Abstract
Strontium ranelate is a new orally administered agent for the treatment of women with postmenopausal osteoporosis that reduces the risk of vertebral and hip fractures. Evidence for the safety and efficacy of strontium ranelate comes from two large multinational trials, the SOTI (Spinal Osteoporosis Therapeutic Intervention) and TROPOS (Treatment Of Postmenopausal Osteoporosis) studies. The SOTI study evaluated vertebral fracture prevention in 1649 postmenopausal women with a mean age of 69 y. The subjects all had at least one previous vertebral fracture and a low spine bone mineral density (BMD) (equivalent to a Hologic spine T-score below −1.9). The strontium ranelate group had a 41% lower risk of a new vertebral fracture than the placebo group over the three-year study period (relative risk [RR]=0.59; 95% confidence interval [CI]: 0.48–0.73; p<0.001). The TROPOS study evaluated non-vertebral fracture prevention in 5091 postmenopausal women with a mean age of 77 y. The subjects were aged 74 y and over (or 70–74 y with one additional risk factor) and a low femoral neck BMD (equivalent to an NHANES III [Third National Health and Nutrition Examination Survey] T-score below −2.2). Over the three-year study period there was a 16% reduction in all non-vertebral fractures (RR=0.84; 95% CI 0.702–0.995; p=0.04) and a 19% reduction at the principal sites for non-vertebral fractures. The TROPOS study was not powered to investigate hip fracture risk. However, in a high risk group of women aged 74 y and over and with an NHANES III femoral neck T-score less than −2.4 there was a 36% reduction in hip fracture risk (RR=0.64; 95% CI: 0.412–0.997; p=0.046). The overall incidence of adverse events did not differ significantly from placebo and were generally mild and transient, the most common being nausea and diarrhea. Strontium ranelate is a useful addition to the range of anti-fracture treatments available for treating postmenopausal women with osteoporosis and is the only treatment proven to be effective at preventing both vertebral and hip fractures in women aged 80 y and over.
Introduction
Strontium ranelate (Les Laboratoires Servier, Neuilly-sur-Seine, France) is a new orally administered agent recently licensed in Europe and Australia for the treatment of postmenopausal osteoporosis to reduce the risk of vertebral and hip fractures. As an alkaline earth element, strontium has close similarities with calcium in its absorption in the gut, incorporation into bone, and relatively high renal tubular reabsorption in the kidneys (CitationMarshall et al 1973). Since strontium is naturally present in trace amounts in the human body (CitationSowden and Stitch 1957), treatment with strontium ranelate is simply making more stable strontium available for incorporation into bone. After 3 years treatment with strontium ranelate, the molar fraction of strontium in bone is about 1%, ie, there is ~1 strontium atom for every 100 calcium atoms in bone tissue (CitationBlake and Fogelman 2005).
Strontium ranelate is a useful addition to the range of anti-fracture treatments available for treating postmenopausal women with osteoporosis (Table ). Its mechanism of action appears to be different from other treatments. Bisphosphonates (BPs) (CitationLiberman et al 1995; CitationBlack et al 1996; CitationHarris et al 1999; CitationChesnut et al 2004) and selective estrogen receptor modulators (SERMs) (CitationEttinger et al 1999) are antiresorptive agents that are believed to work by suppressing bone remodeling (CitationEastell et al 2003). In contrast, human recombinant parathyroid hormone (PTH) is an anabolic agent that that works by promoting bone formation (CitationLindsay et al 1997; CitationNeer et al 2001). Animal models and in vitro studies suggest that strontium ranelate acts in both these ways, having both a mild anabolic effect and a mild antiresorptive effect on bone tissue (CitationMarie et al 1993; CitationCanalis et al 1996). Studies in intact rats given strontium ranelate showed increases in bone resistance, in cortical and trabecular bone volume, and in bone micro-architecture, indicating an improvement in bone quality (CitationAmmann et al 2004). Treatment with strontium ranelate may therefore serve to uncouple bone resorption and bone formation and rebalance bone turnover in favor of bone formation (CitationSPC 2004).
Table 1 Treatments for postmenopausal women with osteoporosis
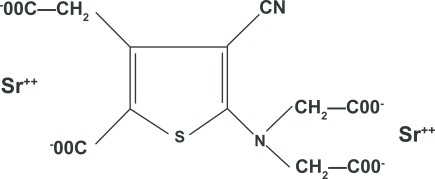
The chemical structure of strontium ranelate is composed of two atoms of stable strontium combined with organic ranelic acid (Figure ). Strontium is the bone active component and makes up 34% by weight of the whole molecule, so each 2 g dose of strontium ranelate delivers 680 mg of elemental strontium. The daily dose comes as granules in a sachet that are suspended in water and drunk usually at bedtime. The summary of product characteristics (SPC) gives a figure of 25% for the gut absorption of strontium. This is consistent with the findings of a recent meta-analysis of strontium gut absorption, which reported a log normal distribution with a geometric mean of 22.3% and 95% confidence interval (CI) 10.9% to 45.6%, or about a factor of 2 around the geometric mean (CitationApostoaei 2002). If strontium ranelate is prescribed with calcium supplements then these latter should be taken at a different time of day (ie, at lunch time) to avoid reducing gut absorption. Similarly, absorption is affected by food, milk, and milk derivatives, and strontium ranelate should be taken at least 2 h after these products. The suspension of strontium ranelate in water is tasteless and the ranelic acid passes through the gut largely unabsorbed (CitationSPC 2004).
Figure 1 Chemical structure of strontium ranelate (CitationMarie et al 1993).
The two most important requirements of any treatment for osteoporosis are firstly that it is proven to be safe and secondly that it is effective in reducing fracture risk. Evidence of the safety and efficacy of strontium ranelate comes from two large multinational clinical trials, the SOTI (Spinal Osteoporosis Therapeutic Intervention) (CitationMeunier et al 2004) and TROPOS (Treatment of Peripheral Osteoporosis) (CitationReginster et al 2005) studies, which were designed to study vertebral and non-vertebral fractures respectively. Further clinical data is available from two earlier Phase 2 trials, the STRATOS (Strontium Ranelate for Treatment of Osteoporosis) (CitationMeunier et al 2002) and PREVOS (Prevention of Osteoporosis) (CitationReginster et al 2002) studies. These latter were dose ranging studies that examined the effects of strontium ranelate treatment on bone mineral density (BMD) and biochemical markers of bone turnover in patients with established vertebral fractures and early postmenopausal women respectively. The STRATOS study also provided some fracture data.
Vertebral fracture prevention by strontium ranelate
The evaluation of the efficacy of strontium ranelate treatment in preventing vertebral fractures was the primary aim of the SOTI trial (CitationMeunier et al 2004). A total of 1649 women (mean age 69 y) were enrolled and randomized to receive either strontium ranelate 2 g/day or placebo. All subjects were also given calcium and vitamin D supplements to ensure they received the recommended daily intakes. The women were aged 50 y and over, at least 5 y postmenopausal, had at least 1 previous vertebral fracture and a lumbar spine bone mineral density (BMD) less than 0.840 g/cm2 (T-score <−2.4 on the Slosman reference range (CitationSlosman et al 1994) used for study inclusion criteria, equivalent to a T-score <−1.9 on the Hologic manufacturer’s spine reference range). Lateral spinal radiographs were performed at baseline and then annually, and were evaluated for evidence of new vertebral fractures using the Genant semi-quantitative method (CitationGenant et al 1993). At the end of the first year of treatment there was a 49% lower risk of a new radiographic vertebral fracture in the strontium ranelate group compared with the placebo group (incidence 6.4% vs 12.2%; relative risk [RR]=0.51; 95% CI: 0.36–0.74; p<0.001). The risk of a clinically symptomatic vertebral fracture was 52% lower (3.1% vs 6.4%; RR=0.48; 95% CI: 0.29–0.80; p=0.003). Over the main three-year study period the strontium ranelate group had a 41% lower risk of a new radiographic vertebral fracture than the placebo group (incidence 20.9% vs 32.8%; RR=0.59; 95% CI: 0.48–0.73; p<0.001), while the incidence of clinically symptomatic vertebral fracture was 38% lower (11.3% vs 17.4%; RR=0.62; 95% CI: 0.47–0.83; p<0.001). Over this period the number of patients treated to prevent one vertebral fracture (NNT) was 9. The four-year SOTI study fracture data were recently published in a conference abstract (CitationReginster et al 2006) and show a 33% reduction in the risk of a new vertebral fracture in the strontium ranelate group compared with placebo (RR=0.67; 95% CI: 0.55–0.81; p<0.001). The 1 y, 3 y, and 4 y data are summarized in Figure .
Figure 2 Results for vertebral fracture reduction by strontium ranelate treatment in the SOTI and TROPOS studies (CitationMeunier et al 2004; CitationReginster et al 2005, Citation2006). Results are shown as the relative risk (RR) and 95% confidence intervals.
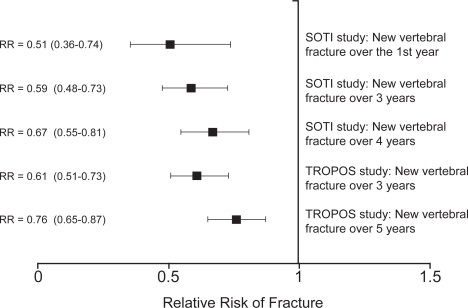
The TROPOS study provided further data on the vertebral fracture efficacy of strontium ranelate (CitationReginster et al 2005). Although not primarily intended to study vertebral fractures, 3640 out of 5091 women enrolled in the TROPOS study (71%) had annual spinal radiographs. Of these 3640 patients, two-thirds (66.4%) had no prevalent vertebral fracture at inclusion. Over three years the overall reduction in vertebral fracture risk was 39% (RR=0.61; 95% CI: 0.51–0.73; p<0.001), and in the first year the reduction was 45% (RR=0.55; 95% CI: 0.39–0.77; p<0.001). The anti-fracture efficacy at 3y was similar for patients with and without vertebral fractures at baseline. The pooled 3-year data from the SOTI and TROPOS studies gave a total population of 5082 women for the evaluation of vertebral fracture risk and showed an overall reduction in fracture risk of 40% over three years (RR=0.60; 95% CI: 0.53–0.69; p<0.001) (CitationRoux et al 2006). The recently presented 5-year TROPOS data show a 24% reduction in vertebral fracture risk (RR=0.76; 95% CI: 0.65–0.87; p<0.001) (CitationReginster et al 2006). Although both the SOTI and TROPOS data show a slight decline in antifracture efficacy the longer treatment is given (Figure ), such a trend is expected since with the Kaplan-Meier analysis patients are censored after their first fracture and with time the remaining subjects in the treated and placebo groups become less well matched.
One interesting aspect of the SOTI and TROPOS studies compared with previous osteoporosis trials was the relatively advanced age of many of the subjects (CitationSeeman et al 2006). Taken together, the two studies enrolled 1556 patients over 80 years at inclusion (23.1% of the entire study population) (CitationSPC 2004). The pooled analysis of the studies for vertebral fracture risk showed no evidence of a treatment-by-age interaction (p=0.652). The RR reduction was 37% (p=0.003) in the younger women (<70 years), 42% (p<0.001) in women 70–80 years of age, and 32% (p=0.013) in the elderly (women ≥80 years of age) (CitationRoux et al 2006).
Some vertebral fracture data was also published for the STRATOS study (CitationMeunier et al 2002). However, this was a much smaller study with only 90 patients in each of four groups (placebo, 0.5, 1 and 2 g/day strontium ranelate) and over the full 2-years of the trial the results were not statistically significant (2 g/day group at 2 y: RR=0.77; 95% CI: 0.54–1.09).
Non-vertebral fracture prevention by strontium ranelate
The efficacy of strontium ranelate treatment in preventing non-vertebral fractures was investigated in the TROPOS trial, a study of 5091 women with a mean age of 77 y (CitationReginster et al 2005). Women were eligible for this study if they had a femoral neck BMD less than 0.600 g/cm2 (T-score <−2.5 on the Slosman reference range [CitationSlosman et al 1994] used for study inclusion criteria, equivalent to a T-score <−2.2 on the widely adopted NHANES III (Third National Health and Nutrition Examination Survey) hip reference range [CitationLooker et al 1998]) and were aged 74 y or over, or were between 70 y and 74 y and had at least one additional risk factor such as a history of previous osteoporotic fracture or a maternal history of fracture. As in the SOTI trial, the subjects were randomized to receive either strontium ranelate 2 g/day or placebo, and also received calcium and vitamin D supplements. All non-vertebral fractures were recorded with the exception of the coccyx, skull, jaw, face, phalanx (fingers and toes) and ankle, since these latter were not regarded as being related to osteoporosis. Over the main 3 year follow-up period there was a 16% reduction in all non-vertebral fractures (incidence 11.2% vs 12.9%; RR=0.84; 95% CI: 0.702–0.995; p=0.04; number needed to treat [NNT]=59) (Figure ). For the principal non-vertebral fracture sites (hip, wrist, pelvis, sacrum, ribs, sternum, clavicle, humerus) there was a 19% reduction in fracture risk (incidence 8.7% vs 10.4%; RR=0.81; 95% CI: 0.66–0.98; p=0.031; NNT=59). Overall the relative risk of hip fracture was reduced by 15%, but this figure was not statistically significant and the trial was not powered to investigate anti-fracture efficacy at this site. However, in a subgroup of high risk patients aged 74 y and over and with an NHANES III femoral neck T-score <−2.4 there was a 36% reduction in hip fracture risk (incidence 4.3% vs 6.4%; RR=0.64; 95% CI: 0.412–0.997; p=0.046; NNT=48) (Figure ). The 5-year results of the TROPOS study show continuing long-term efficacy with a 15% reduction in non-vertebral fractures (RR = 0.85; 95% CI: 0.73–0.99; p=0.03) (CitationReginster et al 2006) (Figure ).
Figure 3 Results for non-vertebral fracture reduction by strontium ranelate in the TROPOS study (CitationReginster et al 2005, Citation2006). Results are shown as the relative risk (RR) and 95% confidence intervals.
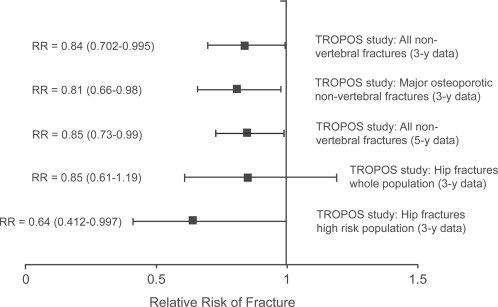
The smaller SOTI study (CitationMeunier et al 2004) was not powered to investigate non-vertebral fracture risk. Non-vertebral fractures were recorded in 234 women (112 in the strontium ranelate group and 122 in the placebo group) over the 3-year study (incidence 15.5% vs 16.8%; RR=0.90; 95% CI: 0.69–1.17). In the analysis of the pooled data from the SOTI and TROPOS studies the overall reduction in non-vertebral fracture risk was 15% (incidence 11.6% vs 13.1%; RR=0.85; 95% CI: 0.74–0.99; p=0.03) (CitationRoux et al 2006). In the cohort of SOTI and TROPOS women aged 80 years and over at enrolment the reduction in non-vertebral fracture risk was 31% (incidence 14.2% vs 19.7%; RR=0.69; 95% CI: 0.52–0.92; p=0.011) (CitationSeeman et al 2006).
Patient safety in the strontium ranelate trials
During the SOTI and TROPOS trials a total of 3352 patients were exposed to strontium ranelate, 2315 of them for at least 36 months (CitationMeunier et al 2004; CitationReginster et al 2005). The overall incidence of adverse events did not differ significantly from placebo and were generally mild and transient. The most common were nausea (6.6% of patients taking strontium ranelate vs 4.3% in those taking placebo) and diarrhea (6.5% vs 4.6%). However, if constipation is reduced the latter effect may be beneficial. The adverse reactions in the two studies thought possibly attributable to strontium ranelate are listed in Table . After 3 months there were no differences between strontium ranelate and placebo groups with respect to nausea and diarrhea. In both studies a small decrease in serum calcium concentration and a small increase in serum phosphate was noted in the strontium ranelate group, but these and other small changes in biochemistry were without clinical consequence (CitationSPC 2004).
Table 2 Adverse events pooled for the SOTI (CitationMeunier et al 2004) and TROPOS (CitationReginster et al 2005) studies
In the two Phase 3 studies strontium ranelate treatment was found to be associated with an increase in the annual incidence of venous thromboembolism (VTE) including pulmonary embolism (CitationSPC 2004). This effect was observed over 4 years and was approximately 0.7% with a RR of 1.42 (95% CI: 1.02–1.98; p=0.036). The cause of this finding is unknown. The SPC advises that strontium ranelate should be used with caution in patients at increased risk of VTE, including patients with a previous history of VTE.
The SPC also states that strontium ranelate is not recommended in patients with severe renal impairment (glomerular filtration rate below 30 mL/min). Plasma strontium levels will be higher in such patients and the implications for bone safety are not known.
The effect of strontium ranelate treatment on bone mineral density
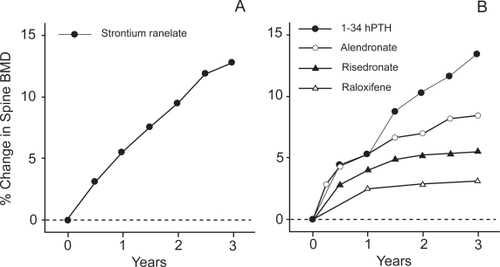
All the clinical trials of strontium ranelate (CitationMeunier et al 2002, Citation2004; CitationReginster et al 2002, Citation2005) show impressive increases in BMD compared with BPs (CitationLiberman et al 1995; CitationBlack et al 1996; CitationHarris et al 1999; CitationChesnut et al 2004) and SERMs (CitationEttinger et al 1999), and comparable with PTH (CitationLindsay et al 1997; CitationNeer et al 2001) (Figure ). After 3 years treatment the SOTI data showed BMD increases compared with placebo of 14.4% at the spine, 9.8% at the total hip site, and 8.3% at the femoral neck (CitationMeunier et al 2004). These large changes may provide a useful way of monitoring patient compliance (CitationFogelman and Blake 2005). However, caution is necessary in interpreting the BMD increases as a real change in bone mass because much of the effect is due to the higher atomic number of strontium (Z=38) compared with calcium (Z=20) (CitationBlake and Fogelman 2005). When BMD is measured by dual energy X-ray absorptiometry (DXA), strontium atoms in bone attenuate X-rays more strongly than calcium causing BMD to be overestimated. This effect was studied quantitatively by CitationPors Nielsen et al (1999) who constructed phantoms containing mixtures of calcium and strontium hydroxyapatite that were scanned on different manufacturers’ DXA systems. They concluded that the same factor (a 1% molar ratio of Sr/(Ca+Sr) causing a 10% overestimation of BMD) applied to all the DXA brands studied. Since 3 years treatment with strontium ranelate results in a molar ratio of ~1%, it is clear that a significant percentage of the BMD increase in the clinical trial data can be explained by the increased attenuation due to the bone strontium content (CitationBlake and Fogelman 2006).
Figure 4 (A) Data from the SOTI study (CitationMeunier et al 2004) showing the effect of 3 years treatment with strontium ranelate (2 g/day) on spine bone mineral density (BMD). The BMD values have not been adjusted for the bone strontium content. (B) Mean percentage changes in spine BMD from baseline to 3 years in patients receiving active treatment in four clinical trials: (i) raloxifene (60 mg/day) (CitationEttinger et al 1999); (ii) risedronate (5 mg/day) (CitationHarris et al 1999); (iii) alendronate (10mg/day) (CitationLiberman et al 1995); (iv) hPTH(1–34) and oestrogen (CitationLindsay et al 1997).
The most detailed discussion of the size of the BMD artefact produced by strontium ranelate treatment is that included in the report of the STRATOS trial (CitationMeunier et al 2002). In this study 17 patients had iliac crest bone biopsies to measure bone strontium content (BSC) at 1 year and 64 patients at 2 years. At each time point a linear relationship was found between the iliac crest BSC and the area under the curve of strontium plasma concentration against time (AUC), and this was used to predict BSC values in patients who did not have a bone biopsy. For the 2 g/day strontium ranelate group it can be inferred from the raw and corrected STRATOS study BMD data that the mean iliac crest BSC was 0.8% at 12 months and 1.3% at 2 years. These BSC values were corrected by a factor of 0.61 for the ratio of spine to iliac crest BSC measured in female monkeys (CitationDahl et al 2001), and then combined with the phantom data of CitationPors Nielsen et al (1999) to adjust the raw spine BMD measurements. The strontium ranelate SPC document cites a factor of 50% for the correction of the measured BMD changes at the end of 3 years of treatment to allow for this effect. This should be taken into account when interpreting BMD changes during treatment with strontium ranelate. Radionuclide studies of the long-term retention of strontium in bone using whole body counters (CitationMarshall et al 1973) suggest that much of this BMD artefact will persist for at least 10 years after patients discontinue treatment (CitationBlake and Fogelman 2006).
The effect of strontium ranelate treatment on biochemical markers of bone turnover
Follow-up measurements of changes in bone resorption and bone formation have played an important part in characterizing the clinical response in trials of other treatments for postmenopausal osteoporosis (CitationBlack et al 1996; CitationLindsay et al 1997; CitationHarris et al 1999; CitationEttinger et al 1999; CitationEastell et al 2003; CitationChesnut et al 2004). For example, with the more potent BPs there is an almost immediate (within 1 month) reduction of 30%–50% in biochemical markers of bone resorption followed by a similar but delayed (3 to 6 month) reduction in markers of bone formation (CitationGarnero et al 1994). For the potent anabolic agent human recombinant PTH there is an increase of about 50% in markers of both bone formation and bone resorption within 6 months of patients starting treatment. Based on pre-clinical studies, strontium ranelate is believed to exert an uncoupling effect on bone turnover by enhancing bone formation at the same time that it suppresses bone resorption (CitationMarie et al 1993). It was therefore of interest to see whether the strontium ranelate trials provided confirmation of this behaviour.
Biochemical marker measurements were reported for the SOTI study (CitationMeunier et al 2004) but not TROPOS (CitationReginster et al 2005). Unfortunately, the SOTI biochemical marker data were affected by some unexplained variations affecting the measurements for both the strontium ranelate and placebo groups. Nevertheless, the SOTI study authors inferred that after 3 months of treatment the serum concentration of bone specific alkaline phosphatase (BSAP), a bone formation marker, increased by 8.1% in the strontium ranelate group compared with placebo (p<0.001) while the concentration of serum C-telopeptide cross-links (CTX), a bone resorption marker, was lower by 12.2% (p<0.001), and these changes were sustained throughout the 3-year study. The biochemical marker data from the SOTI study therefore appeared to confirm the expected effect of a mild (compared with a potent anabolic agent) promotion of bone formation, and a mild (compared with potent antiresorptive agents) suppression of bone resorption, and are in line with the results of pre-clinical animal and in vitro studies (CitationMarie et al 1993; CitationCanalis et al 1996; CitationAmmann et al 2004).
Biochemical marker data were also reported for the STRATOS (CitationMeunier et al 2002) and PREVOS (CitationReginster et al 2002) trials. However, both these studies were smaller than the SOTI trial with patients randomized to a range of treatment doses. In the STRATOS study BSAP increased by 11% at 24 months in the 2 g/day group (p<0.05), while urinary excretion of type 1 collagen cross-linked N-telopeptide pyridinoline (NTX) was reduced by 10.1% compared with placebo at the same time point. In the PREVOS study similar trends were seen, but most of the differences were not statistically significant.
The effect of strontium ranelate treatment on bone histomorphometry
A considerable effort was made in the strontium ranelate trials to obtain transiliac bone biopsies to evaluate bone safety and assess the mechanism of action at the bone tissue level (CitationMeunier et al 2002, Citation2004; CitationReginster et al 2005). Bone histomorphometry data from the STRATOS, SOTI, and TROPOS patients at baseline, 1 y, 2 y, 3 y, 4 y and 5 y from the start of treatment were reviewed by CitationArlot et al (2005). Data were available for 49 patients treated with strontium ranelate for between 1 y and 5 y, and for 87 patients either at study baseline or in those randomized to the placebo group. The positive effect of strontium ranelate on bone formation was confirmed by a higher osteoblastic surface area (Ob.S/BS) in treated compared with untreated patients (+38%; p=0.047) and a greater mineral apposition rate (MAR) in trabecular (+8%; p=0.008) and cortical (+11%; p=0.033) bone. There was no significant change in activation frequency. The effect on bone resorption consisted of trends towards lower endosteal eroded surfaces, endosteal and trabecular osteoclast surfaces, and osteoclast numbers, none of which were statistically significant. In terms of bone safety, osteoid thickness was found to be significantly lower and MAR significantly higher in treated patients with no change in osteoid volume or mineralization lag time demonstrating that primary mineralization was not impaired. Overall, the results are consistent with the mild stimulation of bone formation.
Conclusions
The results of the SOTI and TROPOS studies confirm that strontium ranelate is a safe and effective treatment to prevent vertebral fractures and hip fractures in women with postmenopausal osteoporosis. The 40% reduction in vertebral fracture risk found in the pooled analysis (CitationRoux et al 2006) is similar to that found in trials of other osteoporosis treatments including alendronate (CitationBlack et al 1996), risedronate (CitationHarris et al 1999), ibandronate (CitationChesnut et al 2004), and raloxifene (CitationEttinger et al 1999). Strontium ranelate has also been proven to reduce non-vertebral fracture risk by 15% (CitationRoux et al 2006). This includes the prevention of hip fractures in older patients with a femoral neck BMD T-score consistent with the diagnosis of osteoporosis. Interestingly, because of the generally elderly population enrolled in the SOTI and TROPOS studies, strontium ranelate is the only treatment proven to be effective at preventing both vertebral and non-vertebral fractures in patients aged 80 years and over (CitationSeeman et al 2006). Data from bone biopsies and biochemical markers of bone turnover seem to confirm the hypothesis that strontium ranelate may have a dual mode of action that has the effect of rebalancing bone turnover in favor of bone formation. No studies have yet examined the benefit of combining strontium ranelate with another treatment, for example a BP. Theoretically the administration of strontium ranelate in combination with a potent BP could have an adverse effect on both the uptake and the pharmacological action of strontium in bone because of the reduction in osteoblastic activity by the BP. In many patients strontium ranelate treatment has an impressive effect on BMD, and the large BMD increase may prove a useful way of monitoring patient response (CitationFogelman and Blake 2005). However, clinicians should be aware that at least 50% of the observed BMD increase is an artefact caused by the high bone strontium content (CitationSPC 2004). This artefact will persist for many years after the patient discontinues therapy and will need to be taken into account in the interpretation of future BMD scans in patients who have previously been treated with strontium ranelate (CitationBlake and Fogelman 2006).
References
- AmmannPShenVRobinB2004Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female ratsJ Bone Miner Res1920122015537445
- ApostoaeiAI2002Absorption of strontium from the gastrointestinal tract into plasma in healthy human adultsHealth Phys83566512075684
- ArlotMEDelmasPBurt-PichatB2005The effects of strontium ranelate on bone remodelling and bone safety assessed by histomorphometry in patients with postmenopausal osteoporosisJ Bone Miner Res20Suppl 1S223
- BlackDMCummingsSRKarpfDB1996Randomised trial of the effect of alendronate on risk of fracture in women with existing vertebral fracturesLancet3481535418950879
- BlakeGMFogelmanI2005Long-term effect of strontium ranelate treatment on BMDJ Bone Miner Res201901416234961
- BlakeGMFogelmanI2006Theoretical model for the interpretation of BMD scans in patients stopping strontium ranelate treatmentJ Bone Miner Res2114172416939400
- CanalisEHottMDeloffreP1996The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitroBone18517238805991
- ChesnutCHSkagAChristiansenC2004Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosisJ Bone Miner Res191241915231010
- DahlSGAllainPMariePJ2001Incorporation and distribution of strontium in boneBone284465311336927
- EastellRBartonIHannonRA2003Relationship of early changes in bone resorption to the reduction in fracture risk with risedronateJ Bone Miner Res181051612817758
- EttingerBBlackDMMitlakBH1999Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trialJAMA2826374510517716
- FogelmanIBlakeGM2005Strontium ranelate for the treatment of osteoporosisBMJ3301400115961793
- GarneroPShihWCJGineytsE1994Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatmentJ Clin Endocrinol Metab7916937007989477
- GenantHKWuCYvan KuijkC1993Vertebral fracture assessment using a semiquantitative techniqueJ Bone Miner Res81137488237484
- HarrisSTWattsNBGenantHK1999Effects of risedronate treatment on vertebral and non-vertebral fractures in women with postmenopausal osteoporosisJAMA28213445210527181
- LibermanUAWeissSRBrollJ1995Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosisN Engl J Med3331437437477143
- LindsayRNievesJFormicaC1997Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosisLancet35055059284777
- LookerACWahnerHWDunnWL1998Updated data on proximal femur bone mineral levels of US adultsOsteoporosis Int846889
- MariePJHottMModrowskiD1993An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient ratsJ Bone Miner Res8607158511988
- MarshallJHLloydELRundoJ1973Alkaline earth metabolism in adult man. A report prepared by a task group of Committee 2 of the International Commission on Radiological Protection (ICRP Publication 20)Health Phys24129221
- MeunierPJSlosmanDODelmasPD2002Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis – a 2-year randomised placebo controlled trialJ Clin Endocrinol Metab872060611994341
- MeunierPJRouxCSeemanE2004The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosisN Engl J Med3504596814749454
- NeerRMArnaudCDZanchettaJR2001Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med34414344111346808
- Pors NielsenSSlosmanDSorensenOH1999Influence of strontium on bone mineral density and bone mineral content measurements by dual x-ray absorptiometryJ Clin Densitom2371910677790
- ReginsterJYDeroisyRDougadosM2002Prevention of early postmenopausal bone loss by strontium ranelate: the randomised, two-year, double-masked, dose ranging, placebo-controlled PREVOS trialOsteoporosis Int1392531
- ReginsterJYSeemanEDe VernejoulMC2005Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) studyJ Clin Endocrinol Metab9028162215728210
- ReginsterJYMeunierPJRouxC2006Strontium ranelate: an anti-osteoporotic treatment demonstrating vertebral and nonvertebral antifracture efficacy over 5 years in postmenopausal osteoporotic womenOsteoporosis Int17Suppl 2S138
- RouxCReginsterJYFechtenbaumJ2006Vertebral fracture risk reduction with strontium ranelate in women with postmenopausal osteoporosis is independent of baseline risk factorsJ Bone Miner Res215364216598373
- SeemanEVellasBBenhamouC2006Strontium ranelate reduces the risk of vertebral and nonverebral fractures in women eighty years of age and olderJ Bone Miner Res2111132016813532
- SlosmanDORizzoliRPichardC1994Longitudinal measurements of regional and whole body bone mass in young healthy adultsOsteoporosis Int418590
- SowdenEMStitchSR1957Trace elements in human tissue: estimation of the concentrations of stable strontium and calcium in human boneBiochem J67104913471518
- [SPC] Summary of product characteristics2004Summary of product characteristics. Protelos (Strontium ranelate 2g granules)Neuilly-sur-Seine, FranceLes Laboratories Servier