Abstract
Fewer than 45% of women go through life with a full head of hair. Female pattern hair loss is the commonest cause of hair loss in women and prevalence increases with advancing age. Affected women may experience psychological distress and impaired social functioning. In most cases the diagnosis can be made clinically and the condition treated medically. While many women using oral antiandrogens and topical minoxidil will regrow some hair, early diagnosis and initiation of treatment is desirable as these treatments are more effective at arresting progression of hair loss than stimulating regrowth. Adjunctive nonpharmacological treatment modalities such as counseling, cosmetic camouflage and hair transplantation are important measures for some patients. The histology of female pattern hair loss is identical to that of male androgenetic alopecia. While the clinical pattern of the hair loss differs between men, the response to oral antiandrogens suggests that female pattern hair loss is an androgen dependant condition, at least in the majority of cases. Female pattern hair loss is a chronic progressive condition. All treatments need to be continued to maintain the effect. An initial therapeutic response often takes 12 or even 24 months. Given this delay, monitoring for treatment effect through clinical photography or standardized clinical severity scales is helpful.
Introduction
Female pattern hair loss (FPHL) has emerged as the preferred term for androgenetic alopecia in females owing to the uncertain relationship between androgens and this entity (CitationOlsen 2001). It is characterized by a reduction in hair density over the crown and frontal scalp with retention of the frontal hairline. In 1977, Ludwig clearly described the distinctive features of FPHL and classified it into three grades of severity referred to as Ludwig grades I, II, and III (; CitationLudwig 1977). The prevalence increases with age from approximately 12% amongst women aged between 20 and 29 years to over 50% of women over the age of 80 (CitationGan and Sinclair 2005). Hair loss in women is associated with significant psychological morbidity. CitationCash and colleagues (1993) suggested that women place a greater emphasis than men on physical appearances and outward attractiveness. Societal norms dictate that hair is an essential part of a woman’s sexuality and gender identity, and any hair loss generates feelings of low self-esteem and anxiety from a perception of diminished attractiveness. Women are more likely than men to have a lowered quality of life (CitationCash et al 1993), and to restrict social contacts (CitationVan Neste and Rushton 1997) as a result of hair loss. While men do consider androgenetic alopecia an unwanted and stressful event that diminishes their body image satisfaction (studies indicate 50% of men with mild hair loss and 75% with moderate to severe hair loss report concern [CitationCash 1992]), society tends to regard hair loss in men as expected and normal due to the greater visibility of hair loss within men.
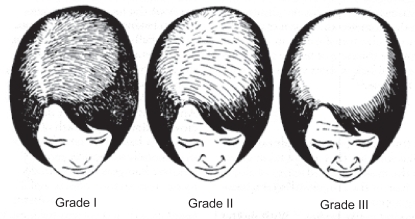
Figure 1 CitationLudwig scale (1977).
Grade I: Perceptible thinning of the hair on the crown, limited in the front by a line situated 1–3 cm behind the frontal hair line.
Grade II: Pronounced rarefaction of the hair on the crown within the area seen in Grade I.
Grade III: Full baldness (total denudation) within the area seen in Grades I and II.
Current management options are limited, and even in positive responders, there is a significant time delay before improvement becomes apparent. Regardless of which option is chosen, sufficient time should be spent counseling the patient. While some women are content to be reassured that their hair loss is not a manifestation of a serious disease, many are sufficiently concerned by the prospect of going bald to seek active treatment.
The two main pharmacological options are antiandrogens and minoxidil. Both treatments need to be continued indefinitely to maintain a response. Nonpharmacological methods may also be appropriate in individual cases, such as changing hairstyles, camouflaging products, and hair replacement (CitationCollins et al 2006).
Epidemiology
While similarities exist between FPHL and androgenetic alopecia in men, the susceptibility, age of onset, rate of progression, and pattern are different in the two sexes. Women are more conscious of subtle degrees of hair loss. Consequently, women often present with hair shedding prior to the development of reduction in hair volume over the crown.
Despite this, the age of onset of FPHL is later than that seen in men. Twelve percent of women first develop clinically detectable FPHL by age 29 years, 25% by age 49 years, 41% by 69 years, and over 50% have some element of FPHL by 79 years. Only 43% of women aged 80 years and above show no evidence of FPHL (CitationGan and Sinclair 2005).
While hair loss is common, severe hair loss as defined by Ludwig grade III (CitationLudwig 1977) or Sinclair grade 5 (CitationCollins et al 2006; CitationYip and Sinclair 2006) () is uncommon and affects less than 1% of women. Severe bitemporal recession as observed in male androgenetic alopecia is also uncommon in women in which the frontal hairline is usually preserved.
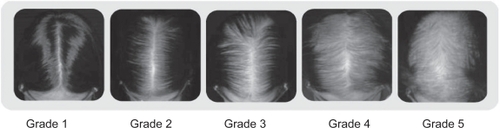
Figure 2 Sinclair Scale (CitationCollins et al 2006; CitationYip and Sinclair 2006).
Grade 1: is normal. This pattern is found in all girls prior to puberty but in only forty-five percent of women aged eighty or over.
Grade 2: shows a widening of the central part.
Grade 3: shows a widening of the central part and thinning of the hair on either side of the central part.
Grade 4: reveals the emergence of a diffuse hair loss over the top of the scalp.
Grade 5: indicates advanced hair loss.
Pathology and pathogenesis of FPHL
The histological hallmark common to male pattern hair loss (MPHL) and FPHL is miniaturization of hair follicles with a progressive transformation of terminal hair follicles into vellus-like follicles. Terminal hair follicles have a shaft diameter of greater than 0.06 mm, whereas vellus-like follicles are defined as hairs with a hair shaft diameter of 0.03 mm or less and are thinner than the hair’s inner root sheath. In addition, women with FPHL have more follicles in the telogen or shedding phase of the hair cycle, and fewer in the anagen or growth phase (CitationHeadington 1984).
The pathogenesis of MPHL involves activation of hair follicle cytoplasmic androgen receptors (AR) (CitationDeplewski and Rosenfield 2000). Both testosterone and dihydrotestosterone (DHT) activate the AR, however DHT binds 5 times more avidly than testosterone (CitationGrino et al 1990) and DHT is thought to be the principle androgen involved in MPHL. Testosterone is converted into DHT by 5α-reductase (CitationBartsch et al 2000), and DHT in turn is converted into estrogen by the cytochrome P450 enzyme aromatase (CitationSawaya and Price 1997). Two isoforms of 5α-reductase have been identified and separated on pH optima, substrate affinity, and tissue distribution (CitationJenkins et al 1992). The type 2 isoform is found in the dermal papilla of the hair follicle and inhibition of this isoenzyme with finasteride is the principle treatment for MPHL (CitationKaufman et al 1998).
The histology of FPHL is indistinguishable for that seen in MPHL. While the role of androgens in the pathogenesis of MPHL has been clearly established, the role of androgens in FPHL is less clear. There are some women with FPHL who do not have elevated androgen levels and other androgen-independent mechanisms are likely to be involved in the development of FPHL (CitationOrme et al 1999). This may explain why post-menopausal women respond to finasteride less well than men. The pattern of hair loss seen in women is materially different to men. Bitemporal recession is less pronounced and vertex bald spots are almost never seen.
The lesser importance of androgen hormone activity in FPHL may be explained by the finding that, compared with men, women have lower circulating androgen levels, lower AR concentration in scalp skin, a lower concentration of 5α-reductase enzymes and a concentration of aromatase, which functions to deactivate local androgens through conversion to estrogens (CitationSinclair and Dawber 2001).
Clinical features, diagnosis, and natural history
Clinical features
The essential feature of FPHL is the pattern of the hair loss. Women develop diffuse thinning over the mid-frontal scalp with relative sparing of the anterior hair line. The thinning is most easily seen when the hair is parted in the midline, and the exposed scalp may resemble a Christmas tree.
Hair loss may begin at any age after the onset of adrenarche and may precede pubarche and menarche. FPHL may present initially with either episodic or continuous hair shedding, prior to any noticeable reduction in hair volume. Alternatively some women present with diffuse thinning over the crown unaware of any increase in hair shedding (CitationSinclair and Dawber 2001).
Examination of the scalp shows a widening of the central part with a diffuse reduction in hair density over the frontal scalp rather than baldness per se. Although these areas show the most marked reduction in hair density, there is usually some evidence of global reduction in hair density throughout the scalp. As a result, many women are not suitable for hair transplantation due to poor donor hair population on the occipital scalp (CitationOlsen 1994) Typically the frontal hairline is preserved although some women also develop bitemporal recession. Bitemporal recession is usually mild, starting postpubertally, and may run a course independent of mid-frontal hair loss.
Traditionally, FPHL severity has been graded using the Ludwig scale mentioned above (), which divides the severity of hair density reduction over the crown into three grades (CitationLudwig 1977). More recently, we developed a 5 point visual analogue scale (the Sinclair Scale; CitationCollins et al 2006; CitationYip and Sinclair 2006) which assesses the degree of hair loss using the midline part (). This is a simplification of the widely accepted CitationSavin (1994) density scale (), which classifies FPHL into 8 stages of increasing crown balding, in addition to a special subcategory to detect frontal anterior recession.
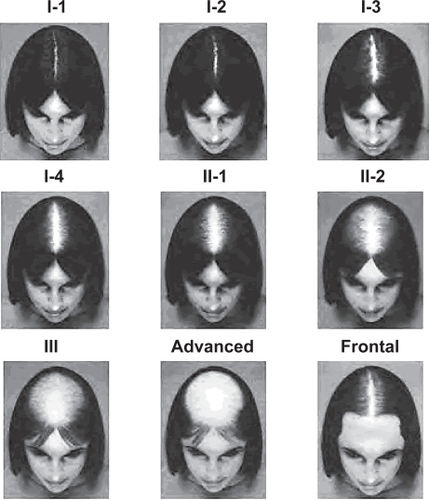
Figure 3 Savin Scale (CitationSavin 1994).
The Savin scale measures overall thinning of the crown scalp, and consists of 8 crown density images reflecting a range from no hair loss to severe hair loss (Stages I-1, I-2, I-3, I-4, II-1, II-2, III, advanced). The ninth and final image in the scale demonstrates frontal anterior recession.
Diagnosis
Increased hair shedding is common in the early stages of FPHL. When women present with increased hair shedding, but little or no reduction in hair volume over the mid-frontal scalp, various differential diagnoses should be considered, in particular acute and chronic telogen effluvium. Acute telogen effluvium is a self-limiting event, often triggered by physical illness, surgery, blood loss, or crash dieting. Chronic telogen effluvium (CTE) can be secondary to thyroid disease, systemic lupus, drug ingestion (; CitationSinclair and Dawber 2001) and iron deficiency anemia. Iron deficiency without anemia is not thought to cause hair shedding (CitationTrost et al 2006). CTE also occurs as a primary idiopathic event. Idiopathic CTE is characterized by excessive shedding of hair for at least 6 months without noticeable widening of the central parting. Other causes for diffuse hair shedding in women are included in (CitationSinclair and Dawber 2001).
Table 1 Drug-induced alopecia (CitationSinclair and Dawber 2001)
Table 2 Causes of alopecia in the female (CitationSinclair and Dawber 2001)
Scalp biopsy is the best way to distinguish between CTE and FPHL (CitationSinclair et al 2004). The importance in accurately distinguishing between these two entities lies in the differing natural histories, with CTE generally running a fluctuating course but ultimately not leading to balding (CitationSinclair 2005), whereas FPHL without treatment invariably leads to a progressive reduction in hair volume over the frontal scalp.
If a biopsy is required, the most information is gained from horizontal sectioning of the tissue and calculation of the terminal to vellus hair ratio. A ratio of <4:1 is considered diagnostic of FPHL while a ratio of >8:1 is considered diagnostic of CTE. Ratios of 5:1, 6:1, and 7:1 are considered indeterminate. Multiple scalp biopsies reduce the risk of an indeterminate result or of underestimating FPHL. Underestimation of FPHL can occur with a single biopsy as FPHL is a diffuse process and the most common biopsy taken is 4 mm in diameter.
In one study where diagnostic accuracy using a single scalp biopsy was compared with 3 adjacent scalp biopsies, 21% of single biopsy specimens were indeterminate compared with 2% in the triple biopsy group (CitationSinclair et al 2004). Given that the need for local anesthetic, the time to do the procedure, and the morbidity for the patient are not increased when three immediately adjacent scalp biopsies are performed, the authors recommend triple biopsy routinely.
While some authors have found a hyperandrogenic state underlying FPHL in up to 40% of women (CitationFutterweit et al 1988), fewer than 10% of our patients, who had been primarily referred for assessment of their hair loss and found to have FPHL and hair follicle miniaturization on biopsy had evidence of virilization (CitationSinclair et al 2005). The presence of menstrual irregularity or marked acne or hirsutism in a woman presenting with FPHL should prompt the physician to investigate for an underlying cause, in particular, polycystic ovary syndrome (PCOS). Rarely, virilizing tumors can cause hyperandrogenism with a recent onset of and rapidly progressive and severe hair loss from the scalp.
Suitable screening investigations in women suspected of hyperandrogenism include serum estimation of androgens, prolactin, FSH, LH, 17-hydroxyprogesterone, and estrogen as well as screening tests for fasting hyperglycemia and hyperlipidemia.
Even when there is no clinical evidence of hyperandrogenism, biochemical hyperandrogenism may be present. CitationFutterweit and colleagues (1988) have found that around 25% of women with diffuse alopecia and biochemical hyperandrogenism may have no clinical androgen excess. The clinical relevance of these biochemical findings is uncertain. Some case series and trials have suggested that efficacy of antiandrogen medications at selected doses may be influenced by the biochemical androgen state of the patient (CitationBurke et al 1985; CitationShum et al 2002; CitationThai et al 2002; CitationVexiau et al 2002; CitationTrüeb 2004).
The clinical assessment of the woman with hair loss should incorporate a thorough history including a detailed drug history as well as a general examination for features of hyperandrogenism. It is also useful to enquire whether the patient intends to become pregnant in the near future as antiandrogen drugs are potentially teratogenic. Not all patients need screening blood tests. It would seem reasonable to assess women for thyroid disease, iron deficiency anemia, and consider endocrinological screening for women suspected of hyperandrogenism.
Natural history
Without treatment, FPHL is a progressive condition; however, the rate of its progression is highly variable. Hair shedding is often episodic. Women commonly notice increased hair loss in the shower or in the brush each day for 2–3 months before the shedding returns to normal levels. 3–12 months may elapse before the shedding returns. The condition’s unpredictability and relentless progression contributes greatly to the mental distress of affected women.
Psychological morbidity
Hair loss in women produces greater psychological distress than in men. In a 1993 Glamour magazine survey, over half of the women stated “if my hair looks good, I look attractive no matter what I’m wearing or how I look otherwise,” and “if my hair isn’t right, nothing else can make me feel that I look good” (CitationEtcoff 1999). A women’s hair is central to her femininity, beauty, and sexuality.
While hair loss can be distressing for males, a social acceptance and understanding of this phenomenon generally allows normal psychosocial functioning. In contrast, FPHL is not expected and less understood by society generating feelings of confusion and distress for the woman. A study has shown that 52% of women were very-to-extremely upset by their hair loss, compared with 28% of men (CitationCash 1992; CitationCash et al 1993). This distress results in lower self-esteem, a poor body image, feelings of guilt, problems with sleep and day-to-day function, and restriction of social activities.
After the initial shock of diagnosis, most women adopt a variety of coping mechanisms (CitationCash 2001). “Compensation” refers to efforts to offset the hair loss with other physical improvements such as greater attention to dress in order to create positive body images. “Concealment” of hair loss aims to avoid associated negative body-image feelings. Women may want to avoid the negative reactions from family, friends, and even strangers, and may wear hats or wigs to achieve this. Thirdly, compulsive activities of reassurance designed to minimize negative body-image feelings such as excessive checking or fixing of one’s hair in front of the mirror may occur.
Clearly, the psychological distress resulting from FPHL is an integral part of disease entity and needs to be addressed in any management regime.
Management
Treatment options
Pharmacological
Pharmacological options can be divided into drugs with androgen-independent and androgen-dependent mechanisms of action.
Androgen-independent medications
Minoxidil
Currently the only androgen-independent medication in widespread use is minoxidil topical solution. This medication’s proposed mechanism of action is by affecting hair cycling, causing premature termination of telogen, and probably prolonging anagen (CitationMessenger and Rundegren 2004). Understanding exactly how minoxidil exerts these effects is currently the subject of intense research. Although this is available in both 2% and 5% preparations, only the 2% solution is currently FDA-approved for FPHL (CitationDeVillez et al 1994). A study comparing the efficacy of the two concentrations using target area hair counts at 48 weeks as a primary endpoint showed a mild nonsignificant advantage for the 5% solution (CitationOlsen et al 2002).
Usually 1 ml of minoxidil is applied twice daily to dry scalp with a dropper. This is left for 1 hour before shampooing or wetting of the hair is allowed to maximize medication absorption. Patients should be warned that in the initial 2–8 weeks, a temporary telogen effluvium may occur, which is self-limiting and subsides when subsequent anagen regrowth begins, and should not be a cause for treatment cessation. Common adverse effects of minoxidil include scalp irritation including dryness, scaling, itching, and/or redness (CitationFriedman et al 2002). Specific to its use in FPHL, hypertrichosis may occur, primarily on the cheeks and forehead. If this occurs, it generally disappears within 4 months of ceasing the medication. Treatment should be continued for at least 12 months before an accurate appraisal of efficacy can be made.
Recently, it has been found that most cases (82%) of scalp irritation after use of topical minoxidil results from a contact dermatitis to propylene glycol, one of the vehicles of the solution, rather than the minoxidil itself (CitationLeffek and Herrick 2006). There is emerging data on a new foam vehicle for minoxidil which has significantly reduced levels of propylene glycol indicating at least equivalent efficacy and increased preference by subjects due to the lack of the sticky residue associated with current formulations (CitationLucky et al 2004).
Androgen-dependent medications
The use of all androgen-dependent medications to treat FPHL carries a risk of causing abnormalities in the genitalia of the male fetus. Thus, these medications are contraindicated in women who are pregnant, which leads many physicians to recommend that women start and remain on an oral contraceptive pill throughout their course of treatment with these medications. As with minoxidil, all androgen-dependent medications need to be continued for at least 1 year before an accurate appraisal of efficacy can be made.
Finasteride
Finasteride works by inhibiting 5α-reductase II enzyme, which is responsible for catalyzing the conversion of testosterone to the much more active chemical 5 DHT. Thus, finasteride suppresses overall androgen activity by restricting total circulating androgen activity. Large scale studies on its efficacy are currently limited, with one large multicenter randomized placebo-controlled trial failing to find any change in hair growth or progression of hair loss with finasteride 1 mg/day in postmenopausal women with FPHL over a 1 year follow-up period (CitationPrice et al 2000). However, smaller case reports and case series have demonstrated efficacy for finasteride 1.25 mg/day in 4 cases of FPHL in both pre- and post-menopausal women with hyperandrogenism (CitationShum et al 2002), finasteride 2.5 mg/day in 5 cases of FPHL in post-menopausal women without hyperandrogenism (CitationTrüeb 2004), and finasteride 5 mg/ day in 1 case of FPHL in a post-menopausal woman without hyperandrogenism (CitationThai et al 2002). Finasteride does appear to be successful in some patients, but further large scale studies are required to determine optimal dosing regimes.
Finasteride is generally well tolerated. Infrequent side effects in female patients include breast tenderness and increased libido, which are most common in the first year of therapy, reversible on dose reduction, and tend to diminish over time with continued use.
Cyproterone acetate
This medication inhibits gonadotropin-releasing hormone (GnRH) and blocks androgen receptors. Other uses include prostate cancer, hirsutism, and severe acne. In Australia, it is commonly used combined with estradiol as an oral contraceptive pill named Diane-35.
A number of studies suggest a role for cyproterone acetate, with some suggesting that this role is greater in patients with evidence of hyperandrogenism. One moderately large study of 25 subjects aged between 31 and 35 years with FPHL showed that Diane-35 used for 6–9 months clearly decreased the loss of hair, hair thinning, and seborrhea CitationBrzezinska-Weislo 2003). Another study compared 8 control patients with FPHL receiving placebo treatment with 20 patients with FPHL treated with 50 μg ethinyloestradiol plus 2 mg cyproterone acetate daily with additional 20 mg cyproterone acetate on days 5–20 of the menstrual cycle. The authors found that patients receiving active treatment enjoyed reduced hair shedding without actual increased hair regrowth (CitationLucky et al 2004). The best study investigating cyproterone actetate was a 12-month randomized trial to compare the use of topical minoxidil 2% and cyproterone acetate in 66 women with FPHL. These authors found that minoxidil 2% was more effective in women without evidence of hyperandrogenism, whereas cyproterone acetate was more effective in those with signs of hyperandrogenism (CitationVexiau et al 2002). Comparison of two antiandrogen medications (ie, spironolactone and cyproterone acetate) was undertaken in another study involving 80 women with biopsy confirmed FPHL. The authors found that overall, 88% of women either experienced improvement or had no progression of their hair loss with no significant difference in efficacy between individual medications (CitationFutterweit et al 1988).
In contrast, there is only one significant randomized study which has shown no benefit for cyproterone acetate. This study compared flutamide, finasteride, and cyproterone acetate in 36 hyperandrogenic women and failed to show a significant response for cyproterone acetate (as well as fin-asteride) (CitationCarmina and Lobo 2003). It would appear that the balance of evidence supports a role for cyproterone acetate in FPHL. Further research would need to be done to clarify whether this medication has a greater role in those patients with evidence of hyperandrogenism.
Treatment doses utilized vary but it appears the most effective is 100 mg/day on days 5–15 of the menstrual cycle supplemented by 50 μg ethinyl estradiol on days 5–25 (CitationDawber et al 1982). The side effect profile includes menstrual disturbances, weight gain, loss of libido, depression, breast tenderness, and gastrointestinal upsets.
Spironolactone
Spironolactone is used widely to treat FPHL and hirsutism. It acts as an androgen antagonist by competitively blocking androgen receptors, as well as inhibiting ovarian androgen production (CitationShaw 1996). In the USA it is the most widely used antiandrogen to treat FPHL. The usual daily dose is 100–200 mg. Published studies supporting efficacy are limited but as mentioned above, a recent open intervention study concluded that spironolactone 200 mg/day was equally effective in either restoring hair growth or preventing further progression of hair loss compared with cyproterone acetate at a dose of either 50 mg/day or 100 mg/day given for 10 days every menstrual cycle (CitationSinclair et al 2005).
The side effect profile of spironolactone is perhaps more varied compared with other medications, due partly to its additional actions as an aldosterone antagonist. These include postural hypotension, electrolyte disturbances, menstrual irregularities, fatigue, urticaria, breast tenderness, and hematological disturbances. Because of these known side effects, it is recommended that women have regular blood pressure and electrolyte monitoring, especially in the first few months of treatment. Further caution with its use needs to be exercised in the patient with renal abnormalities since it can potentially cause serious electrolyte disturbances.
Flutamide
Flutamide is a potent antiandrogen, acting via androgen receptor antagonism. As such, it is commonly used to treat advanced prostate cancer and hirsutism. Being one of the newer antiandrogens, there is limited medical literature on its use in FPHL. One randomized study suggested that flutamide at a dose of 250 mg/day could lead to greater improvements in stemming hair loss after 1 year of treatment compared with finasteride and cyproterone acetate (CitationCarmin and Lobo 2003). Another randomized controlled trial found a significant treatment advantage for flutamide over spironolactone in the treatment of hirsutism, the reduction of total acne and seborrhea, and the slowing or halting of hair loss (CitationSinclair et al 2005).
Adverse effects due to flutamide are potentially severe. These include hepatic dysfunction and breast tenderness, both of which are dose-related. The rate of hepatotoxicity has been estimated at 3 in 10,000 users (CitationSinclair et al 2005). It is recommended that serial liver transaminases are monitored in the first few months of treatment, and that flutamide be stopped or not commenced if transaminase levels exceed twice the normal limits.
Cosmetic aids (CitationCollins et al 2006)
Since so much of the morbidity of FPHL lies in body image disturbances, cosmetic aids are an integral part of management options. These incorporate hair styling techniques, camouflage products, hair replacements, hair accessories, and additions.
Hairstyling
Different hairstyling options can be canvassed by a woman’s individual hair stylist, and it may take 3 or 4 different haircuts before a woman finds her optimal look. Some useful tips applicable in the woman with FPHL include: getting greater hair volume and lift from shorter styles; disguising central thinness by a side or zigzagging part; adding soft layers at the top to allow pulled back hair to have more style; taking some weight off the top; using wide-toothed combs or brushes to allow thin hair to flow through without breakage; using a friction-free towel that blots hair and absorbs most of the moisture rather than damaging thin hair with towel drying; coloring hair to make hair look thicker and appear to give it more volume; styling with a body wave with soft or tight curls to give hair more bounce and fullness; and using hair sprays and foams that are light and nondrying to give volume and prevent breakage.
Camouflaging products
Camouflaging products cover exposed areas on the scalp and hide visible hair loss. They also provide lift at the base of the hair shaft, which adds volume. These products are best suited to those women with mild to moderate hair loss, whereas those with more severe hair loss may not achieve a natural appearance after application. Most camouflaging products do not rub off easily, and are resistant to stresses such as perspiration, exercising, and swimming. Removal can be achieved simply by shampooing. In addition, camouflaging products are compatible with topical minoxidil.
The most commonly used products include hair building fibers, scalp spray thickeners, alopecia masking lotion, and topical shading. Hair building fibers are keratin fibers available in a range of natural hair colors. They come in a jar with a pepper-shaker type top. A gentle shake of this jar onto the thinning areas of the scalp creates density throughout the area, reducing the appearance of thinning hair, which begins to work in about 30 seconds. Scalp spray thickeners cover thinning areas by bonding fibers to hair to create density and add color. Although effective, they can be messy to use, so care needs to be exercised to minimize staining of clothes and fingers. Alopecia masking lotion is a tinted lotion that is dabbed onto thinning areas of the scalp to create the 1 of fuller hair. It is manufactured in a tube with a special applicator. One advantage is that it is not sticky or greasy thus will not stain clothing. One tube usually lasts 3–4 months. Topical shading is a tinted pressed powder that is used to cover the scalp in thinning areas and coat hair strands to create a fuller look. Application involves dabbing the powder onto the scalp by using a sponge-tipped applicator.
Different types of scalp skin respond differentially to different types of camouflaging. In general, for oily to normal skin on the scalp, use of a pressed powder allows good absorption and prevents a shiny scalp. For dry scalp skin, a cream prevents further dryness. Because fibers need hair to bind to, individuals with advanced hair loss generally do better with sprays and creams.
Hair replacement
In addition to measures described above, hair extensions can be useful for women with mild hair loss who simply desire more length and volume. These can be clipped on daily or attached permanently. However, potential damage may occur if they are applied to hair that breaks easily, so selection of appropriate hair is paramount.
For those women with moderate to severe hair loss, hair-styling and camouflaging is often not enough. An integration, hairpiece, or wig may be better options.
An integration is made of fabric or skin-like material with replacement hair attached to it and gaps through which native hair can be pulled through. By blending integrations with natural hair from thinning areas, increased volume of hair is created, which creates the appearance of a full head of hair. However, if it is worn for extended periods, it can cause scalp irritation, as well as stress to existing hair, resulting in damage and hair breakage.
For more extensive hair loss that is too fragile to withstand integrations, hairpieces or wigs may be used. Hairpieces contain skin-like breathable material and hold the attached hair securely in position. They are attached to the scalp by either adhesive tape or alternatively hair clips for ease of removal at the end of the day. They can be matched to existing scalp tone, hair texture, and color providing as natural a look as possible. They are robust and can be worn 24 hours a day.
Wigs are for the advanced stages of hair loss. A good quality, well-fitted wig allows unrestricted daily activities, including working out at the gym, swimming, or going out on a windy day. Current manufacturing offers a wide variety of sizes, styles, texture and colors, the choice of being custom-made or machine-made, being made of synthetic or human fibers, and containing a polyester, polyurethane, nylon, or silicone-derived wig base, all of which provide subtle differences tailored to the personal preferences of the individual.
Hair accessories and additions
Fashion accessories can satisfactorily conceal localized or diffuse patches of hair loss and include hats, scarves, bandanas, and turbans. Hair additions and accessories include combs and headbands with hair attached to them, ponytails that easily attach to existing hair, and hair scrunchies.
Surgical treatments
Hair transplantation
Ideal surgical candidates for hair transplantation are those women with high hair-density in the donor site over the occipital scalp and extensive hair loss or thinning of the frontal scalp (CitationOlsen et al 2005). Patients should be encouraged to develop realistic expectations of the potential results achievable. Technical details of transplantation are beyond the scope of this paper, however, in brief, the procedure is done under local anesthetic whereby 1 session would involve the transplantation of 800–1200 grafts. In most women, 1–3 sessions are undertaken depending on the degree of hair loss and the availability of donor sites (CitationUnger and Unger 2003). Each session is undertaken approximately 6 months apart since results can take up to 6 months after a procedure to become evident. Local complications of the procedure include facial edema, scalp erythema, crusting, post-operative bleeding, infection, swelling, temporary headaches, temporary numbing of the scalp, and abnormal scarring of the graft (CitationUnger and Unger 2003). Occasionally with densely packed grafts, temporary telogen effluvium may occur for a few weeks after the procedure. There is some evidence that this can be reliably prevented by the use of a topical minoxidil solution applied twice daily to the recipient and potential donor areas for 1 week before and 5 weeks after operation (CitationAvram et al 2002; CitationUremia et al 2002). Often the option of hair transplantation in FPHL is overlooked, and is not favored as much as in male androgenetic alopecia, but expert opinion suggests that many women can expect results that are at least as good as those seen in transplanting for early male androgenetic alopecia (CitationUnger and Unger 2003).
Scalp reduction
This option is less popular compared with hair transplantation. It involves bringing hair-bearing skin closer together by removing the central scalp affected by the alopecia. Sometimes it is performed in conjunction with hair transplantation to optimize cosmetic outcomes. Disadvantages of this procedure increase with time and include diminishing efficacy due to the unpredictability of subsequent individual hair loss, increasing cosmetic visibility of excision scars, potential gradual widening of scars due to stretching of adjacent scalp skin, and the usual need for more than one scalp reduction to effectively address hair loss (CitationUnger and Unger 2003).
Experimental
Dutasteride
Dutasteride is a more potent 5α-reductase inhibitor than finasteride, working by inhibiting both type I and type II 5α-reductase. Whereas type II 5α-reductase concentrations are much higher than those of type I 5α-reductase, the additional inhibitory effect on androgen activity produced by dutasteride may translate into greater clinical efficacy. It is commonly used to treat benign prostatic hypertrophy but is not approved by the US FDA for the treatment of either male or female androgenetic alopecia. Significant issues with teratogenicity arise from the long biological half life (months), and this drug is best avoided in women who may become pregnant.
Phase 1 and 2 trials conducted exclusively in men demonstrated that at a dose of 2.5–5.0 mg/day, dutasteride suppresses almost 100% of serum DHT activity, whereas finasteride at a dose of 5 mg/day suppresses only 70% of DHT. Phase 2 preclinical trials showed that after 6 months of treatment, there was a 30% increased improvement in hair count when comparing 0.5 mg of dutasteride with 5 mg of finasteride (CitationGSK 2006).
Future agents
Current gene discovery research may identify a number of novel genes that regulate hair growth, hair cycling, and the hormone-induced changes seen at puberty in the near future. This research may lead to topically delivered therapy, targeting critical pathways to stimulate hair growth. If these agents can be incorporated into hair follicle cells, permanent alteration of hair growth, and resultant reliable re-growth of hair may be achieved.
Monitoring clinical response
Monitoring clinical response is an often under-emphasized aspect of management of FPHL. Its importance is underlined by the extended durations of treatment required to achieve often very subtle treatment responses. One practical way of monitoring response is by encouraging periodical scalp photographs preferably by a trained medical photographer before treatment and every 6 months thereafter thus allowing subtle improvements to be easily observed. Less reliable, but logistically easier methods include self-counting the number of hairs lost each day; however, accuracy of this method may be limited by fluctuations in hair loss amongst different seasons, weeks, and even days (CitationSinclair et al 2000). Another way is to temporarily suspend drug treatment after 12 months use and observe for an increased amount of hair shedding 4–6 weeks later, indicating successful treatment. Aside from giving evidence of tangible results to both patient and physician, feedback of positive treatment results improves ongoing compliance of the patient with treatment.
Conclusion
FPHL is an under-recognized entity. Significant hair loss is seen in over ¼ of females over the age of 50. Satisfactory management of this condition requires a knowledge of possible underlying causes, physical comorbidities, possible differential diagnoses, and the various therapeutic modalities available. It also requires an appreciation of the potential psychological effect of hair loss on affected individuals, and sensitivity during patient consultations.
The condition is progressive without treatment. Current pharmacological treatment stems further progression and can also stimulate partial regrowth.
Regardless of which medication is utilized, response is slow, and requires patience and persistence in both patient and clinician. The cosmetic effects of this condition should not be underestimated, thus cosmetic aids and surgical options are both important adjunctive options that need to be discussed with these patients in addition to pharmacotherapy.
References
- AvramMRColeJPGandelmanM2002The potential role of min-oxidil in the hair transplanting settingJ Dermatol Surg28894900
- BartschGRittmasterRSKlockerH2000Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasiaEur Urol373678010765065
- Brzezinska-WcisloL2003Assessment of efficacy of Diane-35 in androgenetic feminine alopeciaWiad Lek56202512923971
- BurkeBMCunliffeWJ1985Oral spironolactone therapy for female patients with acne, hirsutism or androgenic alopeciaBr J Dermatol11212453155956
- CarminaELoboRA2003Treatment of hyperandrogenic alopecia in womenFertil Steril7991512524069
- CashTFPriceVHSavinRC1993Psychological effects of androgenetic alopecia on women: Comparisons with balding men and with female control subjectsJ Am Acad Dermatol29568758408792
- CashTF1992The psychological effects of androgenetic alopecia in menJ Am Acad Dermatol2696231
- CashTF2001The psychology of hair loss and its implications for patient careClin Dermatol19161611397595
- CollinsFBiondoSSinclairR2006Bad hair dayMelbourne, AustraliaLothan Books
- DawberRPSonnexTRalfsI1982Oral antiandrogen treatment of. common baldness in womenBr J Dermatol107Suppl2021
- DeplewskiDRosenfieldRL2000Role of hormones in pilosebaceous unit developmentEndocrine Rev213639210950157
- DeVillezRIJacobsJPSzpunarCA1994Androgenetic alopecia in the female: Treatment with 2% topical minoxidil solutionArch Dermatol13030378129407
- EtcoffN1999Survival of the prettiest: The science of beautyNew YorkDoubleday
- FriedmanESFriedmanPMCohenDE2002Allergic contact dermatitis to topical minoxidil solution: etiology and treatmentJ Am Acad Dermatol463091211807448
- FutterweitWDunaifAYehH-C1988The prevalence of hyperandrogenism in 109 consecutive female patients with diffuse alopeciaJ Am Acad Dermatol1983163192772
- GanDCSinclairRD2005Prevalence of male and female pattern hair loss in MaryboroughJ Investig Dermatol Symp Proc101849
- [GSK] Glaxo Smith Kline2006Glaxo Smith Kline Clinical Trial Register Accessed August 8th, 2006. URL: http://ctr.gsk.co.uk/Summary/dutasteride/studylist.asp.
- GrinoPBGriffinJEWilsonJD1990Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosteroneEndocrinology1261165722298157
- HeadingtonJT1984Transverse microscopic anatomy of the human scalpArch Dermatol120449566703750
- JenkinsEPAnderssonSImperato-McGinleyJ1992Genetic and pharmacological evidence for more than one human steroid 5-alpha-reductaseJ Clin Invest892933001345916
- KaufmanKDOlsenEAWhitingD1998Finasteride in the treatment of men with androgenetic alopeciaJ Am Acad Dermatol39578899777765
- LeffelDJHerrickCThe Dermatology Foundation2006 Dermatology Focus Summer 2006 [online] Accessed November 10th, 2006 URL: http://dermatologyfoundation.org/pdf/pubs/DF_Summer_2006.pdf.
- LuckyAWPiacquadioDJDitreCM2004A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair lossJ Am Acad Dermatol505415315034503
- LudwigE1977Classification of the types of androgenetic alopecia (common baldness) occurring in the female sexBr J Dermatol9724754921894
- MessengerAGRundegrenJ2004Minoxidil: mechanisms of action on hair growthBr J Dermatol1501869414996087
- OlsenE1994Androgenetic alopeciaOlsenEDisorders of hair growthNew YorkMcGraw-Hill25784
- OlsenEADunlapFEFunicellaT2002A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in menJ Am Acad Dermatol473778512196747
- OlsenEAMessengerAGShapiroJ2005Evaluation and treatment of male and female pattern hair lossJ Am Acad Dermatol523011115692478
- OlsenEA2001Female pattern hair lossJ Am Acad Dermatol45S708011511856
- OrmeSCullenDRMessengerAG1999Diffuse female hair loss: are androgens necessary?Br J Dermatol141521310583059
- Peereboom-WyniaJDvan der WilligenAHvan JoostT1989The effect of cyproterone acetate on hair roots and hair shaft diameter in androgenetic alopecia in femalesActa Derm Venereol6939582572103
- PriceVHRobertsJLHordinskyM2000Lack of efficacy of finasteride in postmenopausal women with androgenetic alopeciaJ Am Acad Dermatol437687611050579
- SavinRC1994Evaluating androgenetic alopecia in male and female patientsKalamazoo, MIThe Upjohn Company
- SawayaMEPriceVH1997Different levels of 5alpha-reductase type I and II aromatase and androgen receptor in hair follicles of women and men with androgenetic alopeciaJ Invest Dermatol1092963009284093
- ShawJC1996Antiandrogen therapy in dermatologyInt J Dermatol35770768915726
- ShumKWCullenDRMessengerAG2002Hair loss in women with hyperandrogenism: four cases responding to finasterideJ Am Acad Dermatol47733912399766
- SinclairRCargnelloJCallanA2000Understanding Common Baldness in Women Australian Hair and Wool Research Society [online]. Accessed on September 8th, 2006. URL: http://www.ahwrs.org.au/alopecia/CB.nsf/b331dafa1d45b4a36a2568100080f30e/a0f27d129ba111ceca2568b800051120/$FILE/redbaldness+in+women.PDF.
- SinclairRJolleyDMallariR2004The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in womenJ Am Acad Dermatol511899915280836
- SinclairRWewerinkeMJolleyD2005Treatment of female pattern hair loss with oral antiandrogensBr J Dermatol1524667315787815
- SinclairR2005Chronic telogen effluvium: a study of 5 patients over 7 yearsJ Am Acad Dermatol52S1216
- SinclairRDDawberRPR2001Androgenetic alopecia in men and womenClin Dermatol191677811397596
- ThaiKESinclairRD2002Finasteride for female androgenetic alopeciaBr J Dermatol1478121312366441
- TrostLBBergfeldWFCalogerasE2006The diagnosis and treatment of iron deficiency and its potential relationship to hair lossJ Am Acad Dermatol548244416635664
- TrüebRM2004Finasteride treatment of patterned hair loss in normoandrogenic postmenopausal womenDermatology209202715459533
- UngerWPUngerRH2003Hair transplanting: an important but often forgotten treatment for female pattern hair lossJ Am Acad Dermatol498536014576664
- UremiaSUmarSHLiCH2002Prevention of temporal alopecia following rhytidectomy: the prophylactic use of minoxidil: a study of 60 patientsDermatol Surg16674
- Van NesteDJRushtonDH1997Hair problems in womenClin Dermatol15113259034660
- VexiauPChaspouxCBoudouP2002Effects of minoxidil 2% vs. cyproterone acetate treatment on female androgenetic alopecia: a controlled, 12-month randomized trialBr J Dermatol146992912072067
- YipYSinclairRD2006Antiandrogen therapy for androgenetic alopeciaExp Rev Dermatol12619