Abstract
Aging is a universal process to all life forms. The most current and widely accepted definition for aging in humans is that there is a progressive loss of function and energy production that is accompanied by decreasing fertility and increasing mortality with advancing age. The most obvious and commonly recognised consequence of aging and energy decline is a decrease in skeletal muscle function which affects every aspect of human life from the ability to play games, walk and run to chew, swallow and digest food. There is hence a recognised overall decline of an individuals’ fitness for the environment that they occupy. In Westernised countries this decline is gradual and the signs become mostly noticeable after the 5th decade of life and henceforth, where the individual slowly progresses to death over the next three to four decades. Given that the aging process is slow and gradual, it presents with opportunities and options that may ameliorate and improve the overall functional capacity of the organism. Small changes in function may be more amenable and likely to further slow down and possibly reverse some of the deleterious effects of aging, rather, than when the incremental changes are large. This overall effect may then translate into a significant compression of the deleterious aspects of human aging with a resultant increase in human life expectancy.
Keywords:
Introduction
Understanding the difference between life expectancy and lifespan is the framework upon which any selective review of anti-aging interventions must originate from. Human life expectancy is determined from mortality data tables. Life expectancy is a statistical projection of the length a human being is expected to live based upon probabilities and assumptions of genetic predispositions, living conditions, medical discoveries and advances, natural disasters and other environmental factors. Morbidity statistics track presumed causes of death over time, producing trend data that can be factored into life expectancy tables (CitationMathers and Loncar 2005). Lifespan, however, is defined as the characteristic observed age of death for its very oldest individuals.
Throughout most of recorded human history, it has been recognized that poor socioeconomic and nutritional status, have been strongly associated with decreased life expectancy, a trend that is also very much evident today (US Gov. Report, 2005). Current data estimates, show that on average human life expectancy in western countries is approximately 82 years for women and 80 years for men (US Gov. Report, 2005).
In this brief selective review we present scientific evidence as to what anti aging practices may better serve to increase mean life expectancy and hence the human lifespan.
Lifestyle
CitationWillet (2002) recently emphasized that genetic and environmental factors, including diet and lifestyle, both contribute to cardiovascular diseases, cancers, and other major causes of mortality and that numerous lines of evidence indicate that environmental factors are the most important in determining disease prevention. Hence, environmental factors may certainly have the strongest influence on life expectancy and hence lifespan () (CitationWillett 2002; CitationVitetta et al 2005). Intertwined with nutritional practices and lifestyle is life stressor modification (CitationVitetta et al 2005).
Figure 1 Preventable chronic diseases. Adapted and modified from CitationWillett 2002.
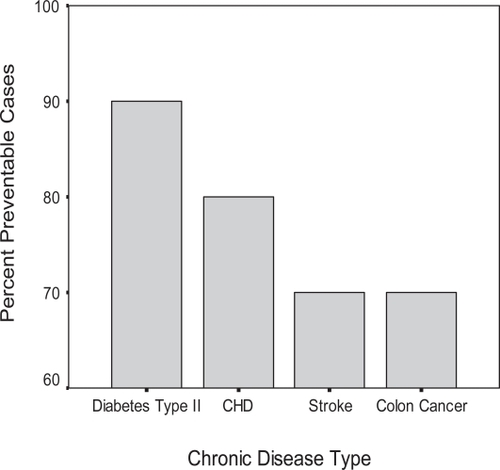
Evidence continues to accumulate that strongly suggests that the state of a human being’s mind – which associates psychosocial factors with emotional states such as depression and with behavioural dispositions that include hostility and psychosocial lifestyle stresses – can directly and significantly influence human physiologic function and, in turn, health outcomes.
Stressors and negative stress-related reactions have been documented and recognized to have multiple ill health related sequalae and exposure to chronic social stress has been associated with many systemic and mental disorders. These have significant deleterious outcomes that significantly decrease life expectancy (CitationAder 2003; CitationVitetta et al 2005).
Different research groups support the notion that health consequences are more likely to occur when unpredictable stressors of a social nature chronically induce physiological and behavioral adjustments that may create wear and tear on underlying physiological functions. When stressors challenge an organism’s integrity, a set of physiological reactions is elicited to counteract the possible threat and adjust the physiological setting of the organism to the new situation. This has become known as the stress response (CitationAder 2003). Stresors and lifestyle choices can be a significant trigger for disease through immunomodulation of the immune system enhancing disease susceptibility, which can then affect life expectancy (CitationAder 2003). Immune function modulation influenced and expressed by stressful life experiences has a significant correlate with CitationWillett’s (2002) environmental trigger view of disease initiation.
Nutrition
Triggers for adverse and inappropriate nutritional practices in childhood have been shown to have psychosocial correlates in adult life with an increase in risk for disease (CitationJackson 2005). Obesity in humans, influenced by poor dietary choices and inactivity, are significantly associated with an increased risk of chronic diseases such as diabetes, high blood pressure, high cholesterol, cardiovascular diseases, asthma, arthritis, some cancers and overall poor health status, that can significantly decrease an individual’s life expectancy.
CR is linked to nutritional choices. CR with nutritionally poor foods (eg, consuming half the quantity of a fast food) is still an unbalanced nutritional choice albeit in smaller portions and still provides no health advantage.
Recently it was reported and further confirmed, that a greater adherence to a traditional Mediterranean diet was associated with a significant reduction in total all cause mortality (CitationTrichopoulou et al 2003). CitationMeyer and colleagues (2006) have demonstrated that associating a CR diet with adherence to a Mediterranean type diet that consisted of whole grains, beans, fish, fruit, olive oil, and many different kinds of vegetables was beneficial for heart health. Hence when optimal nutritional choices are coupled to CR, an increase in life expectancy is made possible. Such nutritional practices can serve to further expand the human life span.
Caloric restriction (CR)
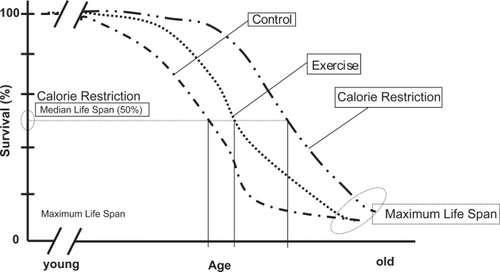
Optimizing nutrition relates food intake to the benefits of prudent CR. That is, CR refers to a dietary regimen low in calories without under nutrition that leads to increased disease risk due to nutritional deficits. Following the pioneering work of CitationMcCay and Maynard (1935) over 70 years ago, CR was then first noted to significantly extend the life span of rodents. Since that time, the increase in longevity has been demonstrated to result from the limitation of total calories derived from carbohydrates, fats, or proteins to a level 25%–60% below that of control animals that were fed ad libitum (CitationSohal and Weindruch 1996; CitationWeindruch and Sohal 1997; CitationWeindruch et al 2001). The extension in lifespan can approach 50% in rodents () (CitationMcCay et al 1935; CitationHolloszt 1997). Moreover, CR has been shown to extend the lifespan in a broad range of organisms that include, yeast, rotifers, spiders, worms, fish, mice, and rats (CitationWeindruch et al 2001). Emerging data show that its effect may also apply to non human primates (CitationLane et al 2001). Also CR has been reported to delay a wide spectrum of diseases in different experimental animals such as kidney disease (CitationLane et al 2001), a variety of neoplasias (CitationFernandes et al 1976; CitationFernandes and Good 1984; CitationKubo et al 1984), autoimmune disease (CitationSarkar et al 1982; CitationEngelman et al 1990) and diabetes (CitationShields et al 1991), and it reduces age associated neuronal loss in most mouse models of neurodegenerative disorders such as Parkinson’s Disease (CitationDuan and Mattson 1999) and Alzheimer’s Disease (CitationZhu et al 1999). The CR regimen also prevents age-associated declines in psychomotor and spatial memory tasks (CitationIngram et al 1997) and loss of dendritic spines necessary for learning (CitationMoroi-Fetters et al 1989) and improves the brain’s plasticity and ability for self repair (CitationMattson 2000).
Figure 2 Effect of CR and Exercise on Survival in rats. Adapted and modified from CitationHolloszy 1995.
Numerous biomarkers of CR have been identified in rodents, such as temperature, and DHEAS, insulin and glucose levels (CitationRoth et al 2002). CitationRoth and colleagues (2002) have recently observed that body temperature and insulin and DHEAS levels were also altered in primates that had been subjected to CR, hence validating the usefulness of these biomarkers in longer-lived species. More importantly, they have also shown that these parameters were altered in longer-lived men. Together these findings support the role these factors have as biomarkers of longevity in humans.
Recently, a trial on the effect of a 6 month CR diet on metabolic biomarkers such as energy expenditure, and oxidative stress in humans was completed (CitationHeilbronn et al 2006). The report showed that prolonged CR significantly reduced two biomarkers of longevity, namely, fasting insulin level and body temperature. This is the first human study to show that CR, in addition to significant reductions in well known biomarkers of aging, also caused a metabolic adaptation in CR individuals and a reduction in DNA fragmentation that reflected less DNA damage (CitationHeilbronn et al 2006).
Important genetic links and regulators of organism life span have been uncovered in the form of sirtuins (CitationSauve et al 2006) Sirtuins are a family of NAD+ – dependent protein deacetylases widely distributed in all phyla of life. In multicellular organisms, sirtuins deacetylate histones and transcription factors that regulate stress, metabolism, and survival pathways. Sir2 is the founding member of a large and diverse family of these protein-modifying sirtuin enzymes, which regulate key pathways throughout biology, in eubacteria, archaea, eukaryotes, and even viruses (CitationSauve et al 2006).
The link between CR and Sir2 and aging has been reviewed elsewhere (cf. for reviews: refer CitationSinclair and Guarente 1997; CitationGuarente and Picard 2005; CitationSuave et al 2006). We further point out that there is an extensive body of evidence that suggests that sirtuins are involved in promoting longevity, particularly longevity associated with CR regimens, in several organisms. The sirtuin – CR relationship remains complex however. Recently Suave and colleagues (CitationSauve et al 2006) hypothesized that mammalian SIRT1 may mediate significant changes in tissues and endocrine systems through the sensing of low CR diets and triggering physiological changes that benefit mammals with health and hence longevity.
Plant factors such as resveratrol have been shown to increase longevity in several organisms by regulating Sir2/SIRT1 (cf. for review: CitationBaur and Sinclair 2006). In mammals, there is growing evidence that resveratrol can prevent or delay the onset of cancer, heart disease, ischaemic and chemically induced injuries, diabetes, pathological inflammation and viral infection. These effects are observed despite extremely low bioavailability and rapid clearance of resveratrol from the circulation (CitationBaur and Sinclair 2006). Resveratrol has been suggested to be associated with the prevention of age-related diseases such as cancer because of its regulation of transcription factors that control tumuor cell survival (CitationGuarente and Kenyon 2000; CitationHowitz et al 2003; CitationNemoto et al 2005; CitationStorz 2005).
Mitochondria and free radicals
That mitochondria have a pivotal role in the effective provision of energy to eukaryotic cells is an undisputed scientific fact. Cellular mechanisms regulating energy utilization must function properly to sustain life. Adenosine 5′–triphosphate (ATP) is the ubiquitous energy storage molecule produced in several cellular processes. Aerobic energy metabolism requires a cellular investment, namely, molecular oxygen. Humans rely on mitochondria to synthesize and export ATP. The process is initiated by the transfer of electrons derived from food sources along a series of mitochondrial respiratory–chain carriers until they are consumed with the production of water from the utilization of oxygen. A single eukaryotic cell may contain between several hundred to thousands of these energy organelles. With increased aging, there is a decrease in mitochondrial energy output (CitationSingh 2006). Hence in aerobic animals, mitochondrial health for effective energy provision is central to life.
The free radical theory of aging, as formulated by Denham CitationHarman (1956), is supported by observations that the life span of most organisms is roughly proportional to their metabolic rate and thus due to the rate at which the organism generates mitochondria derived reactive oxygen species (ROS). This view however, is over simplistic and may require modification within the confines of aging. Cellular generated ROS contributing to the overall production of ROS are apparently traced back to the mitochondria (CitationLenaz et al 2002). ROS have been viewed as mostly deleterious to health and hence aging. Reports that show that in a wide spectrum of animal species, dietary antioxidants or caloric restriction, as well as chemical antioxidants or increased expression of antioxidant proteins, can lower mitochondria ROS production, which translates into an extension of the life span of these species which have served to support the free radical theory of aging (CitationOrr and Sohal 1994; CitationSohal and Weindruck 1996; CitationParkes et al 1998; CitationSun and Tower 1999; CitationTaub et al 1999; CitationSchon 2000; CitationFinkel and Holbrook 2000; CitationXu and Finkel 2002) However, it is known that ROS are generated in multiple cellular compartments and by multiple enzyme systems within the cell and have cellular signaling functions that are critical for the normal physiological function of the cell (CitationRhee et al 2003; CitationLinnane et al 2007a, Citation2007b, Citation2007c).
Important contributions to the production of ROS within cells, include proteins within the plasma membrane, such as the growing family of NADPH oxidases; lipid metabolism within the peroxisomes; protein synthesis within the endoplasmic reticulum; as well as the activity of various cytosolic enzymes such as the cyclo-oxygenases (CitationLinnane and Eastwood 2004; CitationMoldovan and Moldovan 2004; CitationBalaban et al 2005). The generation of mitochondrial ROS is a consequence of oxidative phosphorylation, a process that uses the controlled and regulated oxidation of NADH or FADH to generate a potential energy for protons across the mitochondrial inner membrane (ROS produced by mitochondria have been demonstrated to have important and specific roles in cellular signalling (CitationLinnane and Eastwood 2004). The notion that the mitochondria are the sole most abundant site of ROS formation is currently an area of much discussion and debate (CitationRhee et al 2003; CitationBalaban et al 2005; CitationLinnane et al 2007b).
The disruption of mitochondrial functions has been implicated in more than 40 known diseases, including atherosclerosis, ischemic heart disease, cancer, diabetes, and neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (cf. for reviews: CitationMcKenie 2004; CitationWallace 2005). Together these data indicate that mitochondrial health is an important factor for health and aging. Current and future research would aim to further improve and preserve mitochondrial function. Although further research is warranted, recent reports show that supplementation with coenzyme Q10 shows promise in maintaining the health of mitochondria (CitationKagan et al 1999; CitationCrestanello et al 2002; CitationMcKenzie et al 2004; CitationSomayagulu et al 2005; CitationLinnane et al 2007b).
Further, mimetics of specific cellular antioxidants such as the SOD/catalase mimetic EUK-134 can be used to assist in reducing oxygen radicals and are already in clinical trials (CitationMelov et al 2000).
Hormones
In humans there is a progressive decrease in hormone synthesis as well as a loss of hormone receptors with age. The elderly have demonstrated significantly lower levels of production of most hormones compared to young adults (CitationHertogue 2005). There is a voluminous body of scientific literature concerned with hormonal deficits with aging. That is, levels of growth hormone (GH) and insulin like growth factor-1 (IGF-1), melatonin (nocturnal), TSH, thyroid hormones (T3), calcitonin, DHEA (sulphated form in and its urinary 17-keto-metabolites), aldosterone, estrogens, testosterone in men and women have been demonstrated to progressively decrease with age in adults (for extensive review and individual references refer CitationHertogue 2005).
Hormonal deficits are not merely limited to menopause, andropause (male menopause), somatopause (adult growth hormone deficiency), and other sex hormone related diseases, they are now implicated in conditions such as obesity (CitationCranny et al 2006; CitationPasquali and Gambineri 2006), osteoporosis (CitationBennett 2005), fibromyalgia (CitationCleare 2004) chronic fatigue syndrome (CitationCleare 2004) cancer (CitationSimonenko et al 2006) attention deficit problems (CitationCheng 2005) and possibly others as yet that remain to be elucidated.
Conventional medicine treatments have only severed to address hormone depletion, ignoring the mild and moderate deficiencies; whereas the anti-aging medicine model focuses on mild, moderate, and severe deficiencies. Symptoms and severity of symptoms will be proportional to the level of deficiency for each hormone (CitationAnton et al 2005). Only recently has the science of endocrinology started focusing on advanced testing methods, taking into account reference ranges based on age as well as gender, free or bioavailable hormone levels versus bound hormone levels, and ratios between major antagonistic hormones to titrate patients and achieve an optimum balance. Titration and optimum balance have been used with thyroid and insulin hormones, and has not been universally applied to all other hormones, till recent times. Ongoing research in the field of longevity should include the use of nutrient and hormonal precursors to augment the body’s ability to make more of its own hormonal compounds thus limiting structural and DNA damage in the latter decades of life.
Concluding remarks
The purest use of the term “biological aging” confines it exclusively to molecular changes. CitationHayflick (2003) defines aging in biological systems as a stochastic process that occurs systemically after reproductive maturity in animals that reach a fixed size in adulthood and that it is then caused by the escalating loss of molecular fidelity that ultimately exceeds repair capacity. This then increases the vulnerability to pathology or age-associated diseases. Further, that there is a finitude associated with aging that eventually leads to death (CitationHayflick 2003). CitationGrimley Evans (2000) defined senescent changes as de novo structural and functional alterations that are not part of the developmental program. In this selective and brief review we have summarized the scientific evidence that may prevent disease and maintain the molecular integrity of the organism, thus expanding the mean life expectancy whilst operating within the framework of normal physiological function and repair. We find that whilst aging is not irreversible and inevitable, certain key factors may add significant benefits to human mean life expectancy.
There is a vast array of scientific literature that concludes that lifestyle modification factors that optimize nutrition, physical activity and mental health have significant correlates with reducing the risks of disease in later life that is translates into an increased mean life expectancy. Further, although the correction of all hormonal deficits by an adequate multiple hormone replacement therapy, is not currently possible, hormone therapy does constitute a viable solution to prevent and treat most hormonal deficits due to pathological aging. CitationHertogue (2005) recently reported that even though we are not presently able to abrogate senescence by hormone treatments because of the unavailability as medications of all hormones that decline with age, the future appears promising.
As a final note; with the onset of the 21st century, it is apparent that we stand on the horizon of a revolution in anti aging therapies and technologies. A promising therapeutic modality, the concept of stem cell treatments, will within the next decade, allow a markedly expanded quality of health and possibly also further increase the mean human life expectancy. Recent advances by major corporations, both private and public, have documented that we are already capable of taking stem cells from an individual and selectively copying these cells along with their DNA components. Thus making possible the restoration and rejuvenation properties that human bodies lose as they age. The prospects for use of human embryonic stem cells derivatives in regenerative medicine hence are significant (CitationTrouson 2006). If, the potential contributions to human regenerative medicine can be realized, significant alterations and enhancement of mean life expectancy in humans will be possible.
References
- AderR2003Conditioned immunomodulation: research needs and directionsBrain Behav ImmunSuppl 1S517 Review12615187
- AntonBVitettaLCortizoFSaliA2005Can we delay aging? The biology and science of agingAnn N Y Acad Sci10575253516399917
- BalabanRSNemotoSFinkelT2005Mitochondria, oxidants, and agingCell1204839515734681
- BaurJASinclairDA2006Therapeutic potential of resveratrol: the in vivo evidenceNat Rev Drug Discover5493506
- BennettR2005Growth hormone in musculoskeletal pain statesCurr Pain Headache Rep9331816157062
- ChengSY2005Isoform-dependent actions of thyroid hormone nuclear receptors: lessons from knockin mutant miceSteroids70450415862829
- CleareAJ2004The HPA axis and the genesis of chronic fatigue syndromeTrends Endocrinol Metab1555915036250
- CranneyAPapaioannouAZytarukN2006Parathyroid hormone for the treatment of osteoporosis: a systematic reviewCMAJ17552916818910
- CrestanelloJADolibaNMDolibaNM2002Effect of coenzyme Q10 supplementation on mitochondrial function after myocardial ischemia reperfusionJ Surg Res102221811796022
- DuanWMattsonMP1999Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson’s diseaseJ Neurosci Res5719520610398297
- EngelmanRWDayNKChenRF1990Calorie consumption level influences development of C3H/Ou breast adenocarcinoma with indifference to calorie sourceProc Soc Exp Biol Med19323302294519
- FernandesGYunisEJGoodRA1976Suppression of adenocarcinoma by the immunological consequences of calorie restrictionNature26350471085916
- FernandesGGoodRA1984Inhibition by restricted calorie diet of lymphoproliferative disease and renal damage in MRL/lpr miceProc Natl Acad Sci81614486592606
- FinkelTHolbrookNJ2000Oxidants, oxidative stress and the biology of agingNature4082394711089981
- Global Population Aging in the 21st Century and Its Economic Implications2005 URL: http://www.cbo.gov/ftpdocs/69xx/doc6952/12-12-Global.pdf
- Grimley EvansJ200021st Century: Review: ageing and medicineJ Intern Med2471596710692078
- GuarentelKenyonC2000Genetic pathways that regulate aging in model organismsNature4082556211089983
- GuarenteLPicardF2005Cell1204738215734680
- HarmanD1956Aging: a theory based on free radical and radiation chemistryJ Gerontol1129830013332224
- HayflickL2003Living forever and dying in the attemptExp Gerontol3812314114698802
- HeilbronnLKde JongeLFrisardMI2006Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trialJAMA29515394816595757
- HertogueT2005The “multiple hormone deficiency” theory of aging: is human senescence caused mainly by multiple hormone deficienciesAnn N Y Acad Sci105744865 Review16399912
- HollosztJO1997Mortality rate and longevity of food-restricted exercising male rats: a reevaluationJ Appl Physiol823994039049716
- HowitzKTBittermanKJCohenHY2003Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespanNature425191612939617
- IngramDKWeindruchRSpanglerEL1987Dietary restriction benefits learning and motor performance of aged miceJ Gerontol4278813794202
- JacksonAA2005Integrating the ideas of life course across cellular, individual, and population levels in cancer causationJ Nutr13512 Suppl2927S33S Review16317152
- JohnsonPRSternJSHorwitzBA1997Longevity in obese and lean male and female rats of the Zucker strain: Prevention of hyperphagiaAm J Clin Nutr668909039322565
- KaganTDavisCLinL1999Coenzyme Q10 can in some circumstances block apoptosis, and this effect is mediated through mitochondriaAnn NY Acad Sci887314710668462
- KuboCDayNKGoodRA1984Influence of early or late dietary restriction on life span and immunological parameters in MRL/Mp-lpr/lpr miceProc Natl Acad Sci81583156333026
- LaneMABlackAHandyA2001Caloric restriction in primatesAnn NY Acad Sci9282879511795520
- LenazGBovinaCD’AurelioM2002Role of mitochondria in oxidative stress and agingAnn N Y Acad Sci959199213 Review11976197
- LinnaneAWEastwoodH2004Cellular redox poise modulation; the role of coenzyme Q10, gene and metabolic regulationMitochondrion47798916120432
- LinnaneAWKiosMVitettaL2007aThe essential roles of superoxide radical and nitric oxide formation for healthy agingMitochondrion71517317335
- LinnaneAWKiosMVitettaL2007bHealthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems. The essential roles of superoxide anion and nitric oxideExperimental Gerontology844567
- LinnaneAWKiosMVitettaL2007cCoenzyme Q(10) – its role as a prooxidant in the formation of superoxide anion/hydrogen peroxide and the regulation of the metabolomeMitochondrionSuppl 1S5161
- MathersCDLoncarD2005Updated projections of global mortality and burden of disease, 2002–2030: data sources, methods and results World Health Organization –http://www.who.int/healthinfo/statistics/bodprojectionspaper.pdf
- MattsonMP2000Neuroprotective signaling and the aging brain: Take away my food and let me runBrain Res886475311119686
- McCayCMCromwellMFMaynardLA1935The effect of retarded growth upon the length of life span and upon the ultimate body sizeJ Nutr106379
- McKenzieMLiolitsaDHannaMG2004Mitochondrial disease: mutations and mechanismsNeurochem Res3589600 Review15038606
- MelovSRavenscroftJMalikS2000Extension of life-span with superoxide dismutase/catalase mimeticsScience2891567910968795
- MeyerTEKovacsSJEhsaniAA2006Long-Term Caloric Restriction Ameliorates the Decline in Diastolic Function in HumansJ Amer Coll Cardiol4739840216412867
- MoldovanLMoldovanNI2004Oxygen free radicals and redox biology of organellesHistochem Cell Biol12239541215452718
- Moroi-FettersSEMervisRFLondon1989Dietary restriction suppresses age-related changes in dendritic spinesNeurobiol Aging10317222682315
- NemotoSFergussonMMFinkelT2005SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1αJ Biol Chem280164566015716268
- OrrWCSohalRS1994Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogasterScience2631128308108730
- ParkesTLEliaAJDickinsonD1998Extension of Drosophila lifespan by overexpression of human SOD1 in motorneuronsNat Genet1917149620775
- PasqualiRGambineriA2006Metabolic effects of obesity on reproductionReprod Biomed Online125425116790096
- RheeCGChangTSBaeYS2003Cellular regulation by hydrogen peroxideJ Am Soc Nephrol148 Suppl 3S2115 Review12874433
- RothGSLaneMAIngramDK2002Biomarkers of caloric restriction may predict longevity in humansScience29781112161648
- SarkarNHFernandesGTelangNT1982Low-calorie diet prevents the development of mammary tumors in C3H mice and reduces circulating prolactin level, murine mammary tumor virus expression, and proliferation of mammary alveolar cellsProc Natl Acad Sci797758626296850
- SauveAAWolbergerCSchrammVL2006The Biochemistry of SirtuinsAnnu Rev Biochem754356516756498
- SchonEA2000Mitochondrial genetics and diseaseTrends Biochem Sci255556011084368
- ShieldsBAEngelmanRW1991Calorie restriction suppresses subgenomic mink cytopathic focus-forming murine leukemia virus transcription and frequency of genomic expression while impairing lymphoma formationProc Natl Acad Sci8811138421763029
- SimonenkoVBDulinPAMakaninMA2006Somatostatin analogues in treatment of gastrointestinal and pancreatic neuroendocrine tumorsKlin Med (Mosk)8448
- SinclairDAGuarenteL1997Cell911033429428525
- SinghKK2006Mitochondria damage checkpoint, aging, and cancerAnn N Y Acad Sci106718290 Review16803984
- SohalRSWeindruchR1996Oxidative stress, caloric restriction, and agingScience27359638658196
- SomayajuluMMcCarthySHungM2005Role of mitochondria in neuronal cell death induced by oxidative stress; neuroprotection by Coenzyme Q10Neurobiol Dis186182715755687
- StorzP2005Reactive oxygen species in tumor progressionFront Biosci1018819615769673
- SunJTowerJ1999FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster fliesMol Cell Biol19216289858546
- TaubJLauJFMaC1999A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutantsNature399162610335847
- TrichopoulouACostacouTBamiaC2003Adherence to a Mediterranean diet and survival in a Greek populationN Engl J Med348259960812826634
- TrousonA2006The production and directed differentiation of human embryonic stem cellsEndocr Rev272081916434509
- VitettaLAntonACortizoF2005Mind-body medicine: stress and its impact on overall health and longevityAnn NY Acad Sci105749250516399915
- WallaceDC2005A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicineAnnu Rev Genet39359407 Review16285865
- WeindruchRKeenanKPCarneyJM2001Caloric restriction mimetics: Metabolic interventionsJ Gerontol A Biol Sci Med Sci56Spec No 1203312088209
- WeindruchRSohalRS1997Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and agingNEJM337986949309105
- WillettW2002Balancing life-style and genomics research for disease preventionScience296695811976443
- XuDFinkelT2002A role for mitochondria as potential regulators of cellular life spanBiochem Biophys Res Commun294245812051701
- ZhuHGuoQMattsonMP1999Dietary restriction protects hippocampal neurons against the death-promoting action of presenilin-1 mutationBrain Res842224910526115