receiving ZOL showed lower mortality rates.Citation30 This is a particularly important effect especially in the older and frail populations. However, the mechanisms that explain this effect remain unknown.
Finally, no studies have been pursued in long-term institutions looking at the effectiveness and cost-benefit of ZOL in institutionalized elderly patients. For patients that are in long-term care institutions, the population at higher risk of fractures according to the literature,Citation18,Citation69 ZOL could become an important choice for fracture prevention.
Conclusion
Hip fractures are an increasing global problem as the population ages. The human costs and economic burden will escalate proportionately. Pharmacotherapy to reduce the morbidity and mortality associated with hip fractures is effective and proven although is still limited by convenience, tolerability and thus compliance. Decisions regarding which agent should be based on these factors and sound hip fracture data. Only alendronate,Citation70 risedronateCitation71 and ZOLCitation29,Citation30 have been proven to reduce nonvertebral and in particularly hip fractures in the intention to treat populations from more than one trial and randomized controlled trials of at least 3 years’ duration.Citation65 ZOL offers advantages of convenience, tolerability and thus greater compliance. The findings that ZOL reduces the recurrence of fractures in patients who already sustained a hip fracture and increase survival, means that there is now available therapy for a population that was largely without therapy before these recent trials were pursued.
Acknowledgements
Dr Duque holds a Fellowship from the University of Sydney Medical Research Foundation. Dr Demontiero has a postdoctoral scholarship in aging research from the Rebecca L Cooper Medical Research Foundation.
Disclosures
The authors disclose no conflicts of interest.
References
- RaiszLPathogenesis of osteoporosis: concepts, conflicts, and prospectsJ Clin Invest2005115123318332516322775
- ClowesJEastellRThe role of bone turnover markers and risk factors in the assessment of osteoporosis and fracture riskBaillieres Best Pract Res Clin Endocrinol Metab200014221323211035903
- BurgeRDawson-HughesBSolomonDWongJKingATostesonAIncidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025J Bone Miner Res200722346547517144789
- SirisEMillerPBarrett-ConnorEIdentification and Fracture Outcomes of Undiagnosed Low Bone Mineral Density in Postmeno-pausal Women. Results From the National Osteoporosis Risk AssessmentJAMA2001286222815282211735756
- WilkinsCBirgeSPrevention of osteoporotic fractures in the elderlyAm J Med2005118111190119516271899
- ReginsterJBurletNOsteoporosis: A still increasing prevalenceBone2006382 Suppl 1S4S916455317
- ColeZDennisonECooperCOsteoporosis epidemiology updateCurr Rheumatol Rep2008102929618460262
- JohnellOKanisJEpidemiology of osteoporotic fracturesOsteoporos Int200516Suppl 2S3S715365697
- CooperCAtkinsonEJacobsenSO’FallonWMeltonLrPopulation-based study of survival after osteoporotic fracturesAm J Epidemiol19931379100110058317445
- FarahmandBMichaelssonKAhlbomALjunghallSBaronJSurvival after hip fractureOsteoporos Int2005161583159016217590
- HaentjensPBoonenSExcess mortality after hip fracture among postmenopausal women and ageing men: evidence from data searches and life-table analyses for gender-related differences in absolute risk of death after hip fractureOsteporosis Int200718Suppl 1S5S6
- TorgersonDDolanPPrescribing by general practitioners after an osteoporotic fractureAnn Rheum Dis19985763783799771215
- KamelHHussainMTariqSPerryHMorleyJFailure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fractureAm J Med2000109432632810996585
- KamelHDuthieEThe underuse of therapy in the secondary prevention of hip fracturesDrugs Aging200219111011929323
- AndradeSMajumdarSChanKLow frequency of treatment of osteoporosis among postmenopausal women following a fractureArch Intern Med2003163172052205714504118
- SolomonDFinkelsteinJKatzJMogunHAvornJUnderuse of osteoporosis medications in elderly patients with fracturesAm J Med2003115539840014553876
- Colón-EmericCLylesKHousePRandomized trial to improve fracture prevention in nursing home residentsAm J Med20071201088689217904460
- KlotzbuecherCRossPLandsmanPAbbottTrBergerMPatients with prior fractures have an increased risk of future fractures:a summary of the literature and statistical synthesisJ Bone Miner Res200015472173910780864
- KanisJJohnellOLaetCDA meta-analysis of previous fracture and subsequent fracture riskBone200435237538215268886
- CenterJBliucDNguyenTEismanJRisk of subsequent fracture after low-trauma fracture in men and womenJAMA200729738739417244835
- FriesendorffMVBesjakovJÅkessonKLong-term survival and fracture risk after hip fracture: a 22-year follow-up in womenJ Bone Miner Res200823111832184118597630
- HamletWLiebermanJFreedmanEDoreyFFletcherAJohnsonEInfluence of health status and the timing of surgery on mortality in hip fracture patientsAm J Orthop19972696216279316725
- CenterJNguyenTSchneiderDSambrookPEismanJMortality after all major types of osteoporotic fracture in men and women: an observational studyLancet1999353915687888210093980
- BhattacharyyaTIorioRHealyWRate of and risk factors for acute inpatient mortality after orthopaedic surgeryJ Bone Joint Surg Am200284-A456257211940616
- MagazinerJFredmanLHawkesWChanges in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling agedAm J Epidemiol2003157111023103112777366
- RussellRDeterminants of structure-function relationships among bisphosphonatesBone2007405S21S25
- ShobackDUpdate in osteoporosis and metabolic bone disordersJ Clin Endocrinol Metab200792374775317341572
- BoonenSVanderschuerenDVenkenKMilisenKDelforgeMHaentjensPRecent developments in the management of postmeno-pausal osteoporosis with bisphosphonates: enhanced efficacy by enhanced complianceJ Intern Med2008264431533218823505
- BlackDDelmasPEastellROnce-yearly zoledronic acid for treatment of postmenopausal osteoporosisN Engl J Med2007356181809182217476007
- LylesKColon-EmericCMagazinerJZoledronic acid and clinical features and mortality after hip fractureN Engl J Med2007357181799180917878149
- DunfordJThompsonKFPFCStructure-activity relationships for inhibition of farnesyl disphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonatesJ Pharmacol Exp Ther2001296223524211160603
- RodanGFleischHBisphosphonates: mechanism of actionsJ Clin Invest199697269226968675678
- LehenkariPKellinsalmiMNäpänkangasJFurther insight into mechanism of action of clodronate:inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenosine containing metaboliteMol Pharmacol20026151255126211961144
- FrithJMonkkonenJAuriolaSMönkkönenHRogersMThe molecular mechanism of action of the antiresorptive and antiinflammatory drug clodronate: evidence for the formation in vivo of a metabolite that inhibits bone resorption and causes osteoclast and macrophage apoptosisArthritis Rheum20014492201221011592386
- RussellRBisphosphonates: from bench to bedsideAnn N Y Acad Sci2006106836740116831938
- BergstromJBostedorRMasarachiaPReszkaARodanGAlendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthaseArch Biochem Biophys2000373123124110620343
- LiEDavisLZoledronic acid: a new parenteral bisphosphonateClin Ther200325112669270814693298
- GreenJChemical and biological prerequisites for novel bisphosphonate molecules: Results of comparative preclinical studiesSemin Oncol2001282 Suppl 641011346859
- BenfordHMcGowanNHelfrichMNuttallMRogersMVisualization of bisphosphonateinduced caspase-3 activity in apoptotic osteoclasts in vitroBone200128546547311344045
- BoissierSFerrerasMPeyruchaudOBisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastasesCancer Res200060112949295410850442
- NancollasGTangRGuideSMineral binding affinities and zeta potentials of bisphosphonatesJ Bone Miner Res200217 Suppl 1S368
- NancollasGTangRPhippsRNovel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyappetiteBone200638561762716046206
- HornbySEvansGHornbySPatakiAGlattMGreenJLong-term zoledronic acid treatment increases bone structure and mechanical strength of long bones of ovariectomized adult ratsCalcif Tissue Int200372451952712574877
- BinkleyNKimmelDBrunerJZoledronate prevents the development of absolute osteopenia following ovariectomy in adult rhesus monkeysJ Bone Miner Res19981311177517829797488
- Novartis, Reclast (zoledronic acid)East Hanover, NJ 82007
- GreenJMullerKJaeggiKPreclinical pharmacology of CGP 42’446, a new, potent, heterocyclic bisphosphonate compoundJ Bone Miner Res1994597457518053405
- ChenTBerensonJVescioRPharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastasesJ Clin Pharmacol200242111228123612412821
- BoonenSSellmeyerDLippunerKRenal safety of annual zoledronic acid infusions in osteoporotic postmenopausal womenKidney Int20087464164818509324
- McClungMReckerRMillerPIntravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronateBone200741112212817468062
- ReidIBrownJBurckhardtPIntravenous Zoledronic acid in postmenopausal women with low bone mineral densityN Engl J Med2002346965366111870242
- PatrickMPurcellWBBoydIWBisphosphonates and osteonecrosis of the jawMJA2005182841741815850440
- VieillardMMaesJPenelGThirteen cases of jaw osteonecrosis in patients on bisphosphonate therapyJoint, Bone, Spine: Revue du Rhumatisme20087513440
- BrooksJGilsonASindlerAAshmanSSchwartzKNikitakisNOsteonecrosis of the jaws associated with use of risedronate: report of 2 new casesOral Surg Oral Med Oral Pathol Oral Radiol Endod2007103678078617223592
- MashibaTMoriSBurrDThe effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog ribJ Bone Miner Metab200523 SupplS36S42
- KomatsubaraSMoriSMashibaTLong term treatment of incdronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebraJ Bone Miner Res20031851252012619936
- AllenMIwataKPhippsRothers aResidroante and alendroante similarly suppress remodeling and increase microdamage in beagles after 1 year of treatment at clinical doses27th Annual Meeting of the American Society for Bone and Mineral ResearchNashville, Tennessee, USAJ Bone Miner Res2005S22
- ReckerREnsrudKDiemSNormal Bone Histomorphometry and 3D microarchitecture after 10 years alendronate treatment of post-menopausal women26th Annual meeting of the American Society for Bone and Mineral ResearchSeattle, Washington, USA2004S45
- SaagKLindsayRKriegmanABeamerEZhouWA single zole-dronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral densityBone20074051238124317347063
- BorbaVPaz-FilhoGBone turnover 18 months after a single intravenous dose of zoledronic acidInt J Clin Pract20076161058106217504370
- DevogelaerJBrownJBurkhardtPZoledronic acid efficacy and safety over five years in postmenopausal osteoporosisOsteoporos Int20071891211121817516022
- BruyereOBrandiMBurletNPost-fracture management of patients with hip fracture: a perspectiveCurr Med Res Opin200824102841285118759997
- DuqueGIntravenous zoledronic acid reduced new clinical fractures and deaths in patients who had recent surgery for hip fractureACP J Club200814824018311870
- BlackDCummingsSKarpfDRandomised trial of effect of alendronate on risk of fracture in women with existing vertebral fracturesLancet19963489041153515418950879
- CranneyAWaldeggerLZytarukNRisedronate for the prevention and treatment of postmenopausal osteoporosisCochrane Database Syst Rev20034CD00452314584020
- DuqueGDemontieroOTroenBThe prevention and treatment of senile osteoporosis and hip fracturesMinerva Medica (in press)
- SilvermanSAdherence to medications for the treatment of osteoporosisRheum Dis Clin North Am200632472173117288974
- YoodREmaniSReedJLewisBCharpentierMLydickECompliance with pharmacologic therapy for osteoporosisOsteoporos Int2003141296596814504697
- SirisEHarrisSRosenCAdherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databasesMayo Clin Proc20068181013102216901023
- Colón-EmericCLylesKLevineDPrevalence and predictors of osteoporosis treatment in nursing home residents with known osteoporosis or recent fractureOsteoporos Int200718455355917120179
- WellsGCranneyAPetersonJAlendronate for the primary and secondary prevention of osteoporotic fractures in postmeno-pausal womenCochrane Database Syst Rev20081Art. No.: CD001155
- WellsGCranneyAPetersonJRisedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal womenCochrane Database Syst Rev20081CD00452318254053