Abstract
In elderly women, loss in bone mass and micro-architectural changes are generally attributed to the onset of menopause. Men do not experience menopause, they do, however, experience age-related acceleration in bone loss and micro-architecture deterioration. The incidence of osteoporotic fractures in elderly men, just as in aged women, increases exponentially with age; the rise in men, however, is some 5–10 years later than in women. Up to 50% of male osteoporotics have no identifiable etiology; however elderly males have much higher likelihood of having an identifiable secondary cause than younger men. Therefore, clinical and laboratory evaluation of aged male osteoporotics must be thorough and should be aimed at identifying lifestyle or conditions contributing to bone loss and fragility. It is essential to identify and treat secondary causes and ensure adequate vitamin D and calcium intake before embarking upon treatment with pharmacological agents. The evidence from a limited number of trials suggests that bisphosphonates, especially alendronate and risedronate, are effective in improving BMD, and seem to be the treatments of choice in aged men with osteoporosis. In cases where bisphosphonates are contra-indicated or ineffective, teriparatide or alternatives such as strontium should be considered.
Background
Osteoporosis is a skeletal disorder characterized by compromised bone strength, predisposing a person to an increased risk of fracture (NIH 2000). There is a loss of bone mass and micro-architectural deterioration of bone tissue, resulting in low bone mineral density (BMD). This, in combination with other structural changes, causes an alteration in biomechanical properties and an increased risk of low trauma fractures. Osteoporosis is a common condition that afflicts both men and women, with the lifetime risk of fracture at the age of 50 years being estimated at 50% for women and 20% for men (CitationVan Staa et al 2001; CitationUSDHHS 2004). Up to 20% of symptomatic vertebral fractures, 25% of forearm fractures and 30% of hip fractures occur in men (CitationEastell et al 1998; CitationO’Neill et al 2001; CitationVan Staa et al 2001). Furthermore, these fractures have a profound impact on the individual in terms of morbidity and mortality (CitationPoor et al 1994, Citation1995; CitationCenter et al 1999; CitationScane et al 1999; CitationO’Neill et al 2001; CitationVan Staa et al 2001).
The number of men presenting with these fractures is rising, because of increasing life expectancy and a doubling of the age specific incidence of fractures over the past three decades (CitationBoyce and Vassey 1985; CitationObrant et al 1989; CitationRoyal College of Physicians 1989). Therefore, osteoporosis in men is a major health issue and so insights into its pathogenesis as well as strategies to prevent and treat it are of importance. Despite this, male osteoporosis is both under diagnosed and under treated. In a retrospective case-control study in the USA of 1,171 men with osteoporotic fractures, Feldstein et al found that only 7.1% received medication for osteoporosis and 1.1% had bone mineral measurement (CitationFeldstein et al 2005). CitationKiebzak et al (2002) found similar results in men sustaining low trauma hip fractures, although the rate of treatment did rise to 27% between 1 and 5 years later.
Low BMD is an established risk factor for osteoporotic fractures. The attainment of peak BMD and subsequent maintenance is influenced by genetic, endocrine and environmental factors. The peak bone mass is attained in the second decade of life and is followed by a period of consolidation lasting 5 years, such that peak BMD is achieved in the early to mid twenties and maintained until around the age of 40 years. Men have larger bones and hence a 10%–12% greater peak mass than women. After the age of 35–40 years there is a gradual loss of BMD. Women have a rapid phase of bone loss following the menopause. Men do not experience menopause, but they too undergo age-related bone loss. Therefore, advancing age is one of the most important risk factors for osteoporotic (low trauma) fractures.
Epidemiology of male osteoporosis
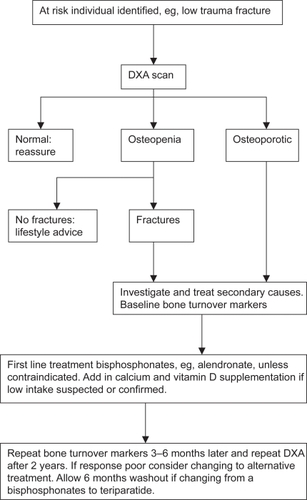
The major osteoporotic fractures are those of the vertebral body, hip and forearm, but fractures of the humerus, tibia, pelvis and ribs are also common. The incidence of most of these fractures rises steeply with age in both sexes (CitationJohansen et al 1997), but the increase occurs earlier in women than men, such that the fracture rate in elderly women is twice that of men of the same age ().
Figure 1 The incidence of forearm, symptomatic vertebral and hip fractures in men and women from Cardiff (CitationJohansen et al 1997).
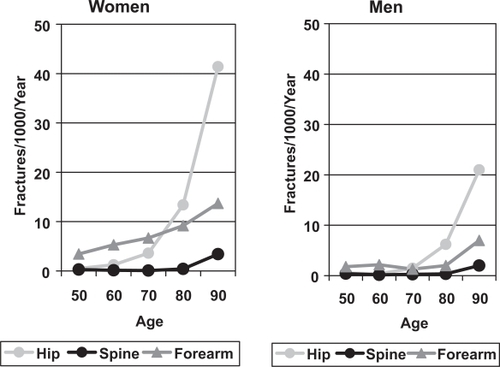
It has been estimated that as few as 1 in 4 vertebral fractures are clinically recognised (CitationEnsrud et al 1999). Some are asymptomatic, but it can also be difficult to distinguish a vertebral fracture from other causes of back pain and vertebral damage including: trauma, degenerative changes, Scheuermann’s disease, congenital anomalies, neoplasia, infection and Paget’s disease. Furthermore, there are a variety of terms and classification systems used with the expressions vertebral fracture, osteoporotic collapse and vertebral deformity often used interchangeably. There is also substantial geographical variation in the prevalence of vertebral fracture in men across Europe, with the highest rates in Scandinavian countries. The European Prospective Osteoporosis Study showed an increased incidence of morphometric vertebral fractures with age in both sexes (), but the rates were higher in women than men (CitationRoy et al 2003). The only significant determinant of vertebral fracture incidence in men was body mass index (BMI), with reduced risk in those with a high BMI.
Figure 2 The incidence of morphometric vertebral fractures in men and women in the European Prospective Osteoporosis Study (CitationRoy et al 2003).
Hip fractures are the most serious consequence of osteoporosis as they have the most severe impact in terms of morbidity and mortality and result in huge costs for health and social services. In the USA, hip fractures cost $15 billion in 1998 and results in 60,000 admissions to nursing homes annually (CitationSeeman 2001). The incidence of hip fractures increases exponentially with age in both sexes in all geographical areas and ethnic groups (CitationJohansen et al 1997; ). There is a greater difference in hip fracture incidence between ethnic groups and countries than between sexes, highlighting the potential importance of environmental, genetic and lifestyle factors in the etiology of hip fractures. The highest incidence of hip fracture is in Scandinavia, with the lowest rates in Mediterranean countries (CitationPande and Francis 2001).
Low trauma distal forearm fracture is widely regarded as a typical early manifestation of post-menopausal osteoporosis in women (CitationEastell 1996; CitationCuddihy et al 1999). Indeed, 50% of women who suffer a distal forearm fracture of Colles’ type will have osteoporosis (CitationEarnshaw et al 1998). Traditionally, this has not been thought to be the case for men. This is partly because the incidence of these fractures is much lower in men than in women at 9 per 10000 person years as opposed to 36.8 per 10000 person years (CitationO’Neill et al 2001) and does not increase with age in the same way. It has been suggested (CitationEastell 1996) that this is because men have a higher peak bone mass at this site than women and have no decrease in distal forearm BMD with age. In normal men CitationButz et al (1994) found a rate of trabecular bone loss of 0.59%/year at the forearm and a similar rate of loss was found by CitationBerntsen et al 2001. The incidence of forearm fracture does rise in the very old (CitationO’Neill et al 2001). Furthermore, low femoral neck bone density has been demonstrated to be a major risk factor for forearm and wrist fractures in men, along with height loss, dietary calcium and history of falls (CitationNguyen et al 2001). CitationCuddihy et al 1999 have shown that men have a 2.7 fold and a 10.7 fold increase in hip and vertebral fractures respectively following a distal forearm fracture. Finally, in a case-control study, 41.8% of men sustaining distal forearm fractures were found to be osteoporotic in at least one site (CitationTuck et al 2002); this is comparable to that seen in women with distal forearm fractures (CitationEarnshaw et al 1998).
Morbidity and mortality
There is considerable disability after hip fracture in men, with only 21% living independently in the community a year later, whereas 26% receive home care and 53% live in an institution (CitationPoor et al 1995). Although not all vertebral fractures come to medical attention, symptomatic fractures typically cause acute episodes of back pain, which usually settle after 6–8 weeks. Men with symptomatic vertebral fractures commonly complain of back pain, loss of height and kyphosis, but also have significantly less energy, poorer sleep, more emotional problems and impaired mobility than age-matched control subjects (CitationScane et al 1999).
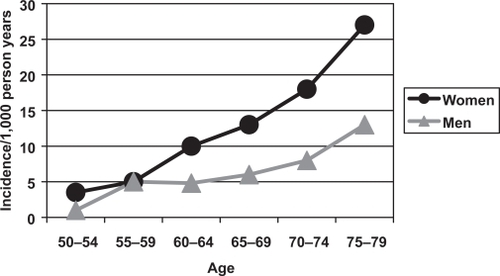
There is an increased mortality after all major fracture in men and women, with much of the excess mortality occurring in the first year. This excess mortality is higher in men than it is in women (). The standardized mortality ratio after a hip fracture is 3.17 in men and 2.18 in women and for vertebral fractures this is 2.38 and 1.66 respectively, but the reason for the higher mortality in men remains uncertain (CitationCenter et al 1999). One possibility is that this may be due to a higher prevalence of co-existing conditions, which are associated with an increased risk of fracture and mortality.
Figure 3 The Age-Standardised Mortality Ratio after fractures in men and women (CitationCenter et al 1999).
Pathogenesis of low trauma fractures in men
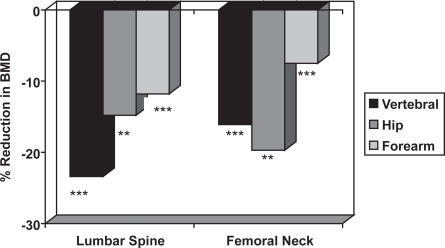
The risk of fracture is determined by skeletal and non-skeletal risk factors. The skeletal risk factors include BMD, bone turnover, trabecular architecture, bone size, and skeletal geometry, whereas non-skeletal risk factors include postural instability and propensity for falling. There is an inverse relationship between BMD and the incidence of vertebral and hip fractures in men (), which is similar to that observed in women (CitationDe La et al 1997; CitationVan der Klift et al 2002). Case-control studies show that men with distal forearm, symptomatic vertebral and hip fractures have lower BMD than age-matched control subjects (CitationScane et al 1999; CitationPande et al 2000; CitationTuck et al 2002) (); therefore, lower BMD is associated with an increased risk of fracture.
Figure 4 The relationship between femoral neck BMD and the incidence of hip fractures in 80 year old men and women in the Rotterdam Study (CitationDe Laet et al 1997).
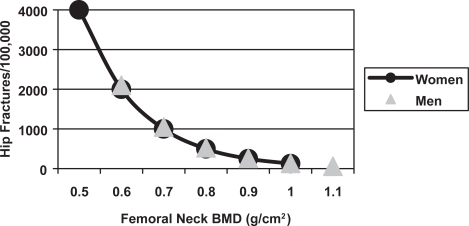
Figure 5 The mean reduction in lumbar spine and femoral neck BMD in men with distal forearm (CitationTuck and Raj 2002), symptomatic vertebral (CitationScane et al 1999) and hip (CitationPande et al 2000) fractures compared with age-matched male control subjects. The statistical significance is indicated (** = p < 0.01, *** = p < 0.001).
Non-skeletal risk factors (falls)
A number of studies, mainly in women, show that the risk of fracture is determined not only by BMD and other skeletal factors, but also by non-skeletal factors associated with physical frailty and an increased risk of falls (CitationCummings et al 1995; CitationDargent-Molina et al 1996). A prospective study from Australia showed that the combination of low BMD and high body sway conferred a greater risk of fracture than either one alone (CitationNguyen et al 1993). In the same study, there was also an increased risk of fracture with quadriceps weakness, falls in past year, previous fractures, low body weight and short stature. In men and women the risk of hip fractures is also increased by conditions predisposing to falls, such as strokes, Parkinsonism, dementia, vertigo, alcoholism and visual impairment (CitationGrisso et al 1991; CitationPoor et al 1995).
Secondary causes
Osteoporosis may be either primary (idiopathic) or secondary to one of a number of identifiable causes. In either case the end result is a low BMD and a propensity for low trauma fractures. The development of osteoporosis may be accelerated by underlying secondary causes of bone loss such as hypogonadism and steroids, which are found in over 50% of men presenting with symptomatic vertebral crush fractures (CitationBaillie et al 1992). A case-control study from the Mayo Clinic investigated 105 men with vertebral fractures and 105 age-matched control subjects with Paget’s disease of bone. This showed a significantly increased relative risk of vertebral fractures with smoking, alcohol consumption and underlying secondary causes of osteoporosis, whilst the risk was reduced in the presence of obesity (CitationSeeman et al 1983). A subsequent case-control study from Newcastle has demonstrated an increased risk of vertebral fractures with oral steroid therapy, anticonvulsant treatment, smoking, alcohol intake >20 units/week, physical inactivity and low free androgen index (CitationScane et al 1999). Case-control studies of hip fractures in men have also shown an increased risk of fracture with disorders associated with secondary osteoporosis (CitationStanley et al 1991). The major secondary causes of osteoporosis in men are given in .
Table 1 Causes of secondary oeteoporosis in men
Diagnosis of male osteoporosis
The use of bone density measurement
Until the development of DXA the diagnosis of osteoporosis in men was based on the history of fractures after minimal trauma. The introduction of DXA bone density measurement allowed a more objective diagnosis of osteoporosis and stimulated interest in making the diagnosis before fractures occur. The World Health Organization (WHO) has defined osteoporosis as a BMD 2.5 standard deviations or more below the mean value for young adults (T score < −2.5), but this has only been established for women.
Although the reference ranges for BMD measurements in men are derived from a smaller sample size than in women, there is a similar inverse relationship between absolute BMD and the incidence of vertebral and hip fractures in both sexes (CitationDe Laet et al 1997; CitationVan der Klift et al 2002). This indicates that the same threshold value of absolute BMD could be used for the diagnosis of osteoporosis in men and women. A T score of −2.5 in women would therefore be equivalent to a T score of −2.8 in men, calculated using gender specific normative data. The prevalence of osteoporosis in men using this diagnostic threshold is too low, whereas the prevalence of T score <−2.5 at the hip, spine or forearm in men over the age of 50 years is broadly comparable to the lifetime risk of fractures at these sites (CitationMelton et al 1998). This suggests that the WHO criteria may be applicable for the diagnosis of osteoporosis in men and women. More recently, CitationDe Laet et al (2002) using mathematical models and data from a large prospective study in Rotterdam, concluded that using male specific T score of −2.5 best fitted the available data. The International Society for Clinical Densitometry Position Development (ISCD) Panel and Scientific Advisory Committee also came to this conclusion in 2002 and is contained in their official positions statement 2005 (CitationBinkley et al 2002). The ISCD’s official positions have been endorsed by the American Society for Bone and Mineral Research (ASBMR) and the International Osteoporosis Foundation.
Although only about 50% of men with apparently low trauma vertebral fractures have densitometric evidence of osteoporosis at the lumbar spine or femoral neck, a further 40% have osteopenia (CitationScane et al 1999). It has therefore been suggested that treatment for osteoporosis should be considered in men with low trauma vertebral or hip fractures and evidence of osteoporosis or osteopenia at the lumbar spine or femoral neck, whereas the possibility of unrecognized antecedent trauma should be explored in those with normal bone density measurements (CitationTuck and Francis 2006).
The fact that only 50% of men are osteoporotic according to BMD measurements by DXA may be partly due to the uncertainties of threshold values for osteoporosis in men. It may also reflect that other aspects of size, structure and geometry may be important in determining fracture risk.
Investigation of osteoporosis in men
Secondary causes of osteoporosis should be sought by careful history, physical examination and appropriate investigation (). Serum testosterone should be measured in a morning sample, because of the diurnal variation in circulating concentration. A recent hip fracture may alter the hypothalamic–pituitary gonadal axis, as well as increasing the alkaline phosphatase, so investigations for secondary osteoporosis should be performed after the patient has recovered from the fracture and subsequent surgery.
Table 2 Investigations for secondary osteoporosis in men
Prostate specific antigen should also be measured in men with vertebral fractures and symptoms of prostatism or evidence of sclerosis on x-rays. In elderly men with osteoporosis, serum 25 hydroxyvitamin D (25OHD) and intact parathyroid hormone (PTH) measurements may be used to exclude vitamin D insufficiency and secondary hyperparathyroidism (CitationAloanzi et al 2006).
These investigations are usually normal in men with idiopathic osteoporosis, apart from a transient rise in serum alkaline phosphatase after fracture. The most frequently encountered causes of secondary osteoporosis in men are oral steroid therapy, hypogonadism, alcohol abuse, myeloma and skeletal metastases. In men with severe unexplained osteoporosis, it may be worthwhile considering 24-hour urine calcium estimation to identify hypercalciuria, 24-hour urine cortisol to exclude Cushing’s syndrome and anti-endomysial antibodies to look for coeliac disease. Although up to 50% of male osteoporotics may have no identifiable etiology, elderly males have much higher likelihood of having identifiable secondary cause than younger men. The younger male osteoporotics have been shown to have several and varied possible secondary causes (CitationVaranasi et al 1999).
Management of osteoporosis in men
The management of osteoporosis should include symptom relief, lifestyle measures to prevent bone loss and decrease the risk of falls and specific treatment to increase BMD and reduce the incidence of fractures. All patients should be offered analgesia of potency appropriate for the severity of their pain. Transcutaneous electrical nerve stimulation (TENS) is also of value in some patients with vertebral fractures. For persistent pain there is now the option of vertebroplasty or kyphoplasty, where this technique is available. Advice from a physiotherapist may help to maintain mobility and prevent falls, as may occupational therapy assessment. Advice and support is also available from self-help groups such as the National Osteoporosis Society (http://www.nos.org.uk).
Men with osteoporosis should be given advice on lifestyle measures to decrease bone loss, including a balanced diet rich in calcium, weight bearing exercise, cessation of smoking, moderation of alcohol intake and maintenance of regular exposure to sunlight in summer months. Where there is a history of recurrent falls, falls assessment and multifactorial intervention strategies may decrease the risk of falls. Hip protectors may potentially decrease the risk of hip fractures in frail elderly patients with recurrent falls, although compliance with their use is poor.
Treatment of osteoporosis in men
Any underlying secondary cause of osteoporosis should be treated if possible, as specific treatment of underlying conditions such as hyperthyroidism, hypogonadism and hyperparathyroidism may increase bone density by 10%–20%. There are a number of therapeutic options for idiopathic osteoporosis in men, including bisphosphonates, teriparatide, calcitonin and calcium and vitamin D supplementation. The best data are available for alendronate and teriparatide: summarizes the data for the available treatments.
Table 3 Summary of the available evidence for treatments of male osteoporosis (A = randomised controlled trials, B = other well designed studies, C = expert opinion/reports)
Established treatments
The established treatments for osteoporosis can be divided, based on underlying molecular physiology, into two broad biological categories: anabolic agents that directly stimulate bone formation and antiresoptive agents that inhibit osteoclast-mediated bone resorption. Both these agents increase BMD and reduce fracture risk. The extent of fracture risk of reduction with antiresoptives is rarely greater than 50% of the baseline risk (CitationRosen and Billzekian 2001). The antiresorptives, such as bisphosphonates, calcitonin, testosterone in men and estrogen for women, reduce remodeling and prolong mineralization duration. Anabolic agents, such as parathyroid hormone and strontium, directly stimulate bone formation.
Bisphosphonates
Bisphosphonates (BPs) are widely used as antiresorptive agents for the treatment of osteoporosis as well as other metabolic bone diseases, including Paget’s disease, and tumor-associated bone disease. All BPs are synthetic pyrophosphates an analog, ie, oxygen in P-O-P has been replaced by a carbon, resulting in a P-C-P backbone structure. They have a high affinity for bone mineral and are resistant to chemical and enzymatic hydrolysis. Two additional chains (R1 and R2, respectively) have been modified to produce different pharmacological properties and potencies (). The antiresoptive action of bisphosphonates is thought to result from their ability to bind strongly to bone and suppress osteoclast-mediated bone resorption. At higher doses however most BPs can inhibit normal mineralization and therefore the relative potency of a particular BP is given as a ratio of its antiresoptive activity to its normal mineralization inhibitory action. The relative potency is a function of the chemical structure, especially of the R1 and R2 chains. The newer compounds have a higher relative potency, as a result of which their relative risk of inhibiting bone mineralization leading to osteomalacia is lower.
Table 4 Relative potencies of bisphosphonates
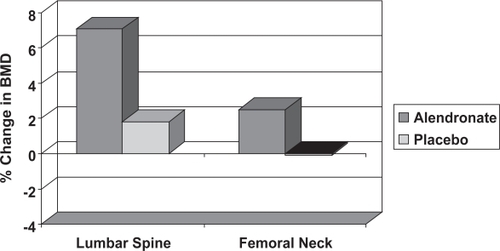
BPs have become the treatment of choice for most men with osteoporosis, following the publication of a large, randomized control trial of oral alendronate. This compared the effect of two years’ treatment with 10 mg daily alendronate (Fosamax) and placebo in 241 men with osteoporosis aged between 31 and 87 years, 36% of whom were hypogonadal (CitationOrwoll et al 2002). This showed significant improvement in lumbar spine and femoral neck BMD with alendronate (), with similar increases in BMD in eugonadal and hypogonadal men. There was also a significant reduction in vertebral fracture incidence and decrease in height loss with alendronate. Similar results were reported by two other RCTs: one in 134 men with primary osteoporosis and another with 77 men (CitationGonelli et al 2003). The daily preparation of alendronate has now been licensed in the UK for the treatment of osteoporosis in men. There is no reason to think that the 70 mg weekly preparation would not be equally effective and many patients find the weekly preparation more convenient.
Figure 6 The change in lumbar spine and femoral neck BMD in men with osteoporosis treated with alendronate or placebo (CitationKurland et al 2000). The statistical significance between the two groups is indicated (*** = p < 0.001).
Observational studies in men with idiopathic and secondary osteoporosis suggest that intermittent cyclical etidronate therapy (Didronel PMO) increases BMD at the lumbar spine by 5%–10%, with smaller increases at the hip. In an uncontrolled study in 42 men with vertebral fractures, cyclical etidronate increased spine BMD by 3% annually, whilst hip bone density showed a non-significant rise of 0.7% per year (CitationAnderson et al 1997). It would therefore appear that cyclical etidronate has comparable effects on bone density in men and women, although the effect on fracture incidence in men remains unclear.
Although there are no published studies of the effect of risedronate (Actonel) in men with idiopathic osteoporosis, there is no reason to suspect that it would be ineffective, particularly as it has been shown to be beneficial in men and women with glucocorticoid-induced osteoporosis. Furthermore, data from CitationRinge et al (2004) have demonstrated that there was a significant increase in BMD and a 60% reduction in new vertebral fractures after 1 year of treatment with risedronate in osteoporotic men, but this has only been published in abstract form. As risedronate has considerable evidence for increasing BMD and reducing fracture rates in women it is probably the second choice bisphosphonate after alendronate.
Further evidence that bisphosphonates are equally effective in men and women is provided by a three year RCT in 677 men and women with osteoporosis and at least one vertebral fracture. There were 84 men randomized to receive clodronate or placebo (CitationMcCloskey et al 1999). Interim analysis at one year showed a significant increase in lumbar spine and total hip BMD with clodronate compared with placebo, with similar changes in men and women. There was also an overall reduction in vertebral fracture incidence with clodronate.
Intravenous BPs are unlicensed for the treatment of osteoporosis, but are nevertheless widely used in patients unable to tolerate oral BPs. There have been few published studies of intravenous BPs and none solely in men or demonstrating antifracture efficacy. One abstract showed a significant increase in lumbar spine BMD in men 12 months after 30 mg of intravenous pamidronate given at monthly intervals (CitationTuck and Fordham 2001). Miller et al demonstrated that IV pamidronate (30 mg every three months) produced similar response rates to standard therapy with oral BPs (CitationMiller et al 2005). Adverse events were uncommon and included myalgias and flu-like symptoms. In a small, pilot study of 14 men with idiopathic osteoporosis intravenous ibandronate produced a significant 6.7% increase in lumbar spine BMD in combination with calcium and vitamin D supplementation (CitationLamy et al 2003). There were also significant falls in bone turnover markers (beta crosslinks and osteocalcin) of 30%–45%. Ibandronate is also available orally as monthly and 3 monthly preparations and increases BMD and reduces fractures in women, but there are no data available in men. Zoledronate is a potent bisphosphonate, which need only given as an infusion annually, but there is no data in men except for those with cancer. The less frequent dosing offered by ibandronate and zoludronate is likely to increase compliance, but anti-fracture evidence is required.
Oral BPs have to be taken during fasting and food must has be avoided for at least thirty minutes after alendronate or risedronate. This is necessary as the gastrointestinal absorption of orally administered BPs is poor, and the absorption can be almost totally abolished by simultaneous ingestion with food, divalent cations and certain medication. Therefore, many of the elderly patients who may also be taking iron or calcium supplements should avoid taking BPs at same time as any of these medications.
Oral BPs also need to be taken either sat upright or standing and are contraindicated in patients with impaired esophageal emptying. This contraindication is to avoid esophageal erosion, which is one of the most serious side effects of oral BPs. This is particularly so for the amino-bisphosphonates and these should be avoided in patients with peptic ulcers and reflux esophagitis. Parental administration can cause acute hypocalcemia, but this is rarely seen in oral therapy. However, alendronate and risderonate may cause mild hypocalcemia and hypophosphatemia, and increase PTH and worsen hyperparathyroidism. There have been reports of non-specific renal damage by BPs, but that is only seen with high doses. BPs should therefore be used cautiously in patients with renal insufficiency.
Teriparatide
Teriparatide is a recombinant fragment of human parathyroid hormone (PTH) composed of 1–34 amino acids and has anabolic properties, which make it useful for treating osteoporosis in men. PTH stimulates both bone formation and resorption, leading to increased or decreased BMD, depending on the mode of administration. Continuous infusion causes persistent elevation of PTH and results in greater resorption than formation, leading to bone loss. In contrast, daily injections of PTH lead to only transient peaks in serum PTH, resulting in greater bone formation and an increase in BMD.
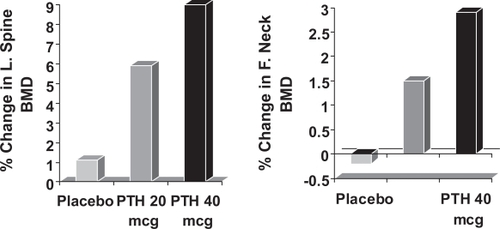
In postmenopausal women with prior vertebral fractures, teriparatide increases BMD and reduces both vertebral and non-vertebral fractures (CitationNeer et al 2001). In a small study of subcutaneous rhPTH (1–34) 400 IU daily in 23 men aged 30–68 years, BMD increased by 13.5% in the lumbar spine and by 2.9% at the femoral neck over 18 months (CitationKurland et al 2000). Another study in 437 osteoporotic men, showed significant increases in lumbar spine and femoral neck after a median of 11 months’ treatment with subcutaneous teriparatide 20 and 40 μg daily (CitationOrwoll et al 2003: ). CitationKaufman et al 2005 continued to observe 355 of these men over a period of 30 months and found that BMD gradually fell after cessation of teriparatide, but remained significantly higher than that at baseline. The rate of new vertebral fracture was also significantly reduced by 51%. Given the fall in BMD after stopping teriparatide, the question remained as to whether BPs would increase the BMD if given in combination or stop the fall if given after teriparatide. Kaufman et al in their observational study found that those men given BPs after teriparatide maintained their BMD and tended towards further increases. CitationKurland et al (2004) reported an observational study of 21 men and also reported that there were significant increases in lumbar spine BMD if BPs were given after cessation of teriparatide. Recently, a trial was reported in which 83 men were randomized to receive alendronate or teriparatide alone or in combination. Those given teriparatide alone had significantly greater BMD than either of the other two groups (CitationFinkelstein et al 2003) It therefore appears that BPs should be given after teriparatide to maintain the BMD, but not used in combination as they attenuate the effects of teriparatide. Side effects of teriparatide include nausea, headache and transient mild hypercalcaemia, but these were reported less commonly with the 20 μg dose. Teriparatide has therefore been licensed for use at a recommended dose of 20 μg daily for an 18 month course of treatment.
Figure 7 The change in lumbar spine and femoral neck BMD on treatment with subcutaneous Teriparatide 20 μg and 40 μg daily or placebo injections in men with osteoporosis. The statistical significance of differences from the placebo group is indicated (CitationKurland et al 2000: * = p < 0.05, *** = p < 0.001).
In Europe, teriparatide is licensed for a treatment course of 18 months and in the USA for 24 months. Teriparatide is contraindicated in patients with Paget’s disease of bone and unexplained elevation of alkaline phosphatase, as they are considered to be at an increased baseline risk for osteosarcoma. It should not be used in patients with metabolic bone disease other than osteoporosis, such as hypercalcemia or metastatic bone metastases. The British Society for Rheumatology also recommends caution in ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis (DISH), lumbar canal stenosis, urolithiasis and gout.
Administration of 20 μg dose of teriparatide leads to transient hypercalcemia, which is seen approximately 2 hours after the dose and reached maximum between 4 to 6 hours. Neer et al observed mild hypercalcemia, defined as total calcium of >10.6 mg/dL, in 11% of women receiving 20 μg dose of teriparatide (CitationNeer et al 2001). Transient episodes of orthostatic postural hypotension are one of the infrequent adverse effects of teriparatide. This typically occurred in the first several doses and within 4 hours of the dose being administered. The transient orthostatic hypotension resolved within a few minutes to a few hours and did not preclude use of the treatment. Other adverse side effects associated with teriparatide administration include nausea, headache, angina pectoris, constipation, depression, dizziness, insomnia, hypertension, and syncope. There is insufficient data to assess the safety of teriparatide in patients with compromised cardiac, hepatic or renal function.
Calcium and vitamin D
The role of calcium and vitamin D supplementation in the management of osteoporosis in men remains unclear. In an RCT in 86 normal men aged 30–87, supplementation with 1,000 mg calcium and 1,000 IU of vitamin D daily had no effect of bone loss from the forearm or spine (CitationOrwoll et al 1990). In contrast, an American RCT in 389 older men and women (mean age 70 years) living at home demonstrated that 700 IU vitamin D3 and 500 mg elemental calcium daily had a modest beneficial effect on bone density and decreased the incidence of non-vertebral fractures (CitationDawson-Hughes et al 1997). Sub-group analysis of the results for the men in this study showed a significant improvement in BMD with calcium and vitamin D, but no reduction in fractures was demonstrated. A recent study of oral vitamin D3 100,000 IU every 4 months in 2,037 men and 649 women, aged between 65 and 85 years living in the community, showed an overall 22% reduction in fracture risk (CitationTrivedi et al 2003). There was no significant reduction in fractures at any specific site or in either gender alone. In the absence of more conclusive studies, it seems reasonable to recommend calcium and vitamin D in frail elderly men, who are likely to have vitamin D deficiency and secondary hyperparathyroidism. Calcium and vitamin D may also be used as an adjunct to other treatments in men with established osteoporosis. It is important to ensure adequate calcium and vitamin D intake before commencing antiresorptive or anabolic treatment for osteoporosis, this is essential to derive optimum benefit and to prevent and mitigate some of the possible adverse side effects of the therapies.
Calcitonin
Calcitonin, a 32 amino acid peptide, is the most powerful physiological inhibitor of the osteoclast activity. It also inhibits tubular reabsorption of calcium and phosphate, leading to increased rates of their loss in urine. These functions in regulating calcium and phosphate metabolism make it a potentially useful treatment for osteoporosis. Intranasal salmon calcitonin formulations miacalcin and fortical were approved by the FDA in 1995 and in 2005 respectively and there is some data for its use in men.
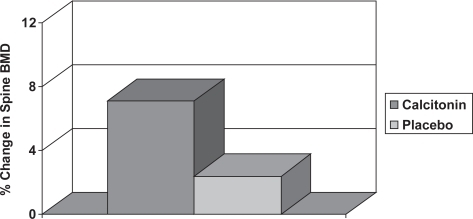
A small study in 28 men with osteoporosis showed that nasal calcitonin 200 units daily for 12 months increased lumbar spine BMD by 4.7% compared with control subjects (), but resulted in no significant change in BMD at the proximal femur (CitationTrovas et al 2002). A larger randomized control trial has subsequently been performed with 71 men suffering idiopathic osteoporosis, which demonstrated significant increases in both lumbar spine and femoral neck BMD of 3.5% and 3.2% respectively compared with controls (CitationToth et al 2005). There is, however, no anti-fracture evidence as yet in men. Calcitonin may also be useful in the management of the patient with acute vertebral fracture. An RCT in 32 men and 68 women with acute vertebral fracture showed that intranasal calcitonin 200 iu daily for 28 days was more effective than placebo at decreasing pain and improving mobility (CitationLyritis et al 1997). The common adverse drug reactions associated with injectable calcitonin include nausea and flushing, but these occur less frequently in patients treated with nasal spray.
Figure 8 The change in lumbar spine BMD in men with osteoporosis treated with calcitonin or placebo (CitationDe Laet et al 1997). The statistical significance between the two groups is indicated.
Alternative agents
Strontium
Strontium ranelate is now an established treatment for post-menopausal osteoporosis. It is a novel agent and a new class of drug having a dual action. It both stimulates bone formation and suppresses bone resorption. It increases BMD at the lumbar spine and hip by 14.4 and 8.3% respectively after three years, although at least half of this increase is the result of the incorporation of metal into bone rather than its effect on bone mineralization (CitationMeunier et al 2004). It also reduces rates of vertebral and non-vertebral fractures, as well as hip fracture in those at high risk (CitationMeunier et al 2004; CitationReginster et al 2005). However, there is no data available for men. Nevertheless, there is no reason to think that it would not work in men and may therefore be worth consideration when BPs fail.
Strontium ranelate is associated with mild adverse effects, such as transient nausea, diarrhea and creatine kinase elevations. There is an increased incidence of deep vein thrombosis and pulmonary embolism associated with strontium administration and it should so be used with caution in patients at increased risk of venous thromboembolism.
Androgens
In addition to improving bone density in men with hypogonadal osteoporosis (CitationBehre et al 1999), testosterone may increase spine bone density in eugonadal men with vertebral fractures and there have been a number of small studies. An uncontrolled study of testosterone treatment in 21 eugonadal men with vertebral osteoporosis showed a significant increase in spine bone density of 5% in six months, but no change in hip bone density was seen (CitationAnderson et al 1997). During treatment there was a 48% increase in serum testosterone, and a 22% reduction in SHBG, leading to an 88% increase in free androgen index. Serum estradiol also increased by 41%. The biochemical markers of bone turnover showed a reduction in bone formation and resorption. Analysis of the changes in bone density and sex steroid concentrations showed a closer relationship between the changes in BMD and serum estradiol than with serum testosterone.
Another small, randomized control trial in 86 osteopoprotic men examined the effects of oral dehydroepiandrosterone (DHEAS) over a 6 month period. As expected there were significantly higher concentrations of DHEAS and IGF-1 (insulin-like growth factor one) in the treatment group. However, DHEAS had only modest effects on BMD of between 2.32 and 3.1%. There were no significant changes in free testosterone, estradiol or PSA (CitationSun et al 2002). A randomized controlled crossover study in 15 men on long-term glucocorticoid treatment showed an increase in spine bone density of 5% after 12 months treatment with testosterone, whilst no change was observed during the control period of 12 months observation (CitationReid et al 1996). Side effect and cardiovascular risk factor profiles were acceptable in these small studies, but androgen treatment needs to be more fully explored in a multicentre randomized controlled trial. Until the results of such studies are available this treatment should also be regarded as experimental.
Calcitriol
Calcitriol, also known as 1,25 dihydroxyvitamin D, is the hormonally active metabolite of vitamin D. Calcitriol promotes calcium absorption from the bowel and may stimulate osteoblastic new bone formation. In a small RCT in 41 men with idiopathic osteoporosis, there was no difference in the change in spine or femoral neck BMD between those treated with calcitriol and the control group taking calcium supplements (CitationEbeling et al 2001).
Fluoride salts
Fluoride salt therapy, mainly as sodium fluoride, has been used for over thirty years to treat osteoporosis. Fluoride is believed to reduce risk of fracture by increasing bone mass and by reducing bone loss. Although fluoride has an ability to increase BMD at lumbar spine, it does not result in a reduction of vertebral fractures. In increasing the dose of fluoride, one increases the risk of non-vertebral fracture and gastrointestinal side effects without any effect on the vertebral fracture rate. (CitationHaguenauer et al 2000) Some clinical trials of fluoride have included men, but it is difficult to ascertain whether responses were in any way gender-specific. A German RCT shows that low dose intermittent monofluorophosphate and calcium increases bone density and decreases the risk of vertebral fractures in men with osteoporosis (CitationRinge et al 1998). On current evidence fluoride salts cannot be recommended for the treatment of osteoporosis.
Emerging novel therapies
Recent advances in the understanding of the functioning and regulation of osteoblast and osteoclast activity has led to the development of a number of novel therapies for the treatment of osteoporosis.
RANKL modulator
Receptor activator of nuclear factor NF-κβ ligand (RANKL) is essential for osteoclast differentiation and activity. It acts by binding to RANK expressed on osteoclast precursors stimulating their differentiation into mature osteoclasts. The human monoclonal antibody denosumab, previously known as AMG 162, specifically binds and inhibits RANKL activity. Denosumab mimics osteoprotegerin, which is a soluble RANKL decoy receptor that binds RANKL. Osteoprotegrin is the key endogenous regulator of the RANKL–RANK pathway. Preliminary evaluation, over a period of 12 months in 412 postmenopausal women with low bone mineral density, suggests that denosumab might be an effective treatment for osteoporosis (CitationMcLung et al 2006). However, further studies are needed to determine its efficacy as well as any potential side effects (CitationSchwartzmann et al 2006).
Intact human recombinant PTH (1–84)
Intact human recombinant PTH (1–84) is also under evaluation as an anabolic therapy for the treatment of osteoporosis. The anabolic effect on BMD and fracture reduction, as well as adverse effects of the full-length PTH and of the truncated 1–34 N-terminal form (teriparatide) appears to be comparable (CitationHodsman et al 2003). Further studies are needed to confirm this observation and to assess the relative efficacy of the two formulations in both women and men.
SERMs
SERMs (Selective Estrogen Receptor Modulators) are non-hormonal agents that modulate the estrogen receptors in some specific tissues. SERMs can have agonist or anatagonist action, ie, mimicking or inhibiting estrogen respectively. This will vary from tissue to tissue and the design of the agent. For example, raloxifene inhibits estrogen receptors in breast and reduce the risk of breast cancer, but stimulate estrogen receptors in bone improving BMD. Raloxifene has been approved for the prevention and treatment of postmenopausal osteoporosis (CitationEttinger et al 1999). Pathophysiological considerations suggest that it may also be effective in particular cases of male osteoporosis. However, large scale clinical trials are required to assess their use in men.
Anabolic steroids
Although agents such as nandrolone decanoate increase bone density transiently in men with osteoporosis, the benefit may be lost in the longer term, possibly because of suppression of the pituitary-gonadal axis, with consequent reduction in endogenous sex hormone production (CitationHamdy et al 1998). Such treatment may also lead to abnormalities in liver function. Anabolic steroids should therefore be regarded as an experimental treatment.
Other developmental therapies
There are a number of other compounds in the development stage that have either been designed or isolated. These modulate specific molecules that play critical roles in bone remodeling; the targets include c-src, cathepsin K and α2 β3 integrin receptor. The efficacy of statins, thiazide diuretics, nitric oxide donors and isoflavones is still being debated and has yet to be established.
Monitoring of treatment
Once a commitment to treat a patient has been undertaken it is important to assess response to treatment. Treatment failure is said to have taken place when there are further fragility fractures despite adherence to treatment for one year and/or BMD declines below pre-treatment baseline (NICE document 87). Approximately 10%–15% of patients fail to respond to treatment (CitationNOS 1998). Therefore, at least one repeat DXA scan is usually recommended to confirm treatment response. Unfortunately, this has to be done after a minimum of two years of treatment, as it takes this long for response to anti-resorptive agents to exceed the least significant change in BMD. Over a period of 2–3 years the BMD can be expected to increase approximately 5%–7% at the lumbar spine with bisphosphonate therapy. Taking into account this change, the precision of 1%–2% for a typical DXA scanner (CitationBlake and Fogelman 2005) and the coefficient of variation means that the least significant change that can be detected is somewhere between 4.4% and 6.9% depending on the site (CitationCummings et al 2000). Therefore, it will take at least 2 years for a sufficiently large change to have taken place to be sure that the result is a true representation of treatment response. Furthermore, the BMD may fall in the first year of treatment only to subsequently gain in the second year: a phenomenon known as regression to the mean (CitationEastell and Bainbridge 2001).
The use of BMD has the disadvantages of taking two years before a lack of response will be noted and also the spine can be affected by degenerative changes, especially in patients over 65. An alternative is to use bone turnover markers, which have a maximum suppression in the order of 50% within three months of starting therapy (CitationEastell and Bainbridge 2001). This would allow earlier identification of non-responders, but they can be difficult to collect, tend to be very variable and are influenced by many factors. For example a recent fracture will cause them to increase and they vary with meals and the time of day. It is therefore important to collect them at the same time of day. Nevertheless, they have been shown to predict further fracture and BMD response. The development of more reliable serum markers has made their use easier. They are increasingly being used to assess early response to treatment and compliance by measuring at baseline and 3–6 months after initiation of treatment.
Conclusion
Osteoporotic fractures are a major public health problem in men and further work is required to clarify the pathogenesis of the condition. Despite the plethora of new and effective treatments for women there is very little evidence for their use in men. Studies are urgently required to address this issue. A possible treatment strategy using the currently available evidence is shown: . The at risk individual, once identified, should have a baseline DXA and investigations for secondary causes undertaken including bone turnover markers. Underlying secondary causes of osteoporosis should be treated where possible, whereas BPs are probably the treatment of choice in other men with osteoporosis. If these are contra-indicated or ineffective, teriparatide or alternatives such as strontium should be considered. Calcium and vitamin D supplements may be useful in frail, elderly men with osteoporosis, who are likely to have vitamin D deficiency and secondary hyperparathyroidism. In the case of BPs, repeating the bone turnover markers 3 to 6 months later could then be used to assess response to therapy and compliance. A DXA scan should be repeated 2 years later. It is hoped that further research will see more treatments licensed for use in men and new agents become available.
References
- Al-oanziZTuckSPRajN2006Assessment of Vitamin D Status in Male OsteoporosisClin Chem522485416339300
- AndersonFHFrancisRMBishopJC1997Effect of intermittent cyclical disodium etidronate therapy on bone mineral density in men with vertebral fracturesAge Ageing26359659351480
- AndersonFHFrancisRMPeastonRT1997Androgen supplementation in eugonadal men with osteoporosis – effects of six months’ treatment on markers of bone formation and resorptionJ Bone Miner Res12472789076591
- BaillieSPDavisonCEJohnsonFJ1992Pathogenesis of vertebral crush fractures in menAge and Ageing21139411575093
- BehreHMvon EckardsteinSKlieschS1999Long-term substitution therapy of hypogonadal men with trans-scrotal testosterone over 7–10 yearsClin Endocrinol5062935
- BinkleyNCSchmeerPWasnichRD2002What are the criteria by which densitometric diagnosis of osteoporosis can be made in males and non-caucasians?J Clin Densitomet5Suppl51927
- CuddihyMTGabrielSECrowsonCS1999Forearm fractures as predictors of subsequent osteoporotic fractureOsteoporosis Int946975
- EarnshawSACauteSAWorleyA1998Colles’ fracture of the wrist as an indicator of underlying osteoporosis in post-menopausal women. A prospective study of bone mineral density and bone turnover rateOsteoporosis Int85360
- BerntsenGKFonneboVSogaardAJ2001Forearm bone mineral density by age in 7,620 men and women: the Tromso study, a population based studyAm J Epidem5346573
- BlakeGFogelmanIThe radiological diagnosis of osteoporosis2005ArdenNOsteoporosis Illustrated2nd edLondonRemedica89116
- BoyceWJVesseyMP1985Rising incidence of fracture of the proximal femurLancetI842115012857223
- ButzSWusterCScheidt-NaveC1994Forearm bone mineral density as measured by peripheral quantitative computed tomography (pQCT) in a German reference populationOsteoporosis Int417984
- CenterJRNguyenTVSchniederD1999Mortality after all major types of osteoporotic fracture in men and women: an observational studyLancet3538788210093980
- CummingsSRNevittMCBrownerWS1995For The Study of Osteoporotic Fractures Research Group. Risk factors for hip fracture in white womenN Eng J Med33276773
- CummingsSRPalermoLBrownerW2000Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research GroupJAMA28313182110714731
- Dargent-MolinaPFavierFGrandjeanH1996Fall-related factors and risk of hip fracture: the EPIDOS prospective studyLancet34814598684153
- Dawson-HughesBHarrisSSKrallEA1997Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age and olderN Engl J Med33767069278463
- De LaetCEVan HoutBABurgerH1997Bone density and risk of hip fracture in men and women: cross-sectional analysisBMJ31522159253270
- De LaetCEVan der KliftMHofmanA2002Osteoporosis in men and women: a story about bone mineral density thresholds and hip fracture riskJ Bone Min Res1722316
- EastellR1996Forearm fractureBone18Suppl 3203575
- EastellRBainbridgePR2001Bone turnover markers for monitoring antiresorptive therapyOsteoporosis Rev915
- EastellRBoyleITCompstonJ1998Management of male osteoporosis: Report of the UK Consensus GroupQuart J Med917192
- EnsrudKENevittMCPalermoL1999What proportion of incident morphometric vertebral fractures are clinically diagnosed and vice versa?J Bone Min Res14S1S138
- EbelingPRWarkJDYeungS2001Effects of calcitriol or calcium on bone mineral density, bone turnover and fractures in men with primary osteoporosis: a two year randomised, double blind, double placebo studyJ Clin Endocrinol Metab86409810311549632
- EttingerBBlackDMMitlakBH1999Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) InvestigatorsJAMA2826374510517716
- FeldsteinACNicholsGOrwollE2005The near absence of osteoporosis treatment in older men with fracturesOsteoporosis Int1695362
- FinkelsteinJSHayesAHunzelmanJL2003The effects of parathyroid hormone, alendronate, or both in men with osteoporosisN Engl J Med34912162614500805
- GonnelliSCepollaroCMontagnaniA2003Alendronate treatment in men with primary osteoporosis: a three year longitudinal studyCalcif Tissue Int73133914565594
- GrissoJAKelseyJLStromBL1991Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study GroupN Engl J Med3241326312017229
- HamdyRCMooreSWWhalenKE1998Nandrolone decanoate for men with osteoporosisAm J Therapeutics58995
- HodsmanABHanleyDAEttingerMP2003Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosisJ Clin Endocrinol Metab8852122014602752
- HaguenauerDRobinsonVASheaBJ2000Fluoride for treating postmenopausal osteoporosis The Cochrane Database of Systematic Reviews, Issue 4. Art No: CD002825
- JohansenAEvansRJStoneMD1997Fracture incidence in England and Wales: A study based on the population of CardiffInjury28655609624346
- KaufmanJMOrwollEGoemaereS2005Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapyOsteoporosis Int1651016
- KiebzakGMBeinartGAPerserK2002Undertreatment of osteoporosis in men with hip fractureArch Intern Med16222172212390065
- KurlandESCosmanFMcMahonDJ2000Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markersJ Clin Endocrinol Metabol85306976
- KurlandESHellerSLDiamondB2004The importance of bispohosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone (1–34)]Osteoporosis Int159927
- LamyOSandiniLPacheI2003Intravenous ibandronate in men with osteoporosis: an open pilot study over 2 yearsJ Endocrinol Invest267283214669826
- LyritisGPPaspatiIKarachaliosT1997Pain relief from nasal salmon calcitonin in osteoporotic vertebral crush fractures. A double blind, placebo-controlled clinical studyActa Orthop Scand275Suppl11214
- McCloskeyESelbyPDaviesM1999Clodronate decreases vertebral fracture incidence in men and women with established osteoporosisCalcif Tissue Int64Suppl 1S82
- McLungMRLewieckiEMChenSB2006Denosumab in post-menopausal women with low bone mineral densityN Engl J Med3548213116495394
- MeltonLJAtkinsonEJO’ConnorMK1998Bone density and fracture risk in menJ Bone Min Res13191523
- MeunierPJRouxCSeemanE2004The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosisN Engl J Med3504596814749454
- MillerRGChretienKCMeoniLA2005Comparison of intravenous pamidronate to standard therapy for osteoporosis: use in patients unable to take oral bisphosphonatesJ Clin Rheumatol112716357689
- National Institute for Clinical Excellence (NICE) technology apparaisal document number 87: Osteoporosis – secondary prevention 2006
- National Osteoporosis Society (NOS)1998Fundamentals of bone densitometry – report of a working partyBathNational Osteoporosis Society
- NeerRMArnaudCDZanchettaJR2001Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Eng J Med344143441
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy2001Osteoporosis prevention, diagnosis, and therapyJAMA2857859511176917
- NguyenTVSambrookPKellyP1993Prediction of osteoporotic fractures by postural instability and bone densityBMJ3071111158251809
- NguyenTVCenterJRSambrookPN2001Risk factors for proximal humerus, forearm and wrist fractures in elderly men and women: the Dubbo Osteoporosis Epidemiology StudyAm J Epidemiol1535879511257067
- ObrantKJBengerUJohnellO1989Increasing age-adjusted risk of fragility fractures: a sign of increasing osteoporosis in successive generations?Calcif Tissue Int445767
- O’NeillTWCooperCFinnJD2001Incidence of distal forearm fractures in British men and womenOsteoporosis Int1255558
- OrwollESOviattSKMcClungMR1990The rate of bone mineral loss in normal men and the effects of calcium and cholecalciferol supplementationAnn Int Med11229342152844
- OrwollEEttingerMWeissS2000Alendronate treatment of osteoporosis in menN Engl J Med3436041010979796
- OrwollESScheeleWHPaulS2003The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosisJ Bone Miner Res1891712510800
- PandeIFrancisRM2001Osteoporosis in menBest Practice and Research in Clinical Rheumatology1541527
- PandeIO’NeillTWPritchardC2000Bone mineral density, hip axis length and risk of hip fractures in men: results from the Cornwall hip fracture studyOsteoporosis Int1186670
- ParsonsTJPrenticeASmithEA1996Bone mineral mass consolidation in young British adultsJ Bone Min Res1126474
- PoorGAtkinsonEJLewallenDG1995Age-related hip fracture in men: clinical spectrum and short-term outcomeOsteoporosis Int541926
- PoorGJacobsenSJMeltonLJ1994Mortality after hip fractureFacts and Research in Gerontology791109
- ReginsterJYSeemanEDe VernejoulMC2005Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) studyJ Clin Endocrinol Metabolism90281622
- ReidIRWattieDJEvansMC1996Testosterone therapy in glucocorticoid-treated menArch Int Med1561173778639011
- RingeJDDorstAKipshovenC1998Avoidance of vertebral fractures in men with idiopathic osteoporosis by a three year therapy with calcium and low-dose intermittent monofluorophosphateOsteoporosis Int84752
- RingeJDFaberHSalemM2004Risedronate therapy reduces the risk of new vertebral fractures in osteoporotic men within 1 yearASBMR, 26th Annual MeetingSeattle (Abs) M424
- RosenCJBilizekianJP2001Anabolic therapy for osteoporosisJ Clin Endocrinol Metab869576411238469
- RoyDKO’NeillTWFinnJD2003Determinants of incident vertebral fracture in men and women: results for the European Prospective Osteoporosis Study (EPOS)Osteoporosis Int141926
- Royal College of Physicians of London1989Fractured neck of femur: prevention and managementLondonRoyal College of Physicians of London
- ScaneACFrancisRMSutcliffeAM1999Case-control study of the pathogenesis and sequelae of symptomatic vertebral fractures in menOsteoporosis Int9917
- SchwartzmanJYazicicYRifkinWD2006Denosumab in post-menopausal women with low bone mineral densityN Engl J Med35423909116738280
- SeemanEMeltonLJIIIO’FallonWM1983Risk factors for spinal osteoporosis in menAm J Med75977836650552
- SeemanE2001Sexual dimorphism in skeletal size, density and strengthJ Clin Endocrinol Metab8645768411600506
- StanleyHLSchmittBPPosesRM1991Does hypogonadism contribute to the occurrence of a minimal trauma hip fracture in elderly men?J Am Geriat Soc39766712071807
- SunYMaoMSunL2002Treatment of osteoporosis in men using dehydroepiandrosterone sulfateChinese Medical Journal115402411940375
- TrivediDPDollRKhawKT2003Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trialBMJ32646912609940
- TrovasGPLyritisGPGalanosA2002A randomized trial of nasal spray calcitonin in men with idiopathic osteoporosis: effects on bone mineral density and bone markersJ Bone Miner Res175212711874243
- TothECsuporEMeszarosS2005The effect of intranasal salmon calcitonin therapy on bone mineral density in idiopathic male osteoporosis without vertebral fractures- an open label studyBone36475115664001
- TuckSPRajNSummersGD2002Is distal forearm fracture in men due to osteoporosis?Osteoporosis Int136306
- TuckSPFordhamJN2001Intravenous Pamidronate for treatment of osteoporosisRheumatology40Suppl 1 Abstracts 268
- TuckSPFrancisRM2005Male OsteoporosisArdenNOsteoporosis Illustrated2nd edLondonRemedica16384
- US Department of Health and Human Resources2004Bone health and osteoporosis. A report of the surgeon generalRockville, MDUSDHHS
- Van der KliftMDe LaetCEMcCloskeyEV2002The incidence of vertebral fractures in men and women: the Rotterdam StudyJ Bone Min Res1710516
- Van StaaTPDennisonEMLeufkensHGM2001Epidemiology of fractures in England and WalesBone295172211728921
- VaranasiSSFrancisRMBergerCEM1999Mitochondrial DNA deletion associated oxidative stress and male osteoporosisOsteoporosis Int101439