Abstract
Osteoarthritis is the most common form of arthritis. It is a progressive joint disease associated with aging. It may be found in the knees, hips, or other joints. It is estimated that costs associated with osteoarthritis exceed 2% of the gross national product in developed countries. Nonsteroidal anti-inflammatory drugs (NSAIDs) are a mainstay in the treatment of inflammatory disease and are among the most widely used drugs worldwide. The main limitation in using NSAIDs consists in their side-effects, including gastrointestinal ulcerogenic activity and bronchospasm. The mechanism of action of these drugs is attributed to the inhibition of cyclooxygenase (COX), and, consequently, the conversion of arachidonic acid into prostaglandins. It is hypothesized that the undesirable side-effects of NSAIDs are due to the inhibition of COX-1 (constitutive isoform), whereas the beneficial effects are related to the inhibition of COX-2 (inducible isoform). Arachidonic acid can also be converted to leukotrienes (LTs) by the action of 5-lipoxygenase (5-LOX). Licofelone, a LOX/COX competitive inhibitor, decreases the production of proinflammatory leukotrienes and prostaglandins (which are involved in the pathophysiology of osteoarthritis and in gastrointestinal (GI) damage induced by NSAIDs) and has the potential to combine good analgesic and anti-inflammatory effects with excellent GI tolerability. Preliminary data with this drug seem promising, but further well-designed clinical trials of this agent in the elderly will be necessary before a final evaluation is possible.
Introduction to degenerative processes in osteoarthritis
Osteoarthritis is the most common form of arthritis and the most common indication for total hip and total knee replacement. It is a progressive joint disease associated with aging. Many elderly people have some degree of osteoarthritis. It may be found in the knees, hips, or other joints. Risk factors for the development and/or progression of osteoarthritis identified in epidemiological studies can be broadly divided into systemic factors that increase the susceptibility to the disease and local biomechanical factors that influence the development of osteoarthritis at the particular joint. It is estimated that costs associated with osteoarthritis exceed 2% of the gross national product in developed countries. In this context, the clear need for a better understanding of the disease process has rendered undeniable the importance of finding drugs that can reduce or stop its progression.
The progression of the structural changes that occur during the course of the disease is related to a number of complex pathways and mechanisms, among which the excess production of proteolytic enzymes that can degrade the cartilage matrix and soft tissues surrounding the joint is believed to be of particular importance (CitationMartel-Pelletier et al 2004). The degradation of the osteoarthritis cartilage matrix has been shown to be related to the excess synthesis of a large number of proteases and, more particularly, to that of the matrix metalloproteinases (MMPs) and thioldependent families. Among the MMPs, two collagenases, MMP-1 and MMP-13, have been the subject of extensive investigation and were found likely to be the primary enzymes involved in the breakdown of type II collagen in osteoarthritis cartilage (CitationMartel-Pelletier et al 2001). Cathepsin K, a thiol-dependent enzyme that works preferentially under acidic pH conditions, has also been demonstrated to be synthesized by osteoarthritis chondrocytes and is likewise believed to play an important role in the breakdown of the osteoarthritis cartilage collagen network (CitationKonttinen et al 2002) as well as the aggrecans, and thus likely involved in degrading the cartilage extracellular matrix. The mechanisms involved in the degradation of the aggrecans in osteoarthritis cartilage have also been extensively explored and studied, which has led to the identification of a number of proteolytic enzymes that can specifically degrade aggrecans (CitationNagase and Kashiwagi 2003). Comprehensive investigation has indicated that the MMPs, including MMP-13, aggrecanase-1 (a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS-4), and aggrecanase-2 (ADAMTS-5), are the proteolytic enzymes that seem the most likely to be involved in the degradation of aggrecans in osteoarthritis cartilage (CitationLark et al 1997; CitationChambers et al 2001).
Recent studies have revealed new and interesting information regarding the role played by eicosanoids in the pathophysiology of arthritic diseases, including osteoarthritis (CitationHansen et al 1990; CitationPrete and Gurakar-Osborne 1997; CitationGay et al 2001; CitationJovanovic et al 2001; CitationMartel-Pelletier et al 2003, 2004). For instance, leukotriene-B4 (LTB4) has proven to be an important regulating factor in the synthesis of IL-1β by osteoarthritis synovium (CitationRainsford et al 1996; CitationJovanovic et al 2001; CitationHe et al 2002). Both in vitro and in vivo studies have demonstrated that the excess production of IL-1β in osteoarthritis tissue is a key factor in its destruction and in the progression of the disease itself (CitationPelletier et al 2001; CitationMartel-Pelletier et al 2004). The endogenous production of LTB4 in osteoarthritis synovium is a crucial element in the upregulation of IL-1β synthesis in this tissue (CitationHe et al 2002). The synthesis of LTB4, and subsequently of IL-1β, can be significantly increased by nonsteroidal anti-inflammatory drugs (NSAIDs) (CitationParades et al 2002; CitationMarcouiller et al 2005). It has been hypothesized that this could be related to a ‘shunt’ of the arachidonic acid cascade from the cyclooxygenase (COX) to the lipoxygenase (LOX) pathway (CitationMartel-Pelletier et al 2003). These findings could help explain how some NSAIDs accelerate the progression of clinical osteoarthritis (CitationHuskinsson et al 1995). A recent study has demonstrated that, in in vivo experimental osteoarthritis, licofelone, a drug that can inhibit both the COX and 5-LOX pathways, was capable of reducing the development of osteoarthritis structural changes while simultaneously reducing the synthesis of LTB4 and IL-1β by the osteoarthritis synovium (CitationJovanovic et al 2001). These findings are in strong support of the in situ role played by LTB4 in the structural changes that occur in osteoarthritis.
Review of pharmacology, mode of action, and pharmacokinetics of licofelone
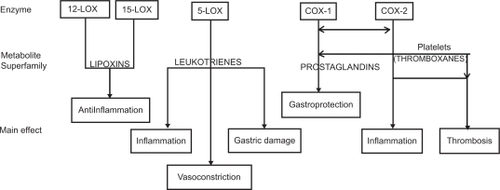
Arachidonic acid (C20:4), a ω-6 essential fatty acid, is metabolized by several enzymes, in different tissues. After the cleavage from membrane phospholipids, arachidonic acid generates a lot of compounds with a wide range of clinical effects involved in inflammation. It is very complicated to quantitatively determine the final effect of arachidonic acid metabolism resulting from the sum of different actions of its metabolites. In fact, just to calculate COX and LOX products’ global effect, we have to consider more than 25 active substances, each with two to five different actions, having different cellular or extracellular tissue and organ targets, depending on their different baseline and under-stimulus production rate () (CitationBertolini et al 2002). A detailed description of the arachidonic acid metabolism and the function of its metabolites is not part of the scope of this overview; therefore, the different effect of inhibitory drugs on COX-1, COX-2, and/or LOX appears to be clinically relevant (). The anti-inflammatory mechanism of action of NSAIDs is attributed to the inhibition of COX, and, consequently, the conversion of arachidonic acid into prostaglandins. It is hypothesized that the main undesirable side-effects of NSAIDs are due to the inhibition of COX-1 (constitutive isoform), whereas the beneficial effects are related to the inhibition of COX-2 (inducible isoform). This is why selective COX-2 inhibitors seem to be safer with regard to gastric damage (CitationWolfe et al 2002). Arachidonic acid can also be converted to LTs by the action of 5-LOX. LT-C4, LT-D4 and LT-E4 are potent bronchoconstrictors, whereas LT-B4 is chemotactic for leukocytes and plays an important role in the development of gastrointestinal ulcers by contributing to the inflammatory process. Thus, developing dual inhibitor compounds that will simultaneously inhibit COX and 5-LOX could enhance their individual anti-inflammatory effects and reduce the undesirable side-effects associated with NSAIDs, especially of the gastrointestinal (GI) tract (CitationFiorucci et al 2001). Moreover, lipoxins are also LOX-derived prostanoids, though their biological actions differ dramatically from LTs. In fact, lipoxins inhibit bronchoconstriction and carry local anti-inflammatory signals. Therefore, inhibitors of 5-LOX/COX which do not block the 12-LOX and 15-LOX pathways should allow unbridled synthesis of lipoxins to resolve inflammation and attenuate any remaining LT effect (CitationSerhan 2001). Several chemically distinct compounds are in different phases of preclinical or clinical development: BF-389, RWJ 63556, PGV 20229, BW755C, CI-986, SK&F 105809, FPL 62064, PD 127443, PD 137968, L-652,343, CBS-1108, licofelone (ML3000), but none already marketed (CitationCelotti and Laufer 2001). The first one currently involved in phase III clinical trials is licofelone (ML3000) ([2,2-dimethy l-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid) (CitationReginster et al 2002).
Figure 1 Actions of eicosanoids: from the enzymes to the main effects through the superfamily of metabolic products (simplified pathway) Copyright © 2002. Modified with permission from CitationBertolini A, Ottani A, Sandrini M. 2002. Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarks. Curr Med Chem, 9:1033–43.
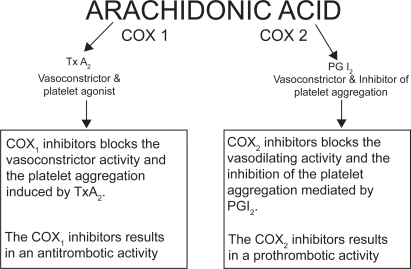
Figure 2 Effect of COX-1 and COX-2 inhibition on mechanisms involved in CHD pathogenesis Copyright © 2000. Modified with permission from Whelton 2000.
Abbreviations: COX, cyclooxygenase; LOX, lipoxygenase.

Preclinical data
Preclinical data show that licofelone has anti-inflammatory, analgesic, antipyretic, and antiplatelet (CitationLaufer et al 1994, Citation1995; CitationAbraham et al 1997; CitationRotondo et al 2004; CitationSingh et al 2006) activities. Licofelone has been shown in vitro to suppress the polymorphonuclear (PMN) leukocyteplatelet transcellular metabolism of arachidonic acid, prevent PMN aggregation and activation, and reduce PMN and platelet adhesion (CitationRotondo et al 2002), all of which are relevant to the pathogenesis of inflammation.
General toxicological studies in animals have also demonstrated that licofelone is devoid of deleterious effects on the autonomic nervous system, CNS, and cardiovascular system, being safe at doses far above pharmacologically active doses (CitationAlgate et al 1995). In addition, licofelone has no genotoxic potential (CitationHeidemann et al 1995).
Further studies in animals have demonstrated that licofelone has a lower ulcerogenic potential than aspirin, indometacin, and diclofenac (CitationWallace et al 1994).
Interestingly, in contrast to COX-2 inhibitors, licofelone does not exacerbate gastric mucosal damage, even in aspirin-treated rats, thus creating the possibility of concomitant treatment with aspirin and licofelone in osteoarthritic patients with cardiovascular risk who require long-term aspirin therapy (CitationFiorucci et al 2003). A clinical study confirmed that the co-administration of low-dose, enteric-coated aspirin and licofelone did not produce a clinically relevant increase in the occurrence of gastrointestinal ulcers (CitationBuchner et al 2003). However, the long-term effect of this association on cardiovascular outcomes has yet to be determined.
Licofelone also appears to have specific anti-arthritic activity in animals. In a rat experimental model of adjuvant arthritis, licofelone given at doses ranging between 20 mg/kg and 80 mg/kg for 26 days significantly reduced erythema and oedema, as well as arthritis-associated splenomegaly (CitationGay et al 2001). Histological examination of the joints also revealed reduced synovial cell proliferation and bone/cartilage erosions, suggesting selective activity of the drug on synovial fibroblasts.
Prevention of cartilage degradation by licofelone has also been observed in an experimental dog model of osteoarthrosis in which the anterior cruciate ligament of the stifle joint was surgically removed (CitationJovanovic et al 2001; CitationMoreau et al 2006). Administration of licofelone 2.5–5 mg/kg/day for 8 weeks significantly reduced the size and grade of the cartilage lesions. In the low-dose group (2.5 mg/kg/day), the size of lesions was lowered by 39% on femoral condyles and by 45% on tibial plateaus in comparison with placebo-treated animals, whereas in the high-dose group (5 mg/kg/day) the reductions were 64% and 54%, respectively. Moreover, while the synovium from placebo-treated dogs was hypertrophic and evidently discolored, in dogs treated with licofelone the synovium was thinner and the discoloration was less intense. Anti-arthritic activity was accompanied not only by a combined reduction in the synovial synthesis of PGE2 and LTB4, but also by a reduction in two major catabolic factors involved in cartilage degradation, namely interleukin (IL)-1β and collagenase 1. Only intra-articular injections of corticosteroid (CitationPelletier et al 1995) and NSAIDs such as tenidap (CitationSipe et al 1992) have produced similar results, with reduced synthesis of IL-1β and IL-6; intra-articular injections of the NSAIDs naproxen (CitationSipe et al 1992), carprofen (CitationPelletuer et al 2000), and tiaprofenic acid (CitationPelletier and Martel-Pelletier 1991) did not produce these effects. Furthermore, under the same experimental conditions, aspirin may actually accelerate cartilage destruction (CitationPalmoski and Brandt 1983). Since LTB4 plays an important role in the regulation of the synthesis of IL-1β, IL-6, and IL-8 in the synovial membrane (CitationParedes et al 2002), the anti-arthritic activity of licofelone may be mediated primarily through inhibition of LTB4 synthesis. Indeed, selective inhibition of 5-LOX and LTB4 synthesis is associated with a reduced production of these cytokines in vitro (CitationRainsford et al 1996).
All therapeutic potentialities of licofelone, studied by international literature, are resumed in .
Table 1 Therapeutic potentialities of licofelone
Efficacy studies and safety of licofelone in humans
First studies in humans indicate a very good gastric safety, a positive effect on bone cell remodelling and on osteoarthritis, as well. In an endoscopic study, 121 subjects with normal gastric and duodenal mucosa were randomly assigned a 4-week treatment with licofelone 200 mg twice daily (bid) or 400 mg bid, placebo or naproxen 500 mg bid. Groups were matched with respect to Helicobacter pylori status (19% positive). After 4 weeks the mucosa of the stomach and duodenum was endoscopically evaluated. Mean ± standard deviation gastric score with both 200 mg and 400 mg bid of licofelone were similar to placebo and statistically less than with naproxen: 0.13 ± 0.57 (200), 0.14 ± 0.45 (400), 0.20 ± 0.66 (placebo), 1.47 ± 1.31 (naproxen), respectively. The gastric mucosa was completely normal in 93%, 89%, 90%, and 37% of subjects, respectively.
Duodenal scores were worst with naproxen but not significantly different among groups in the given sample size: 0.00, 0.11 ± 0.57, 0.00, 0.20 ± 0.81. The results were qualitatively similar in H. pylori positive and negative subgroups. Ulcer with unequivocal depth occurred significantly more frequently in naproxen-treated subjects (6/30 = 20%; five gastric ulcers, one duodenal ulcer) than in other groups; no ulcer were present in either licofelone group or the placebo group (CitationKlesser et al 2002). In another clinical study, 148 patients with knee osteoarthritis were treated for 12 weeks with licofelone 200 mg bid or naproxen 500 mg bid. Efficacy was evaluated using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index. Responders were defined to show a 30% improvement versus baseline. With licofelone, the mean WOMAC index was improved by 23.3 mm and with naproxen there was an improvement by 21.5 mm. For the WOMAC index, 69.4% of licofelone-treated patients were responders (68.4% with naproxen). GI adverse events were reported by 13.9% (licofelone) and by 26.3% (naproxen) of patients (CitationReginster et al 2002). A 52-week trial was performed to determine the long-term tolerability and efficacy of licofelone compared with naproxen. This trial demonstrated that licofelone was at least as effective as naproxen in osteoarthritis treatment (CitationBlanco et al 2003). Patients with symptomatic osteoarthritis of the knee (as defined by American College of Rheumatology guidelines), who had discontinued NSAID therapy 3–14 days prior to the baseline visit, were randomized to receive licofelone 100 mg bid, licofelone 200 mg bid or naproxen 500 mg bid in this multicentre, double-blind, phase III trial. Licofelone treatment was associated with a dose-dependent improvement in WOMAC pain scores from baseline. The efficacy of licofelone 200 mg was similar to that of naproxen during the study. Mean changes in WOMAC pain scores, from a mean baseline value of 63.9 mm, were 27.1 mm, 30.2 mm, and 27.7 mm for licofelone 100 mg, licofelone 200 mg and naproxen, respectively. Adverse event rates again confirmed the superior tolerability of licofelone compared with naproxen, with patients in the 100 mg and 200 mg licofelone treatment groups experiencing fewer events than those in the naproxen group (59.2%, 56.3%, and 66.7%, respectively). In particular, lower frequencies of peripheral oedema and aggravated hypertension were recorded for licofelone compared with naproxen. Long-term licofelone therapy did not result in any clinically relevant variations in laboratory parameters or vital signs.
Licofelone 200 mg twice daily was also compared with celecoxib 200 mg once daily in a 12-week clinical study of patients with symptomatic osteoarthritis of the knee (CitationPavelka et al 2003). Licofelone was as effective as celecoxib, but showed better tolerability with a lower incidence of adverse events.
Conclusion: place in therapy
LOX/COX inhibitors equally block both the COX-1 and COX-2, and the 5-LOX metabolic pathways, inhibiting the formation of prostaglandins, thromboxanes, and LTs (CitationReginster et al 2002). The main reason for the search for a new class of anti-inflammatory agents is the need for safer drugs, because traditional and more recent ones have failed to show a high safety profile, especially in “frail” patients, as the elderly.
The pharmacological profile of LOX/COX inhibitors is quite similar to that of glucocorticoids drugs, which inhibit PLA2 and thereby prevent arachidonic metabolism by both COX and 5-LOX, without the severe adverse events on the endocrine system and on the metabolism intermediates of PLA2, normally associated with the use of these steroid drugs (CitationParente 2001). What is especially interesting is that these drugs seem to positively affect the cartilage metabolism thus contrasting the degenerative process of osteoarthritis (CitationJovanovich et al 2001), while significantly reducing pain and pain-related disability. At present time, this seems to be one of the more interesting paths for the future development of potent-and-safe drugs that can reduce damage derived from inappropriate and widespread use of conventional NSAIDs. From this point of view, the new class of the LOX/COX inhibitors present several beneficial and promising therapeutic effects that could be very important in the elderly (CitationSkelly and Hawkey 2003), and mainly:
potentially higher anti-inflammatory and anti-pain efficacy;
positive action on the bronchial tract (inhibition of bronchoconstriction);
reduced adverse effects on the upper GI tract, even with chronic treatment;
reduced interaction with ASA.
Moreover, dual COX/LOX inhibition could reduce the incidence or the progression of many cancers, as assessed by many preclinical studies (CitationLeval et al 2002). The main limitations of these conclusions are related to the fact that we have yet a lack of published data about clinical effectiveness and safety of COX/LOX dual inhibitors (the most part of those reported in this review have been found in posters presented at international meetings) and that only licofelone has reached the phase III study level. First results on licofelone use in humans are very encouraging (Reginster et al 2003), but larger clinical trials need to define its efficacy and safety and the possibilities of widespread use in the elderly. In fact, clinical studies need to be done, aimed at confirming these clinical effects and safety profile of LOX/COX inhibitors in the elderly. This last point is very important, since elderly will be the first class of NSAIDs consumers in the future. Therefore, it is necessary to adequately study these drugs, especially in the pathological conditions of the fragile elderly patient or the pluripathologic patient (with a polipharmacotherapy), avoiding publication bias caused by missing data.
References
- AbrahamWMLauferSTriesS1997The effects of ML 3000 on antigen-induced responses in sheepPulm Pharmacol Ther10167739514627
- AlgateDRAugustinJAttersonPR1995General pharmacology of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2,3-dihydro-1H-pyrrolizine-5-yl]-acetic acid in experimental animalsArzneimittelforschung45159657710439
- BertoliniAOttaniASandriniM2002Selective COX-2 inhibitors and dual acting anti-inflammatory drugs: critical remarksCurr Med Chem910334312733982
- BlancoFBuchnerABiasP2003Licofelone, an inhibitor of COX-1, COX-2 and 5-LOX, is as effective as naproxen and shows improved safety during 12 months of treatment in patients with osteoarthritis of the knee [abstract]Ann Rheum Dis62Suppl. 1262FRI0217.
- BuchnerABiasPLammerichA2003Twice the therapeutic dose of licofelone–an inhibitor of COX-1, COX-2 and 5-LOX–results in a significantly lower gastrointestinal ulcer incidence than naproxen in osteoarthritis patients, when administered with or without concomitant low-dose aspirinAnn Rheum Dis621 Suppl.26112594116
- CelottiFLauferS2001Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition conceptPharmacol Res434293611394934
- ChambersMGCoxLChongL2001Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort miceArthritis Rheum4414556511407708
- FiorucciSDistruttiEDe LimaOM2003Relative contribution of acetylated cyclo-oxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirinFASEB J1791171312709408
- FiorucciSMeliRBucciM2001Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy?Biochem Pharmacol621433811728379
- GayRENeidhartMPatakyF2001Dual inhibition of 5-lipoxygenase and cyclooxygenase 1 and 2 by ML3000 reduces joint destruction in adjuvant arthritisJ Rheumatol282060511550975
- HansenESFoghKHjortdalVE1990Synovitis reduced by inhibition of leukotriene B4. Carrageenan-induced gonarthritis studied in dogsActa Orthop Scand61207122164743
- HeWPelletierJPMartel-PelletierJ2002The synthesis of interleukin-1beta, tumour necrosis factor-a and interstitial collagenase (MMP-1) is eicosanoid dependent in human OA synovial membrane explants: Interactions with anti-inflammatory cytokinesJ Rheumatol295465311908571
- HeidemannATriesSLauferS1995Studies on the in vitro and in vivo genotoxicity of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2, 3-dihydro-1H-pyrrolizine-5-yl]-acetic acidArzneimittelforschung45486907779147
- HuskissonECBerryHGishenP1995Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. LINK Study Group. Longitudinal investigation of nonsteroidal antiinflammatory drugs in knee osteoarthritisJ Rheumatol22194168991995
- JovanovicDVFernandesJCMartel-PelletierJ2001In vivo dual inhibition of cyclooxygenase and lipoxygenase by ML-3000 reduces the progression of experimental osteoarthritis: suppression of collagenase 1 and interleukin-1beta synthesisArthritis Rheum4423203011665972
- KlesserBBiasPBuchnerA2002Licofelone (ML3000), an inhibitor of COX-1, COX-2 and 5-LOX, has little or no effect on the gastric mucosa after 4 weeks of treatmentAnn Rheum Dis61Suppl. 1121211796397
- KonttinenYTMandelinJLiTF2002Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritisArthritis Rheum469536011953972
- LarkMWBayneEKFlanaganJ1997Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid jointsJ Clin Invest100931069202061
- LauferSTriesSAugustinJ1995Acute and chronic anti-inflammatory properties of [2,2-dimethyl-6-(4-chlorophenyl)-7-phenyl-2, 3-dihydro-1H-pyrrolizine-5-yl]-acetic acidArzneimittelforschung4527327893265
- LauferSTriesSAugustinJ1994Pharmacological profile of a new pyrrolizine derivative inhibiting the enzymes cyclooxygenase and 5-lipoxygenaseArzneimittelforschung44629368024637
- LevalXJulemontFDelargeJ2002New trends in dual 5-LOX/COX inhibitionCurr Med Chem99416211966455
- MarcouillerPPelletierJPGuévremontM2005Leukotriene and prostaglandin synthesis pathways in osteoarthritic synovial membranes: regulating factors for IL-1beta synthesisJ Rheumatol327041215801029
- Martel-PelletierJLajeunesseDPelletierJP2004Etiopathogenesis of osteoarthritis. In: Koopman WJ (ed). Arthritis and allied conditions. A textbook of rheumatologyBaltimore: Lippincott, Williams & Wilkins2199226
- Martel-PelletierJLajeunesseDReboulP2003Therapeutic role of dual inhibitors of 5-LOX and COX, selective and nonselective non-steroidal anti-inflammatory drugsAnn Rheum Dis62501912759283
- Martel-PelletierJWelschDJPelletierJP2001Metalloproteases and inhibitors in arthritic diseasesWoolfADBaillière’s best practice and research clinical rheumatologyEast Sussex, UKBaillière Tindall80529
- MoreauMBoileauCMartel-PelletierJ2006Licofelone reduces progression of structural changes in a canine model of osteoarthritis under curative conditions: effect on protease expression and activityJ Rheumatol3311768316652435
- NagaseHKashiwagiM2003Aggrecanases and cartilage matrix degradationArthritis Res Ther59410312718749
- PalmoskiMJBrandtKD1983In vivo effect of aspirin on canine osteo-arthritic cartilageArthritis Rheum2699410016882492
- ParadesYMassicotteFPelletierJP2002Study of the role of leu-kotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibitionArthritis Rheum4618041212124864
- ParenteL2001Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: is two better then one?J Rheumatol28237538211708405
- PavelkaKBiasPBuchnerA2003Licofelone, an inhibitor of COX-1, COX-2 and 5-LOX, is as effective as celecoxib and shows improved tolerability during 12 weeks of treatment in patients with osteoarthritis of the kneeAnn Rheum Dis62Suppl. 126112594116
- PelletierJPDi BattistaJARaynoudJP1995The in vivo effects of intraarticular corticosteroid injections on cartilage lesions, stromelysin, interleukin-1 and oncogene protein synthesis in experimental osteoarthritisLab Invest72578867745952
- PelletierJPLajennesseDJovanovicDV2000Carprofen simultaneously reduces progression of morphological changes in cartilage and subchondral bone in experimental dog osteoarthritisJ Rheumatol27289390211128682
- PelletierJPMartel-PelletierJAbramsonSB2001Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targetsArthritis Rheum4412374711407681
- PelletierJPMartel-PelletierJ1991In vivo protective effects of prophylactic treatment with tiaprofenic acid or intraarticular corticosteroids on osteoarthritic lesions in the experimental dog modelJ Rheumatol2712730
- PretePEGurakar-OsborneA1997The contribution of synovial fluid lipoproteins to the chronic synovitis of rheumatoid arthritisProstaglandins54689989440132
- RainsfordKDYingCSmithF1996Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture; comparison with interleukin-1-synthesis inhibitorsJ Pharm Pharmacol4846528722494
- RainsfordKDYingCSmithF1996Effects of 5-lipoxygenase inhibitors on interleukin production by human synovial tissues in organ culture: comparison with interleukin-1-synthesis inhibitorsJ Pharm Pharmacol4846528722494
- ReginsterJYBiasPBuchnerA2002First clinical results of licofelone (ML3000), an inhibitor of COX-1, COX-2 and 5-LOX, for the treatment of osteoarhtritisAnn Rheum Dis61Suppl. 111617
- RotondoSDell’ElbaGKrauze BrzoskoK2002Licofelone, a dual lipoxygenase-cyclooxygenase inhibitor, downregulates polymorphonuclear leukocyte and plateletEur J Pharmacol453131912393068
- RotondoSKrauze BrzoskoKManariniS2004Licofelone, an inhibitor of cyclooxygenase and 5-lipoxygenase, specifically inhibits cyclooxygenase-1-dependent platelet activationEur J Pharmacol488798315044038
- SerhanCN2001Lipoxins and aspirin-triggered 15-epi-lipoxins are endogenous components of antiinflammation: emergence of the counter-regulatory sideArch Immunol Ther Exp4917788
- SinghVPPatilCSKulkarniSK2006Anti-inflammatory effect of licofelone against various inflammatory challengesFundam Clin Pharmacol20657116448396
- SipeJDBartleLMLooseLD1992Modification of proinflammatory cytokine production by the antirheumatic agents tenidap and naproxen: a possible correlate with clinical acute phase responseJ Immunol14848041729367
- SkellyMMHawkeyCJ2003COX–LOX inhibition: current evidence for an emerging new therapyInt J Clin Pract57301412800462
- WallaceJLCarterLMcKnightW1994ML 3000 reduces gastric prostaglandin synthesis without causing mucosal injuryEur J Pharmacol271525317705453
- WolfeFAndersonJBurkeTA2002Gastroprotective therapy and risk of gastrointestinal ulcers: risk reduction by COX-2 therapyJ Rheumatol294677311908558