Abstract
COPD is a progressive illness with worldwide impact. Patients invariably reach a point at which they require palliative interventions. Dyspnea is the most distressing symptom experienced by these patients; when not relieved by traditional COPD management strategies it is termed “refractory dyspnea” and palliative approaches are required. The focus of care shifts from prolonging survival to reducing symptoms, increasing function, and improving quality of life. Numerous pharmacological and non-pharmacological interventions can achieve these goals, though evidence supporting their use is variable. This review provides a summary of the options for the management of refractory dyspnea in COPD, outlining currently available evidence and highlighting areas for further investigation. Topics include oxygen, opioids, psychotropic drugs, inhaled furosemide, Heliox, rehabilitation, nutrition, psychosocial support, breathing techniques, and breathlessness clinics.
Introduction
COPD is an international health problem with a worldwide prevalence of at least 9.34/1000 in men and 7.33/1000 in women (1990 estimate) (CitationMurray and Lopez 1996a, Citation1996b). It is the fourth leading cause of death worldwide (CitationWHO 2000). The burden of COPD is expected to increase, such that by 2020, it will be the fifth leading cause of lost disability-adjusted life years worldwide (CitationMurray and Lopez 1996a, Citation1996b).
The 2001 Global Strategy for the Diagnosis, Management, and Prevention of COPD (GOLD) guidelines recommend a step-wise approach to care as lung function declines and patients become more symptomatic with the addition of therapeutic interventions including bronchodilators (short- and long-acting), anticholinergic agents, pulmonary rehabilitation, inhaled glucocorticoids, and oxygen (CitationPauwels et al 2001). While this strategy has improved management, the only intervention that slows progression of disease is smoking cessation, as has been documented in the Lung Health Study I, EUROSCOP, the Copenhagen City Lung Study, and the ISOLDE trial (CitationBurrows et al 1987; CitationPauwels et al 1999). Additionally, while oxygen improves survival in hypoxemic patients with COPD, survival is no different from similar patients with no hypoxemia (CitationMurray and Nadel 2000). As a result, COPD remains a progressive, incurable illness.
Predicting survival and timing of disability in COPD is difficult. Lung function decreases with age and age is inversely proportional to survival. Decline in lung function is not always continuous. The course of COPD is punctuated by acute exacerbations with deterioration in lung function that is less likely to completely resolve as disease progresses (CitationBurrows 1990). Exacerbations increase in frequency as lung function declines and some individuals experience a more rapid deterioration than others. These “rapid decliners” have been identified in prospective studies but it is not clear what sets them apart (CitationGottlieb et al 1996). Other variables that influence mortality include forced expiratory volume in one second (FEV1), nutritional status, and associated co-morbid disease (CitationMelbostad et al 1997). Mortality increases as FEV1 declines (CitationMannino et al 2003), although mortality is best predicted by a multifactorial staging system that includes factors in addition to FEV1 (CitationCelli et al 2004). Overall, patients with COPD follow a trajectory of illness characterized by intermittent worsening followed by improvements in performance status with an overall progression towards complete disability and then death (CitationLunney et al 2002, Citation2003) ().
Figure 1 The trajectory of illness of COPD follows a progressive course with declines in function alternating with periods of improvement. As the disease progresses, the focus of care shifts from life-prolonging therapies to palliative interventions aimed at reducing symptoms, improving function, and improving quality of life. Reprinted from CitationLunney JR, Lynn J, Hogan C. 2002. Profiles of older medicare decedents. J Am Geriatr Soc, 50:1108–12. Copyright © 2002 with permission from Blackwell Publishing Ltd.
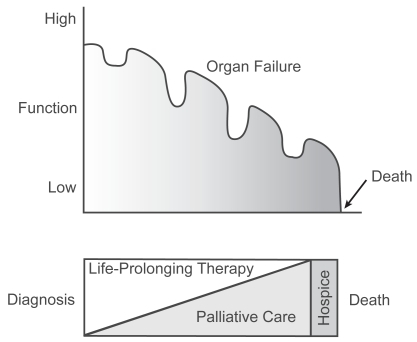
As time passes, quality of life (QOL) diminishes, most often as a result of increasing dyspnea that eventually becomes completely disabling. Conventional methods of alleviating dyspnea by modifying the disease begin to fail and it becomes more difficult to optimize comfort. Care of these patients is made all the more difficult by the challenge in identifying those that are “end-stage” and/or would derive benefit from palliative interventions.
Need for dyspnea management and palliative care
There is increasing interest in identifying COPD patients who would benefit from palliative interventions and palliative care (CitationSkilbeck et al 1998; CitationGore et al 2000; CitationEdmonds et al 2001). The studies are limited by the lack of a clear definition of “end-stage” COPD but do uncover important issues. CitationSkilbeck et al (1998) looked at 63 patients with COPD who were over the age of 55 and had been admitted to the hospital with an exacerbation in the preceding 6 months. There were high levels of morbidity and significant functional limitations. Ninety-five percent of patients reported that breathlessness was their most significant debilitating symptom, with 60% indicating the highest severity level on the scale. Other symptoms causing disability included pain, fatigue, difficulty sleeping, and thirst. Decreased physical, social, and emotional functioning were also described. The authors concluded that COPD patients had a high burden of poorly controlled symptoms, most likely as a result of a focus on crisis intervention with management of acute exacerbations as opposed to continued improvement in baseline functioning.
Two subsequent studies looked at the care of “end-stage” COPD patients as compared with the care of patients with lung cancer. CitationGore et al (2000) studied 50 patients over age 60 with COPD (defined as FEV1 <0.75 L and at least one admission for hypercapneic respiratory failure) and 50 patients over age 60 with inoperable non-small cell lung cancer. The study assessed QOL by several measures as well as the quantity and quality of medical and social care received. All data were obtained by a single interviewer. Patients with COPD had consistently lower (ie, worse) scores on the MOS Short Form-36 Health Survey (SF-36) than patients with lung cancer on all subscales (p ≤ 0.05) with the exception of those measuring emotional and physical roles. COPD patients also scored higher (ie, worse) than lung cancer patients on the both the depression (10.18 [SD 3.95] vs 7.22 [SD 5.16], p<0.01) and anxiety (11.44 [SD 4.76] vs 7.20 [SD 5.25], p<0.0001) subscales of the Hospital Anxiety and Depression Scale (HADS). Finally, 82% of COPD patients were housebound, compared with 36% of those with lung cancer. Despite these findings, none of the COPD patients had received or been offered specialist palliative care (CitationGore et al 2000). Some methodological flaws weaken these results (CitationHill and Muers 2000). There were more females than males in the COPD group; female sex is associated with higher levels of symptomatology and lower health status (Citationvan Wijk and Kolk 1997) as well as higher scores on the HADS (CitationAass et al 1997). Additionally, the lung cancer patients were a particularly well group whose survival exceeded the typical median survival for such patients in the UK (CitationHill and Muers 2000). Even in light of these discrepancies, the data produced by CitationGore et al (2000) support the view that there is a need for better palliative care for patients with COPD. These data are in agreement with those of CitationSkilbeck et al (1998) and were further supported by a later study published by CitationEdmonds et al (2001).
CitationEdmonds et al (2001) looked at the needs of patients with chronic lung disease or lung cancer in their final year of life as reported by a surrogate (relative, friend, or caregiver). Patients with COPD had troublesome symptoms, including pain, cough, anorexia, mouth problems, and insomnia. Breathlessness was most common, with 94% of respondents reporting this symptom during the final year of life and 76% rating it as “very distressing.” This contrasts with the lung cancer patients, 78% of whom reported breathlessness (p<0.001) with 60% describing it as “very distressing” (p=0.024). COPD patients had significantly more frequent (p<0.001) and severe (p<0.024) breathlessness than the lung cancer group. Similar to the findings of CitationGore et al (2000), patients with lung cancer in the CitationEdmonds et al (2001) study were more likely to have received hospice care (30%) as compared with those with COPD (0%, p<0.001).
Together, these data highlight that the symptoms of patients with COPD are at least as severe as those of patients with lung cancer and that these patients would benefit from better symptom management, particularly in the area of dyspnea. Despite the degree of symptomatology, COPD patients less frequently access palliative care.
Definition and mechanisms of dyspnea
Dyspnea is a symptom that is difficult to understand and difficult to define, for both physicians and patients. The experience of dyspnea is the combination of a sensation involving activation of neural pathways and a perception on the part of the patient (CitationATS 1999). Descriptions of dyspnea vary widely and depend, at least in part, on a patient’s underlying disease, ethnic–racial background, previous experiences, and emotional state. In an attempt to incorporate each of these ideas, an American Thoracic Society consensus panel proposed that dyspnea is “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity. The experience derives from interactions among multiple physiological, psychological, social, and environmental factors, and may induce secondary physiological and behavioral responses” (CitationATS 1999 p 322).
The pathophysiological mechanisms that govern the development of dyspnea are poorly understood. Dyspnea seemingly results from a mismatch of respiratory motor activity and incoming afferent activity, including information from chemo-, vagal, mechano-, pulmonary stretch, and muscle receptors (CitationATS 1999). The interaction between these individual components is only starting to be understood and there are clearly situations that cannot be defined by looking at only these factors. For example, increases in PaCO2 plus decreases in PaO2 and/or pH detected by peripheral chemoreceptors result in dyspnea. However, not all patients with abnormal blood gases experience dyspnea and many dyspneic patients have normal blood gases (CitationMountain et al 1978; CitationStulbarg et al 1989; CitationKikuchi et al 1994).
Researchers have tried to define the key parameters of breathlessness. Fifteen years ago, Simon and colleagues tried to look at correlates of dyspnea with underlying induced pathophysiology in normal volunteers (CitationSimon et al 1989), and in disease pathophysiology in people with established single causes of dyspnea (CitationSimon et al 1990). There were some clusters defined. A decade later, that idea was further developed by CitationHarver et al (2000) in their work which defined three key domains of breathlessness: depth and frequency of breathing, perceived urge of need to breathe, and difficulty breathing and phase of respiration.
Goals of palliative interventions for intractable dyspnea: relief of breathlessness and improving function
Patients often complain of dyspnea that seems out of proportion to apparent physical disability or functional impairment. It can be difficult to care for these patients as the usual dictum to treat the dyspnea by treating the underlying disease cannot guide therapy. There is also a substantial – and growing – population of patients whose COPD has been maximally treated but who are still breathless. Patients whose dyspnea cannot be alleviated through further treatment of their COPD have intractable dyspnea or refractory dyspnea (CitationAbernethy et al 2003).
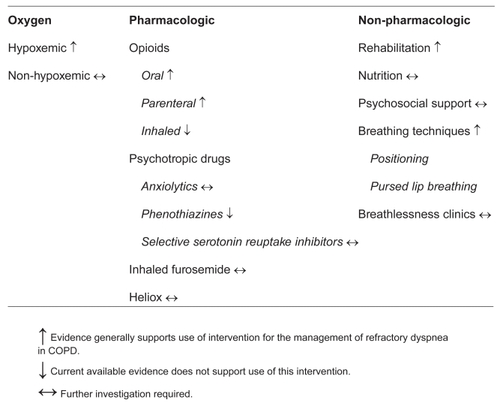
There are palliative options that can improve symptoms and QOL for COPD patients with intractable dyspnea (). The purpose of this review is to outline these options, discuss the data behind them, and provide practical guidance. Incorporating this information into clinical practice first requires that the goals of therapy are clearly articulated. We assume here that extending survival is not the primary goal of therapy. Palliative interventions should not reduce life expectancy, but can be beneficial even when they do not extend survival. The therapeutic interventions will therefore be examined in terms of their impact on relief of the sensation of breathlessness and/or their impact on functional status.
Oxygen therapy
Home oxygen therapy is commonly prescribed for patients with severe COPD. Two randomized controlled trials, one by the Medical Research Council Working Party (CitationMRCWP 1981) and the other by the Nocturnal Oxygen Therapy Trial Group (NOTT) (CitationNOTTG 1980), demonstrated that long-term oxygen therapy (LTOT) prolongs survival in COPD patients with hypoxemia (PaO2 <55 mmHg). LTOT oxygen is administered at home for at least 12–15 hours per day. In the NOTT trial, supplemental criteria for the use of LTOT in moderately hypoxemic patients (PaO2 55–59 mmHg) included pulmonary hypertension, cor pulmonale, and secondary polycythemia (hematocrit ≥ 55%). These two studies form the basis on which LTOT is funded by third-party payers for the management of hypoxemic patients worldwide. The goal is prolonging survival. The studies do not focus on symptomatic treatment of dyspnea, improved function in activities of daily living, and QOL outcomes, nor do they guide treatment of patients with disabling dyspnea who are not hypoxemic.
Palliative oxygen is prescribed when the primary goal is relief of breathlessness. In this setting the data are less clear. CitationMcDonald et al (1995) examined the effects of palliative oxygen in non-hypoxemic patients with severe COPD. The effect of medical air vs oxygen on exercise performance and QOL was examined in a 12-week, double-blind, randomized crossover study involving 26 patients with severe COPD, mean resting PaO2 69 mmHg (range 58–82 mmHg), and exertional dyspnea severe enough to interfere with daily activities. There were small, statistically significant (p<0.05) increases in 6-minute walk distance with oxygen. Both oxygen and medical air improved QOL from baseline but did not affect the sensation of breathlessness. The authors concluded that there was little evidence to support prescription of home oxygen for COPD patients with only mild hypoxemia.
CitationBooth et al (2004) systematically reviewed the published English-language literature through 2003 regarding the use of palliative oxygen for the management of breathlessness in cancer, COPD, and heart failure. They focused on studies in which breathlessness was measured by intensity scales such as the visual analog scale (VAS) or Borg scales. Studies involving the use of supplemental oxygen in patients with COPD were divided as follows: 1) those investigating the use of oxygen at rest; 2) those investigating the use of oxygen before, during and after exercise (ie, “short burst” oxygen therapy); and 3) those investigating LTOT where breathlessness was an outcome in addition to survival. A complete list of the studies included is provided at the end of this article.
Most of the studies were small (range 5–50 patients) and poorly controlled. The results were inconsistent and remarkably patient dependent. The authors concluded that oxygen at rest may be one part of a palliative treatment plan for the care of patients with COPD, but that it should be used only after an “n of 1” assessment as described by CitationBruera et al (1992). The goal of the “n of 1” individualized patient trial is to assess the impact of the oxygen in terms of both breathlessness and personal QOL. The recommendations for “short burst” oxygen were similar, reflecting the potential for some patients to benefit. For example, one study demonstrated great relief of dyspnea with oxygen for some participants, although the cumulative results for all patients in the trial were not statistically significant (CitationBooth et al 1996). Another study showed that dyspnea before and after exertion in patients with COPD could be relieved (CitationKillen and Corris 2000). Ambulatory oxygen therapy was recommended for those that desaturated on exercise but its continuous use was not as clear cut. Again, the impact of oxygen needed to be assessed on an individual basis (CitationEaton et al 2002). Even those who appeared to derive short-term benefit may not realize sustained benefit in the long-term and/or may not wish to continue therapy (CitationEaton et al 2002). Oxygen therapy is not without burden; people may have a sense of being tied to a machine, develop anxiety about power supply, and limit excursions outside the house.
Despite a lack of convincing evidence for benefit, palliative oxygen for the management of breathlessness is commonly prescribed. In a recently reported email survey of all 648 registered palliative care specialists and respiratory physicians in Australia and New Zealand (CitationAbernethy et al 2005), 58% of the 214 respondents (69% of palliative medicine clinicians and 48% of respiratory physicians surveyed) reported a belief that palliative oxygen is beneficial and 65% of respondents cited intractable dyspnea as the most common reason for prescription. Canadian clinicians reported similar convictions (CitationStringer et al 2004), with over 40% of patients receiving LTOT not meeting current guidelines (CitationGuyatt et al 2000). There is clearly a need for a definitive randomized trial. We are members of an international collaborative investigating the role of palliative oxygen vs medical air for the relief of breathlessness in patients with intractable dyspnea and PaO2 ≥ 55 mmHg in a definitive, multi-center, randomized, double-blind controlled trial.
Until these data become available, current evidence suggests that it is reasonable to prescribe this therapy on a case by case basis. Our current clinical standard for those patients who do not enroll in the trial and do not meet criteria for LTOT is to first establish the goals of therapy in terms of relief of dyspnea, improvement in function, and improvement in QOL. Whenever possible we conduct a blinded “n of 1” trial with oxygen vs air at 2 L/min. A respiratory therapist or nurse administers the gas, randomly choosing oxygen or air without revealing the assignment to the clinician or patient. For practical reasons the gas is administered for 15 minutes only. The patient undergoes a 5-minute wash-out period and then the other gas is administered. If access to medical air is not available, the test can be conducted using a fan as the comparison, although it will not be blinded. Dyspnea is scored on a VAS or numerical rating scale (NRS). If function is a major concern, we conduct a baseline exertional test consistent with the patient’s best functional status (ie, shuttle test [CitationBooth and Adams 2001], walking 3 m, reading a paragraph). The same functional test is repeated after 10 minutes on each gas. Unblinded results are reported to the clinician and patient. If the patient has superior results on oxygen, a prescription for palliative oxygen is written after a careful discussion about the funding for oxygen in the relevant health system. If air is superior, a fan is prescribed. If both are equally helpful, then the patient is given the option to use whichever he/she prefers. If oxygen is prescribed, the patient is then asked to evaluate QOL improvements and limitations related to the therapy over the next week to ascertain if the intervention should be continued.
Pharmacological interventions
There has been a great deal of interest in treating refractory dyspnea with non-specific drug therapies aimed at altering the perception of dyspnea (CitationBurdon et al 1994). Investigators have looked at oral, parenteral, and inhaled opioids, psychotropic drugs, inhaled furosemide, and Heliox. A list of references for each of these interventions is provided at the end of this article.
Oral and parenteral opioids
The use of opioids in the treatment of refractory breathlessness has been the subject of investigation for many years and remains controversial. Most of this revolves around safety concerns (predominantly respiratory depression) amidst the perception of inadequate evidence for the efficacy of opioids in relieving breathlessness in COPD (CitationPauwels et al 2001; CitationCurrow et al 2003)
The mechanism of action of opioids is poorly understood and these agents may act centrally, peripherally, or by reducing anxiety (CitationLeach 2005). It is well known that opioids reduce ventilatory response to carbon dioxide (CitationEckenhoff and Oech 1960), hypoxia (CitationWeil et al 1975; CitationSantiago et al 1977), inspiratory flow-resistive loading (CitationKryger et al 1976), and exercise (CitationSantiago et al 1979). Additionally, it is known that morphine decreases oxygen consumption both at rest and with exercise in healthy individuals (CitationSantiago et al 1979). These observations led to the evaluation of oral opioids in individuals with breathlessness due to chronic airway obstruction. Most of the initial studies conducted prior to 1998 only included normocapneic individuals because of concern over respiratory depression. The studies were small (7–19 patients), used several different opioids (including dihydrocodeine, codeine, diamorphine, morphine, and sustained-release morphine) in a variety of doses, and produced conflicting results. Additionally, most of the studies were primarily interested in exercise tolerance as opposed to relief of the sensation of breathlessness and only one study by CitationPoole et al (1998) assessed QOL as measured by the Chronic Respiratory Disease Questionnaire (CRQ).
Two early studies with dihydrocodeine demonstrated decreased breathlessness with exercise as measured by VAS as well as increased exercise tolerance as measured by treadmill or pedometer in the absence of significant adverse effects (CitationWoodcock et al 1981c; CitationJohnson et al 1983). However, these early reports were followed by two negative studies that demonstrated significant adverse effects and raised the issue of safety (CitationRice et al 1987; CitationEiser et al 1991).
CitationJennings et al (2002) systematically reviewed all data on the use of opioids through 2001 in an attempt to evaluate the evidence as a whole. In their assessment of oral and parenteral opioids, they included eight studies that evaluated oral morphine, as well as one study that evaluated subcutaneous morphine. Seven of the nine studies were of patients with COPD. Meta-analysis demonstrated a highly statistically significant effect of oral and parenteral opioids on the sensation of breathlessness (overall pooled effect size −0.31, 95% confidence internval [CI] −0.50 to −0.13, p=0.0008). Exercise tolerance was a secondary outcome. Meta-analysis demonstrated a trend towards an improved effect on exercise tolerance, but the CI crossed zero (−0.20, 95% CI −0.42 to +0.03, p not stated). The CitationPoole et al (1998) study found no difference in QOL between treatment and placebo. CitationJennings et al (2002) concluded that there was a definite and highly statistically significant effect of oral or parenteral opioids on the sensation of breathlessness but that the clinical effect was relatively small (approximately 8 mm on a 100 mm VAS with baseline levels of dyspnea of 50 mm). They gave several reasons for this small effect, including that the opioid doses were often small, doses were not titrated, dosing intervals were long, and single dose studies would not have achieved steady state. Additionally, their evaluation of the data did not suggest that the use of opioids was associated with changes in arterial blood gas measurements or oxygen saturation.
Following the CitationJennings et al (2002) systematic review, CitationAbernethy et al (2003) published results of a trial using oral morphine in opioid-naïve adults with refractory dyspnea. Forty-eight outpatients with refractory dyspnea despite optimal management were enrolled in an adequately powered 8-day randomized, double-blind, crossover study in which they received either 20 mg oral morphine sulfate or placebo. The morphine product used was a 24-hour sustained-release preparation. The primary outcome was the sensation of breathlessness measured on a 100 mm VAS; exercise tolerance was not measured. While participants were not restricted to those with COPD, 88% had COPD as the predominant cause of dyspnea. Participants were elderly (mean age 76, standard deviation [SD] 5) and 71% were on oxygen. The mean baseline morning dyspnea score was 43 (SD 26). In an intention to treat analysis, there was a significant decrease in dyspnea with morphine, with mean improvements in dyspnea intensity of 6.6 mm in the morning (p=0.011) and 9.5 mm in the evening (p=0.006). Relative improvement over baseline dyspnea was 15%–22%. These results were similar to the estimate of efficacy for oral and parenteral opioids generated by CitationJennings et al (2002). Morphine did not depress the respiratory rate (RR; mean RR for morphine vs placebo = 20 [SD 5] vs 21 [SD 4], p=0.143) and no episodes of severe sedation or obtundation were recorded. The main side-effect was constipation (9 vs 1, p=0.021), but neither treatment caused more vomiting, confusion, sedation, or appetite suppression. Those who received morphine also described better sleep at night (p=0.039) despite the fact that the medication was administered each morning. We concluded that oral sustained-release morphine could provide relief to patients with refractory breathlessness, even for patients who were elderly, had COPD, and were not previously receiving background opioids. We do caution, however, that this was not a safety study and was not powered to detect infrequent significant side-effects, so these agents should be used with care.
Current data do not clarify the ideal dose of morphine, the additive effect of opioids, whether all opioids provide equal effect, or whether the effect would be as substantial in diseases other than COPD. We used a 20-mg dose of morphine as that was the lowest dose once daily sustained-release product available to us at the time of study design. A dose ranging study exploring 10–30 mg sustained-release morphine is ongoing. This study will report on long-term effectiveness and will also clarify whether there is an additional benefit to increasing the dose sequentially. A study to evaluate additive effect of additional medication in opioid-tolerant patients is also planned. In the CitationJennings et al (2002) meta-analysis, all of the studies of oral and parenteral opioids had an effect size in favor of opioids except the two studies of diamorphine. This suggests that opioids have a beneficial effect but that all opioids are not the same. A study of morphine vs oxycodone is also planned. Once adequate data are available, sub-group analyses considering COPD vs other causes of dyspnea will be conducted.
In practise, we prescribe oral morphine for COPD patients with refractory dyspnea. When the patient is opioid-naïve, we start with a 20-mg, once-daily, sustained-release morphine preparation if the drug is available and the patient does not have a contraindication to morphine. When not available, we start with the 15-mg, twice-daily, long-acting morphine product, initially prescribing it once a day and increasing to twice daily after 5–7 days if the patient tolerates the medication and has residual breathlessness. When the patient has a contraindication to morphine, we choose long-acting oxycodone starting at 10 mg once a day and increase to twice a day after 5–7 days as tolerated and needed. We have also used hydromorphone dosed around the clock in analgesic doses equal to 20–30 mg of morphine. We avoid starting with the 25-μg fentanyl transdermal patch as this provides over 80 morphine equivalents per day. If an opioid-tolerant patient is already on a regular dose of morphine or another opioid, we sequentially increase the opioid by 20% of the total daily dose every 3–5 days until the breathlessness is relieved or side-effects occur (CitationBruera et al 1993).
Nebulized opioids
The role of nebulized opioids in the management of refractory dyspnea has also been studied. The mechanism by which these agents may act is currently unclear but it has been proposed that there may be a direct local effect on peripheral neural receptors in small airways. Much like the data with oral opioids, the studies are small and the results inconsistent. However, on the whole, the majority of studies failed to demonstrate a benefit, as reported by CitationJennings et al (2002) in their meta-analysis. At this point, we do not feel that there is adequate evidence to support the use of nebulized opioids in the treatment of refractory breathlessness. Since the size of nebulized droplets is crucial for the activity of inhaled opioids, in the future, newer methods of microdroplet delivery of opioids may result in rapid systemic effects and improved efficacy of this method of drug delivery.
Psychotropic drugs
Use of psychotropic agents including anxiolytics, phenothiazines, and selective serotonin reuptake inhibitors (SSRIs) for the treatment of refractory dyspnea has received some attention based on the observation that there is often a large psychological component to dyspnea and that anxiety significantly contributes to functional impairment in COPD (CitationKim et al 2000). Diazepam was initially reported to be beneficial by CitationMitchell-Heggs et al (1980) when they published the results of an exploratory study in four patients. However, these results could not be replicated in two subsequent studies (CitationWoodcock et al 1981a; CitationSen et al 1983) which also raised the issue of safety. Several other investigators chose to re-look at the use of benzodiazepines but with alprazolam, a shorter-acting and potentially less-sedating medication. In one controlled study (CitationMan et al 1986), the investigators found no improvement in breathlessness. However, a benefit was found in a subsequent single case report (CitationGreene et al 1989).
Anxiolytics
Buspirone, a serotonergic anxiolytic agent, has been shown to be a respiratory stimulant in animals (CitationGarner et al 1989; CitationMendelson et al 1990). Two small studies (CitationArgyropoulou et al 1993; CitationSingh et al 1993) evaluated the effects of buspirone on breathlessness, exercise tolerance, and anxiety in patients with severe COPD. These studies included slightly different patient populations in that CitationSingh et al (1993) required baseline anxiety as measured by the Speilberger State-Trait Anxiety Inventory Scale (STAI) for study enrollment and CitationArgyropoulou et al (1993) did not require any baseline anxiety assessment. The results of the studies conflicted with those of the CitationArgyropoulou et al (1993) study documenting improvement in all three domains and the other study finding no difference.
Taken together, these data, while inconsistent, suggest that there may be a role for anxiolytic agents in selected patients with refractory dyspnea. Further studies are needed. In practise, we perform “n of 1” trials for patients where we plan to use these agents, carefully monitoring for benefit in terms of relief of the sensation of breathlessness and improvement in function without overwhelming adverse effects. When we do not have identical appearing placebos available, we conduct single-sided “n of 1” trials carefully documenting outcomes to be reviewed by both clinician and patient.
Phenothiazines
There are also limited, conflicting data on the use of phenothiazines. One early study comparing diazepam and promethazine reported increased exercise tolerance and decreased breathlessness with promethazine (CitationWoodcock et al 1981a) but these results were not supported by a subsequent study (CitationRice et al 1987). Additionally, the CitationRice et al (1987) study raised concerns about side-effects, including increases in PaCO2 and drowsiness. CitationLight et al (1996) evaluated both promethazine and prochlorperazine in combination with morphine in a double-blind, placebo-controlled study that included seven male patients with severe COPD (mean FEV1 0.99 L [SD 0.30]). The authors found that treatment with morphine and promethazine in combination increased exercise tolerance compared with placebo (mean increase in workload of 10 W [SD 6.3] vs 1.4 W [SD 6.9], p<0.05). Morphine and prochlorperazine had an intermediate effect (mean increase in workload of 6.4 W [SD 6.3]; p not reported). The cognitive effects of both combinations were also assessed. Morphine and prochlorperazine had a large negative effect on patients’ mental status while morphine and promethazine produced effects no different from placebo or morphine alone. The authors suggested that the increased exercise tolerance seen with the combination of morphine and promethazine was due to a decreased level of dyspnea at a given workload despite the fact that there was no significant difference in Borg scores. Available data, therefore, do not support the routine use of phenothiazines in the management of refractory dyspnea, referring to the beneficial effect of morphine instead.
SSRIs
In recent years, there has been interest in the use of SSRIs in the treatment of patients with COPD and breathlessness. A pilot study by CitationPapp et al (1995) reported that six patients (three of whom had psychiatric disorders) felt better after 6 weeks of sertraline, suggesting that further study was warranted. CitationSmoller et al (1998) reported a case series of seven patients with obstructive lung disease treated with sertraline by their pulmonologists. All patients were evaluated by a psychiatrist, one in a prospective fashion using the Structured Clinical Interview for the DSM-III-R and the other six retrospectively with modules for depression, dysthymia, panic, agoraphobia, and generalized anxiety disorder (GAD). Four of the seven patients did not meet criteria for a psychiatric diagnosis although several experienced panic and/or anxiety during episodes of dyspnea. Of the remaining three patients, one met criteria for panic disorder with agoraphobia, one for both dysthymia and GAD, and one for major depression. All patients reported a “decrease” in breathlessness and several also reported “improvements” in exercise tolerance. There were, however, no formal measures performed. It is not clear whether SSRIs improve dyspnea and exercise tolerance by relieving anxiety symptoms or by direct effects on respiration. Many patients with dyspnea out of proportion to their pulmonary compromise experience depression and/or anxiety and there are data to suggest that treatment of these symptoms relieves dyspnea (CitationBurns and Howell 1969). However, there are also animal data suggesting that serotonin acts at the level of the brainstem respiratory center and it may be this action that affects the sensation of dyspnea (CitationMueller et al 1982). There have been no subsequent studies of SSRIs and currently there is no substantive evidence on which to recommend their use in COPD patients with refractory dyspnea in the absence of an underlying psychiatric indication. This is an important area for further study. In patients where there is concern for an underlying psychiatric disorder (eg, anxiety, depression), there should be a concerted effort to confirm the diagnosis and initiate effective therapy.
Emerging data
Inhaled furosemide
There are two additional interventions currently under investigation. Inhaled furosemide has been studied in healthy subjects (CitationNishino et al 2000) and in patients with COPD (CitationOng et al 2004). The rationale behind the use of furosemide includes its inhibitory effect on the cough response induced by low chloride content solutions (CitationVentresca et al 1990) and a preventive effect of furosemide on bronchoconstriction in asthma (CitationBianco et al 1988, Citation1989; CitationRobuschi et al 1989). Inhaled furosemide may also act indirectly on vagally mediated sensory nerve ending in airway epithelium (CitationChung and Barnes 1992). In healthy subjects, inhaled furosemide has prolonged breathholding time and the period of no respiratory sensation and has also slowed the development of discomfort during loaded breathing (CitationNishino et al 2000). Does inhaled furosemide have a role in the palliation of refractory dyspnea? A randomized study evaluated the effects of inhaled furosemide vs placebo in exercise-induced dyspnea (as assessed by VAS) in 19 patients with moderate to severe COPD (FEV1 <70%) and moderate-to-severe chronic breathlessness (CitationOng et al 2004). Mean dyspnea VAS scores after exercise were lower after inhalation of furosemide (34mm [SD 25] vs 42 [SD 24], p=0.014). Exercise-related FEV1 and FVC also improved after furosemide inhalation (p=0.038 and 0.005, respectively) but not after placebo. Interestingly, no increase in exercise endurance time was noted. Both this study and the study by CitationNishino et al (2000) suggest that inhaled furosemide deserves further investigation in the relief of refractory breathlessness.
Heliox28
A second agent that may have a role in the therapy of refractory breathlessness is Heliox28, a helium–oxygen mixture containing 72% helium and 28% oxygen. A Phase II crossover study assessed the palliative role of Heliox28 in 12 lung cancer patients with refractory dyspnea (CitationAhmedzai et al 2004). Breathlessness during 6-minute walk test was evaluated while patients were breathing Heliox28, 28% oxygen, or medical air. The VAS measurements were significantly lower when comparing Heliox28 with medical air (40.2% [SD 4.8] vs 59.3% [SD 5.3], p<0.05) but there was no significant difference between Heliox28 and oxygen (47.0% [SD 5.6]) or between oxygen and medical air. There were also no significant differences in Borg scores. Patients walked farther breathing Heliox28 than breathing oxygen (214.2 m [SD 9.6] vs 174.6 m [SD 11.2], p<0.05) or medical air (128.8 m [SD 10.3], p<0.0001). The use of Heliox28 in the management of COPD has been explored (CitationGrape et al 1960; CitationIshikawa and Segal 1973; CitationJaber et al 2000) but there are no studies evaluating its use in patients with COPD and refractory dyspnea. The results obtained by CitationAhmedzai et al (2004) suggest that further investigation of Heliox28 in the therapy of COPD patients with refractory dyspnea may be warranted. Issues of cost and feasibility would need to be considered in any such investigation.
Non-pharmacological interventions
Pulmonary rehabilitation
In 1994, “pulmonary rehabilitation” was defined by the National Institutes of Health Workshop on Pulmonary Rehabilitation Research as “a multidimensional continuum of services directed to persons with pulmonary disease and their families, usually by an interdisciplinary team of specialists, with the goal of achieving and maintaining the individual’s maximum level of independence and functioning in the community” (CitationFishman 1994 p 826). It is a well-established component of the care of COPD patients that is included in the recommendations of most COPD care guidelines, and there have been numerous studies demonstrating its value in reducing dyspnea, improving QOL, and increasing independence–exercise capacity (CitationLacasse et al 1996; CitationGuell et al 2000). There is also an evidence-based guideline examining its scientific basis (CitationACCP/AACVPR 1997). Pulmonary rehabilitation is typically introduced early in the illness trajectory so, while it certainly has a role in the care of patients with advanced COPD, a detailed exploration of the data supporting its use is beyond the scope of this review.
Nutrition
Malnutrition is not uncommon in patients with COPD and weight loss can become particularly severe as the disease advances. Consequences of this loss include weakness of inspiratory and expiratory muscles which can lead to impaired lung function (CitationWilson et al 1986). Studies have demonstrated a link between malnutrition and impaired pulmonary status (CitationSahebjami et al 1993) as well as with increased mortality (CitationWilson et al 1989). It therefore stands to reason that nutritional support would constitute a simple, effective intervention in the management of patients with severe COPD. The data, however, do not support this idea.
CitationFerreira et al (2005) performed a meta-analysis of eleven randomized, controlled trials looking at the effects of nutritional supplementation in patients with severe COPD. All studies evaluated the use of liquid oral supplements, including Pulmocare® (Ross Laboratories, Columbus, OH, USA), Build-up® (Nestle, Croydon, Surrey, UK), Sustacal® (Mead Johnson Nutritionals, Bristol Myers Squibb, New York, NY, USA), Isocal® (Mead Johnson Nutritionals, Bristol Myers Squibb, NY, NY, USA), and Respifor® (Nutricia, Zoetermeer, Netherlands). CitationFerreira et al (2005) identified no significant effect on any outcome variable, including anthropomorphic measures, pulmonary function, respiratory muscle strength, functional exercise capacity, and health-related QOL. Because only 3 (CitationEfthimiou et al 1988;CitationOtte et al 1989;CitationSteiner et al 2003) of the 11 studies evaluated QOL or dyspnea, it is difficult to draw conclusions regarding the effect of nutrition on outcomes most important to patients with end-stage COPD who require palliation. A well-designed clinical trial of nutritional supplementation in end-stage COPD patients with dyspnea and reduced QOL would be useful to assess the effects of this simple intervention.
Psychosocial support
Providing support is a fundamental part of caring for any patient with chronic illness, but it becomes more important as patients begin to decline and experience more functional limitations. Patients often need help coping with their illness and the changes occurring in their lives. At the same time, relatives and family members may require support as relationships and family roles change as a result of disease progression and increasing symptom burden (CitationLeach 2005).
There are several qualitative studies that highlight the specific need for support of those caring for individuals with breathlessness. CitationBooth et al (2003) sought to investigate the experience of living with breathlessness both in patients with cancer or COPD and in those individuals providing informal care. The investigators interviewed 10 COPD patients and their caregivers in their homes; 30% of interviews were conducted separately. Six of the patients were male; they were all Caucasian and ranged in age from 51 to 80 years (mean = 70 years). The study found that many caregivers found taking care of a breathless person to be “pre-occupying, restricting, and a major cause of anxiety” (CitationBooth et al 2003 p 341). Anxiety was noted to be particularly bad a night and several caregivers described spending time listening to their spouse breathing. The investigators concluded that clinicians must not forget about the needs of their patients’ families and that patients with disabling symptoms are often best cared for using a multidisciplinary approach. A similar study described the experiences of 10 patients with COPD and their caregivers (CitationSeamark et al 2004). The caregivers were married to the patient in seven of the 10 cases and ranged in age from 60 to 93 years (mean 72 years). They expressed losses similar to those of the patients and felt burdened by the multiple roles that they were forced to fulfill. There was also a sense that it was not fair that the illness affected both patient and caregiver and this often contributed to tension in the relationships.
There are no specific guidelines available regarding specific support for either patients or their informal caregivers. Clinicians caring for these patients must be conscious of the potential need for increased support as well as responsive to patient–family concerns when they arise. To date there have been no reports of systematic evaluation of isolated behavioral or psychosocial interventions for COPD patients with intractable dyspnea. This is an important area for future study.
Breathing techniques
Several controlled breathing techniques including positioning and pursed lip breathing (PLB) are useful in the management of breathlessness. The most commonly used position is one in which the patient leans forward and supports his/her weight with the arms–upper body. This serves to increase abdominal pressure and may improve respiratory muscle function (CitationATS 1999). Use of the leaning forward position has also been reported to improve inspiratory muscle strength (CitationO’Neill and McCarthy 1983) and diaphragmatic function (CitationSharp et al 1980), reduce the use of accessory muscles, and decrease abdominal breathing (CitationBarach and Beck 1954; CitationBarach 1974; CitationSharp et al 1980). PLB involves inhalation through the nose followed by a slow exhalation, usually 4–6 seconds, through pursed lips. It has been shown to decrease air trapping by stenting the airways and preventing dynamic airway collapse (CitationTiep et al 1986) and often helps to avert panic attacks that accompany severe breathlessness (CitationMadge and Edmond 2001). Unfortunately, despite studies demonstrating the benefit of these techniques in relieving dyspnea, the results in practice are variable (CitationATS 1999). In practise, instruction in positioning and PLB is provided to all of our patients with dyspnea, and the skills reviewed during subsequent clinic visits.
Breathlessness clinics
“Breathlessness clinics” are another potential intervention for dyspneic patients with advanced COPD. Nurse practitioners who run these clinics lead weekly sessions that focus on counseling, breathing re-training, relaxation, and coping–adaptation strategies for dealing with breathlessness. The “breathlessness clinic” model combines education and psychosocial support, and has thus far focused on patients with lung cancer.
The initial pilot study demonstrating benefit of these clinics was published in 1996 (CitationCorner et al 1996). It involved 34 lung cancer patients with breathlessness randomized to the nursing intervention or control. The intervention group attended weekly 1-hour sessions for 3–6 weeks. During these sessions, the nurse practitioner assessed patients’ breathlessness, disease, and feelings. He/she gave advice and support about managing breathlessness, including activity pacing, as well as education on breathing and relaxation techniques. The control group was encouraged to talk about their disease but received no counseling or breathing training. Outcomes were assessed at baseline, one month and three months by three separate VAS scales, the Functional Capacity Scale, and the HADS. The study stopped recruiting at 34 patients at the request of the clinic staff who noted benefit in the intervention group. Despite the small number of participants and the loss of 14 patients, leaving only 20 patients for analysis, 3 months after study entry the intervention group saw significant improvements in worst breathlessness (p=0.02), distress caused by breathlessness (p=0.02), and functional capacity (p=0.03). Symptoms in the control group either remained static or worsened over the course of the study.
Two subsequent studies (CitationBredin et al 1999; CitationHately et al 2003) have further validated this concept. In a multicenter study involving 119 patients, CitationBredin et al (1999) found similar improvements in breathlessness at best (p=0.03), performance status (p=0.02), depression (p=0.02), physical symptoms (p=0.04), and activity (ie, climbing stairs, walking outdoors, and going shopping, p=0.05). A smaller study by CitationHately et al (2003) of 30 breathless patients with lung cancer also demonstrated improvements. The authors saw a decrease in frequency of respiratory symptoms (p<0.001) as well as an improvement in functional capacity (p<0.001) and QOL scores, with the greatest improvement in “patients’ ability to do what they want” (p=0.001) and in overall QOL (0.004). Breathlessness scores were improved with significant reductions in VAS scores for breathing at best (median reduction = 1, p=0.001), breathing at worst (median reduction = 3, p<0.001) and distress caused by breathing (median reduction = 4, p<0.001). Qualitative data demonstrated the value of the intervention on patient’s adjustment to their cancer diagnosis as well as their sense of well-being.
These three studies (CitationCorner et al 1996; CitationBredin et al 1999; CitationHately et al 2003) suggest a role for a nursing intervention incorporating psychosocial support and education in the care of breathless patients with lung cancer. There no studies evaluating such an intervention in patients with end-stage COPD, though this is a population that would likely derive similar benefit. Practically, patients with lung cancer and COPD may be referred to a “breathlessness clinic” when such a specialty clinic is available. However, these types of tertiary referral clinics are often not the ideal setting to see a sick patient and his/her family on a regular basis, so continued follow-up by the referring physician with attention to continued symptom management is required. Future goals should focus on evaluating this model in other diseases and developing sustainable models that can be rolled out worldwide.
Conclusions and future directions
COPD is a common disease with a course that deteriorates in a relentless fashion until culminating in severe functional limitation and symptom burden. Intractable or refractory dyspnea is a substantial contributor to this suffering. As individuals continue to live longer, the number of “end-stage” COPD patients will increase. Their needs are similar to those of lung cancer patients but COPD patients are offered and use fewer palliative interventions. The reason for this is not clear but it may be due, at least in part, to a differential focus of the individuals caring for these two groups of patients. Palliative interventions for patients with advanced COPD have the potential to reduce the suffering caused by breathlessness. The goal of palliative interventions in “end-stage” disease is to decrease symptoms, and improve functional status and QOL.
In this paper we have outlined the interventions currently available for the global treatment of refractory dyspnea. Oral or parenteral opioids are beneficial with the most evidence available for morphine. Palliative oxygen may be beneficial for some patients; an “n of 1” trial is the best approach until further information is available. Anxiolytics and SSRIs have limited and sometimes conflicting data; they should be approached cautiously and with explicit expected outcomes until more information is available. Inhaled furosemide is an interesting potential intervention that needs more study. Heliox28 may or may not be beneficial but its use may be most limited by feasibility concerns. Nutrition needs further study, as does counseling, behavioral therapies, and breathlessness clinics for COPD. Instruction in positioning and PLB is a simple intervention with potential to be very effective but without prior systematic study. Maintaining physical activity, whether via a structured pulmonary rehabilitation program or via another intervention, remains an important intervention, albeit one that is less well studied in individuals with refractory breathlessness.
It is clear that there is more work to be done as many of the options have little or limited evidence to support their use. We have tried to identify areas that are currently under investigation as well as those that require further exploration. We are currently involved in a multi-center, randomized clinical trial investigating the role of palliative oxygen vs medical air in non-hypoxemic patients with intractable dyspnea. Additionally, there is an ongoing study evaluating varying doses of sustained-release morphine. A study of newer psychotropic agents is planned. Finally, non-pharmacologic interventions such as breathlessness clinics have proven benefit in lung cancer patients but need specific evaluation in COPD.
All of this translates to the needs for better palliative care for patients with advanced COPD and for the difficult problems that create suffering like refractory dyspnea. Whether this care is best provided by the patient’s long-standing primary care provider or pulmonologist with or without specialist palliative care support or by primary palliative care teams who take over care remains to be seen. Hospices, inpatient palliative care units, and symptom management consults are also important supports for the care of the COPD patient with unrelenting dyspnea.
Selected reference list
Studies included in CitationBooth et al (2004) systematic review
Use of oxygen at rest: (CitationLiss and Grant 1988; CitationSwinburn et al 1991; CitationBooth et al 1996; CitationO’Donnell et al 2001)
Use of oxygen before, during and after exercise (ie “short burst” oxygen therapy): (CitationWoodcock et al 1981b; CitationWaterhouse and Howard 1983; CitationSwinburn et al 1984; CitationEvans et al 1986; CitationLane et al 1987; CitationDavidson et al 1988; CitationMcKeon et al 1988a, Citation1988b; CitationKollef and Johnson 1990; CitationDean et al 1992; CitationLeach et al 1992; CitationDewan and Bell 1994; CitationRoberts et al 1996; CitationO’Donnell et al 1997; CitationMarques-Magallanes et al 1998; CitationKillen and Corris 2000; CitationKnebel et al 2000; CitationRevill et al 2000; CitationJolly et al 2001; CitationMaltais et al 2001; CitationO’Donnell et al 2001; CitationSomfay et al 2001; CitationEaton et al 2002)
Use of LTOT where breathlessness was an outcome in addition to survival: (CitationMcDonald et al 1995; CitationRooyackers et al 1997; CitationGarrod et al 2000; CitationEaton et al 2002)
Studies on pharmacologic interventions
Opiods – oral: (CitationWoodcock et al 1981c; CitationJohnson et al 1983; CitationRice et al 1987; CitationLight et al 1989, Citation1996; CitationEiser et al 1991; CitationPoole et al 1998; CitationJennings et al 2002; CitationAbernethy et al 2003)
Opioids – parenteral: (CitationBruera et al 1993)
Opiods – inhaled: (CitationYoung et al 1989; CitationBeauford et al 1993; CitationFarncombe et al 1994; CitationHarris-Eze et al 1995; CitationMasood et al 1995; CitationLeung et al 1996; CitationJankelson et al 1997; CitationNoseda et al 1997; CitationJennings et al 2002; CitationForal et al 2004)
Psychotropic agents – anxiolytics: (CitationMitchell-Heggs et al 1980; CitationWoodcock et al 1981a; CitationSen et al 1983; CitationEimer et al 1985; CitationMan et al 1986; CitationGreene et al 1989; CitationArgyropoulou et al 1993; CitationSingh et al 1993)
Psychotropic agents – phenothiazines: (CitationWoodcock et al 1981a; CitationO’Neill et al 1985)
Psychotropic agents – SSRIs: (CitationPapp et al 1995; CitationSmoller et al 1998)
Inhaled furosemide: (CitationNishino et al 2000; CitationOng et al 2004)
Heliox28: (CitationAhmedzai et al 2004).
Studies included in CitationJennings et al (2002) systematic review
Studies evaluating oral morphine: (CitationWoodcock et al 1981c, Citation1982; CitationJohnson et al 1983; CitationEiser et al 1991; CitationLight et al 1996; CitationChua et al 1997; CitationPoole et al 1998)
Study evaluating subcutaneous morphine: (CitationBruera et al 1993).
Studies evaluating inhaled morphine: (CitationYoung et al 1989; CitationBeauford et al 1993; CitationDavis et al 1994, Citation1996; CitationHarris-Eze et al 1995; CitationMasood et al 1995; CitationLeung et al 1996; CitationJankelson et al 1997; CitationNoseda et al 1997)
Studies included in CitationFerreira et al (2005) meta-analysis
(CitationLewis et al 1987; CitationEfthimiou et al 1988; CitationKnowles et al 1988; CitationOtte et al 1989; CitationFuenzalida et al 1990; CitationWhittaker et al 1990; CitationDeLetter 1991; CitationSchols et al 1995; CitationSteiner et al 2002a, Citation2002b, Citation2003; CitationGoris et al 2003)
Acknowledgments
Dr Uronis’ salary is generously supported by the Agency for Healthcare Research and Quality (grant number 5 T32 HS000079). Dr Abernethy’s salary is generously supported through a Clinical Scientist Development Award from the Doris Duke Charitable Foundation of New York, NY, USA (grant number 20010439).
References
- AassNFossaSDDahlAA1997Prevalence of anxiety and depression in cancer patients seen at the Norwegian Radium HospitalEur J Cancer3315976049389921
- AbernethyAPCurrowDCFrithP2005Prescribing palliative oxygen: a clinician survey of expected benefit and patterns of usePalliat Med191687015810762
- AbernethyAPCurrowDCFrithP2003Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoeaBMJ327523812958109
- [ACCP/AACVPR] American College of Chest Physicians/American Association of Cardiovascular and Pulmonary Rehabilitation1997Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based guidelines. ACCP/AACVPR Pulmonary Rehabilitation Guidelines PanelChest1121363969367481
- AhmedzaiSHLaudeERobertsonA2004A double-blind, randomised, controlled Phase II trial of Heliox28 gas mixture in lung cancer patients with dyspnoea on exertionBr J Cancer903667114735178
- ArgyropoulouPPatakasDKoukouA1993Buspirone effect on breathlessness and exercise performance in patients with chronic obstructive pulmonary diseaseRespiration60216208265878
- [ATS] American Thoracic Society1999Dyspnea. Mechanisms, assessment, and management: a consensus statementAm J Respir Crit Care Med159321409872857
- BarachAL1974Chronic obstructive lung disease: postural relief of dyspneaArch Phys Med Rehabil554945044441261
- BarachALBeckGJ1954The ventilatory effects of the head-down position in pulmonary emphysemaAm J Med16556013114235
- BeaufordWSaylorTTStansburyDW1993Effects of nebulized morphine sulfate on the exercise tolerance of the ventilatory limited COPD patientChest10417588325064
- BiancoSPieroniMGRefiniRM1989Protective effect of inhaled furosemide on allergen-induced early and late asthmatic reactionsN Engl J Med3211069732797066
- BiancoSVaghiARobuschiM1988Prevention of exercise-induced bronchoconstriction by inhaled frusemideLancet225252899239
- BoothSAdamsL2001The shuttle walking test: a reproducible method for evaluating the impact of shortness of breath on functional capacity in patients with advanced cancerThorax561465011209105
- BoothSKellyMJCoxNP1996Does oxygen help dyspnea in patients with cancer?Am J Respir Crit Care Med153151588630595
- BoothSSilvesterSToddC2003Breathlessness in cancer and chronic obstructive pulmonary disease: Using a qualitative approach to describe the experience of patients and carersPalliat Support Care13374416594223
- BoothSWadeRJohnsonM2004The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative MedicineRespir Med98667714959816
- BredinMCornerJKrishnasamyM1999Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancerBMJ318901410102851
- BrueraEMacEachernTRipamontiC1993Subcutaneous morphine for dyspnea in cancer patientsAnn Intern Med11990678215003
- BrueraESchoellerTMacEachernT1992Symptomatic benefit of supplemental oxygen in hypoxemic patients with terminal cancer: the use of the N of 1 randomized controlled trialJ Pain Symptom Manage736581517652
- BurdonJGPainMCRubinfeldAR1994Chronic lung diseases and the perception of breathlessness: a clinical perspectiveEur Respir J7134297925915
- BurnsBHHowellJB1969Disproportionately severe breathlessness in chronic bronchitisQ J Med38277945343604
- BurrowsB1990Airways obstructive diseases: pathogenetic mechanisms and natural histories of the disordersMed Clin North Am74547592186232
- BurrowsBKnudsonRJCamilliAE1987The “horse-racing effect” and predicting decline in forced expiratory volume in one second from screening spirometryAm Rev Respir Dis135788933565926
- CelliBRCoteCGMarinJM2004The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med35010051214999112
- ChuaTPHarringtonDPonikowskiP1997Effects of dihydrocodeine on chemosensitivity and exercise tolerance in patients with chronic heart failureJ Am Coll Cardiol29147528996307
- ChungKFBarnesPJ1992Loop diuretics and asthmaPulm Pharmacol5171591461
- CornerJPlantHA’HernR1996Non-pharmacological intervention for breathlessness in lung cancerPalliat Med102993058931065
- CurrowDAbernethyAFrithP2003Morphine for management of refractory dypsnoeaBMJ32712889
- DavidsonACLeachRGeorgeRJ1988Supplemental oxygen and exercise ability in chronic obstructive airways diseaseThorax43965713238640
- DavisCHodderCLoveS1994Effect of nebulised morphine and morphine 6-glucuronide on exercise endurance in patients with chronic obstructive pulmonary diseaseThorax49393
- DavisCLPennKA’HernR1996Single dose randomised controlled trial of nebulised morphine in patients with cancer related breathlessnessPalliative Med106465
- DeanNCBrownJKHimelmanRB1992Oxygen may improve dyspnea and endurance in patients with chronic obstructive pulmonary disease and only mild hypoxemiaAm Rev Respir Dis14694151416422
- DeLetterM1991A nutritional intervention for persons with chronic airflow limitation [details at proof stage]
- DewanNABellCW1994Effect of low flow and high flow oxygen delivery on exercise tolerance and sensation of dyspnea. A study comparing the transtracheal catheter and nasal prongsChest105106158162725
- EatonTGarrettJEYoungP2002Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled studyEur Respir J203061212212960
- EckenhoffJEOechSR1960The effects of narcotics and antagonists upon respiration and circulation in man. A reviewClin Pharmacol Ther148352413819208
- EdmondsPKarlsenSKhanS2001A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancerPalliat Med152879512054146
- EfthimiouJFlemingJGomesC1988The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis1371075823057956
- EimerMCableTGalP1985Effects of clorazepate on breathlessness and exercise tolerance in patients with chronic airflow obstructionJ Fam Pract21359622865326
- EiserNDenmanWTWestC1991Oral diamorphine: lack of effect on dyspnoea and exercise tolerance in the “pink puffer” syndromeEur Respir J4926311783082
- EvansTWWaterhouseJCCarterA1986Short burst oxygen treatment for breathlessness in chronic obstructive airways diseaseThorax41611153538488
- FarncombeMChaterSGillinA1994The use of nebulized opioids for breathlessness: a chart reviewPalliat Med8306127529103
- FerreiraIMBrooksDLacasseY2005Nutritional supplementation for stable chronic obstructive pulmonary diseaseCochrane Database Syst RevCD00099815846608
- FishmanAP1994Pulmonary rehabilitation researchAm J Respir Crit Care Med149825338118655
- ForalPAMaleskerMAHuertaG2004Nebulized opioids use in COPDChest125691414769753
- FuenzalidaCEPettyTLJonesML1990The immune response to short-term nutritional intervention in advanced chronic obstructive pulmonary diseaseAm Rev Respir Dis14249562368979
- GarnerSJEldridgeFLWagnerPG1989Buspirone, an anxiolytic drug that stimulates respirationAm Rev Respir Dis139946502930071
- GarrodRPaulEAWedzichaJA2000Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemiaThorax555394310856310
- GoreJMBrophyCJGreenstoneMA2000How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancerThorax551000611083884
- GorisAHVermeerenMAWoutersEF2003Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementationBr J Nutr897253112720592
- GottliebDJStonePJSparrowD1996Urinary desmosine excretion in smokers with and without rapid decline of lung function: the Normative Aging StudyAm J Respir Crit Care Med154129058912738
- GrapeBChanninETylerJM1960The effect of helium and oxygen mixtures on pulmonary resistances in emphysemaAm Rev Respir Dis81823913828986
- GreeneJGPucinoFCarlsonJD1989Effects of alprazolam on respiratory drive, anxiety, and dyspnea in chronic airflow obstruction: a case studyPharmacotherapy93482922358
- GuellRCasanPBeldaJ2000Long-term effects of outpatient rehabilitation of COPD: A randomized trialChest1179768310767227
- GuyattGHMcKimDAAustinP2000Appropriateness of domiciliary oxygen deliveryChest1181303811083678
- Harris-EzeAOSridharGClemensRE1995Low-dose nebulized morphine does not improve exercise in interstitial lung diseaseAm J Respir Crit Care Med152194058520759
- HarverAMahlerDASchwartzsteinRM2000Descriptors of breathlessness in healthy individuals: distinct and separable constructsChest1186799010988189
- HatelyJLaurenceVScottA2003Breathlessness clinics within specialist palliative care settings can improve the quality of life and functional capacity of patients with lung cancerPalliat Med17410712882259
- HillKMMuersMF2000Palliative care for patients with non-malignant end stage respiratory diseaseThorax559798111083879
- IshikawaSSegalMS1973Re-appraisal of helium-oxygen therapy on patients with chronic lung diseaseAnn Allergy31536424583314
- JaberSFodilRCarlucciA2000Noninvasive ventilation with helium-oxygen in acute exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med161119120010764311
- JankelsonDHosseiniKMatherLE1997Lack of effect of high doses of inhaled morphine on exercise endurance in chronic obstructive pulmonary diseaseEur Respir J10227049387952
- JenningsALDaviesANHigginsJP2002A systematic review of the use of opioids in the management of dyspnoeaThorax579394412403875
- JohnsonMAWoodcockAAGeddesDM1983Dihydrocodeine for breathlessness in “pink puffers”BMJ28667576402199
- JollyECDi BoscioVAguirreL2001Effects of supplemental oxygen during activity in patients with advanced COPD without severe resting hypoxemiaChest1204374311502641
- KikuchiYOkabeSTamuraG1994Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthmaN Engl J Med3301329348152444
- KillenJWCorrisPA2000A pragmatic assessment of the placement of oxygen when given for exercise induced dyspnoeaThorax55544610856311
- KimHFKunikMEMolinariVA2000Functional impairment in COPD patients: the impact of anxiety and depressionPsychosomatics414657111110109
- KnebelARBentzEBarnesP2000Dyspnea management in alpha-1 antitrypsin deficiency: effect of oxygen administrationNurs Res49333811093698
- KnowlesJBFairbarnMSWiggsBJ1988Dietary supplementation and respiratory muscle performance in patients with COPDChest93977833282825
- KollefMJohnsonR1990Transtracheal gas administration and the perception of dyspneaRespir Care357919
- KrygerMHYacoubODosmanJ1976Effect of meperidine on occlusion pressure responses to hypercapnia and hypoxia with and without external inspiratory resistanceAm Rev Respir Dis11433340973724
- LacasseYWongEGuyattGH1996Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary diseaseLancet348111598888163
- LaneRCockcroftAAdamsL1987Arterial oxygen saturation and breathlessness in patients with chronic obstructive airways diseaseClin Sci (Lond)7269383595075
- LeachRMDoyleDHanksGChernyN2005Palliative medicine and non-malignant, end-stage respiratory diseaseOxford textbook of palliative medicine3rd edNew YorkOxford University Press
- LeachRMDavidsonACChinnS1992Portable liquid oxygen and exercise ability in severe respiratory disabilityThorax4778191481177
- LeungRHillPBurdonJ1996Effect of inhaled morphine on the development of breathlessness during exercise in patients with chronic lung diseaseThorax515966008693440
- LewisMIBelmanMJDorr-UyemuraL1987Nutritional supplementation in ambulatory patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis135106283579004
- LightRWMuroJRSatoRI1989Effects of oral morphine on breathlessness and exercise tolerance in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis139126332492170
- LightRWStansburyDWWebsterJS1996Effect of 30 mg of morphine alone or with promethazine or prochlorperazine on the exercise capacity of patients with COPDChest109975818635380
- LissHPGrantBJ1988The effect of nasal flow on breathlessness in patients with chronic obstructive pulmonary diseaseAm Rev Respir Dis137128583144198
- LunneyJRLynnJFoleyDJ2003Patterns of functional decline at the end of lifeJAMA28923879212746362
- LunneyJRLynnJHoganC2002Profiles of older medicare decedentsJ Am Geriatr Soc5011081212110073
- McDonaldCFBlythCMLazarusMD1995Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemiaAm J Respir Crit Care Med152161697582304
- MadgeSEdmondGEsmondG2001End-stage management of respiratory diseaseRespiratory nursingLondonBailliere Tindall22940
- MaltaisFSimonMJobinJ2001Effects of oxygen on lower limb blood flow and O2 uptake during exercise in COPDMed Sci Sports Exerc339162211404656
- ManGCHsuKSprouleBJ1986Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary diseaseChest9083263780329
- ManninoDMBuistASPettyTL2003Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up studyThorax583889312728157
- Marques-MagallanesJAStorerTWCooperCB1998Treadmill exercise duration and dyspnea recovery time in chronic obstructive pulmonary disease: effects of oxygen breathing and repeated testingRespir Med9273589713632
- MasoodARReedJWThomasSH1995Lack of effect of inhaled morphine on exercise-induced breathlessness in chronic obstructive pulmonary diseaseThorax50629347638804
- McKeonJTomlinsonJTarrantE1988aPortable oxygen in patients with severe chronic obstructive pulmonary diseaseAust N Z J Med1812529
- McKeonJLMurree-AllenKSaundersNA1988bEffects of breathing supplemental oxygen before progressive exercise in patients with chronic obstructive lung diseaseThorax435363353874
- MelbostadEEduardWMagnusP1997Chronic bronchitis in farmersScand J Work Environ Health23271809322818
- MendelsonWBMartinJVRapoportDM1990Effects of buspirone on sleep and respirationAm Rev Respir Dis1411527302350096
- Mitchell-HeggsPMurphyKMintyK1980Diazepam in the treatment of dyspnoea in the ‘Pink Puffer’ syndromeQ J Med499206776586
- MountainRZwillichCWeilJ1978Hypoventilation in obstructive lung disease. The role of familial factorsN Engl J Med2985215625307
- [MRCWP] Medical Research Council Working Party1981Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working PartyLancet168166110912
- MuellerRALundbergDBBreeseGR1982The neuropharmacology of respiratory controlPharmacol Rev34255856185961
- MurrayCJLopezAD1996aEvidence-based health policy—lessons from the Global Burden of Disease StudyScience27474038966556
- MurrayCJLLopezAD1996bThe Global Burden of Disease: A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020Cambridge, MAHarvard University Press
- MurrayJFNadelJA2000Textbook of respiratory medicinePhiladelphiaSaunders
- NishinoTIdeTSudoT2000Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspneaAm J Respir Crit Care Med1611963710852774
- NosedaACarpiauxJPMarksteinC1997Disabling dyspnoea in patients with advanced disease: lack of effect of nebulized morphineEur Respir J101079839163650
- [NOTTG] Nocturnal Oxygen Therapy Trial Group1980Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trialAnn Intern Med9339186776858
- O’DonnellDEBainDJWebbKA1997Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitationAm J Respir Crit Care Med15553059032190
- O’DonnellDED’ArsignyCWebbKA2001Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary diseaseAm J Respir Crit Care Med163892811282762
- O’NeillPAMortonPBStarkRD1985Chlorpromazine—a specific effect on breathlessness?Br J Clin Pharmacol1979374027121
- O’NeillSMcCarthyDS1983Postural relief of dyspnoea in severe chronic airflow limitation: relationship to respiratory muscle strengthThorax385956006612651
- OngKCKorACChongWF2004Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16910283314977622
- OtteKEAhlburgPD’AmoreF1989Nutritional repletion in malnourished patients with emphysemaJPEN J Parenter Enteral Nutr1315262651744
- PappLAWeissJRGreenbergHE1995Sertraline for chronic obstructive pulmonary disease and comorbid anxiety and mood disordersAm J Psychiatry15215317573598
- PauwelsRABuistASCalverleyPM2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summaryAm J Respir Crit Care Med16312567611316667
- PauwelsRALofdahlCGLaitinenLA1999Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary DiseaseN Engl J Med34019485310379018
- PoolePJVealeAGBlackPN1998The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1571877809620921
- RevillSMSinghSJMorganMD2000Randomized controlled trial of ambulatory oxygen and an ambulatory ventilator on endurance exercise in COPDRespir Med947788310955754
- RiceKLKronenbergRSHedemarkLL1987Effects of chronic administration of codeine and promethazine on breathlessness and exercise tolerance in patients with chronic airflow obstructionBr J Dis Chest81287923311120
- RobertsCMBellJWedzichaJA1996Comparison of the efficacy of a demand oxygen delivery system with continuous low flow oxygen in subjects with stable COPD and severe oxygen desaturation on walkingThorax5183148795673
- RobuschiMGambaroGSpagnottoS1989Inhaled frusemide is highly effective in preventing ultrasonically nebulised water bronchoconstrictionPulm Pharmacol1187912520344
- RooyackersJMDekhuijzenPNVan HerwaardenCL1997Training with supplemental oxygen in patients with COPD and hypoxaemia at peak exerciseEur Respir J101278849192929
- SahebjamiHDoersJTRenderML1993Anthropometric and pulmonary function test profiles of outpatients with stable chronic obstructive pulmonary diseaseAm J Med94469748498391
- SantiagoTVJohnsonJRileyDJ1979Effects of morphine on ventilatory response to exerciseJ Appl Physiol4711218468650
- SantiagoTVPuglieseACEdelmanNH1977Control of breathing during methadone addictionAm J Med6234754842554
- ScholsAMSoetersPBMostertR1995Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trialAm J Respir Crit Care Med1521268747551381
- SeamarkDABlakeSDSeamarkCJ2004Living with severe chronic obstructive pulmonary disease (COPD): perceptions of patients and their carers. An interpretative phenomenological analysisPalliat Med186192515540670
- SenDJonesGLeggatPO1983The response of the breathless patient treated with diazepamBr J Clin Pract3723236882655
- SharpJTDrutzWSMoisanT1980Postural relief of dyspnea in severe chronic obstructive pulmonary diseaseAm Rev Respir Dis122201117416599
- SimonPMSchwartzsteinRMWeissJW1990Distinguishable types of dyspnea in patients with shortness of breathAm Rev Respir Dis1421009142240820
- SimonPMSchwartzsteinRMWeissJW1989Distinguishable sensations of breathlessness induced in normal volunteersAm Rev Respir Dis140102172508520
- SinghNPDesparsJAStansburyDW1993Effects of buspirone on anxiety levels and exercise tolerance in patients with chronic airflow obstruction and mild anxietyChest10380048449072
- SkilbeckJMottLPageH1998Palliative care in chronic obstructive airways disease: a needs assessmentPalliat Med12245549743823
- SmollerJWPollackMHSystromD1998Sertraline effects on dyspnea in patients with obstructive airways diseasePsychosomatics392499538672
- SomfayAPorszaszJLeeSM2001Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patientsEur Respir J18778411510809
- SteinerMCBartonRLSinghSJ2002aThe effect of nutritional supplementation on body weight and composition in COPD patients participating in rehabilitationEur Respir J20262s
- SteinerMCBartonRLSinghSJ2002bThe effect of nutritional supplementation on body weight and composition in COPD patients participating in rehabilitationEur Respir J20211s
- SteinerMCBartonRLSinghSJ2003Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trialThorax587455112947128
- StringerEMcParlandCHernandezP2004Physician practices for prescribing supplemental oxygen in the palliative care settingJ Palliat Care20303715690833
- StulbargMSWinnWRKellettLE1989Bilateral carotid body resection for the relief of dyspnea in severe chronic obstructive pulmonary disease. Physiologic and clinical observations in three patientsChest95112382495905
- SwinburnCRMouldHStoneTN1991Symptomatic benefit of supplemental oxygen in hypoxemic patients with chronic lung diseaseAm Rev Respir Dis14391352024842
- SwinburnCRWakefieldJMJonesPW1984Relationship between ventilation and breathlessness during exercise in chronic obstructive airways disease is not altered by prevention of hypoxaemiaClin Sci (Lond)6751596478752
- TiepBLBurnsMKaoD1986Pursed lips breathing training using ear oximetryChest90218213731893
- van WijkCMKolkAM1997Sex differences in physical symptoms: the contribution of symptom perception theorySoc Sci Med45231469225411
- VentrescaPGNicholGMBarnesPJ1990Inhaled furosemide inhibits cough induced by low chloride content solutions but not by capsaicinAm Rev Respir Dis14214362368961
- WaterhouseJCHowardP1983Breathlessness and portable oxygen in chronic obstructive airways diseaseThorax3830266867984
- WeilJVMcCulloughREKlineJS1975Diminished ventilatory response to hypoxia and hypercapnia after morphine in normal manN Engl J Med292110361128555
- WhittakerJSRyanCFBuckleyPA1990The effects of refeeding on peripheral and respiratory muscle function in malnourished chronic obstructive pulmonary disease patientsAm Rev Respir Dis14228382116747
- [WHO] World Health Organization2000World Health ReportWHOGeneva
- WilsonDORogersRMSandersMH1986Nutritional intervention in malnourished patients with emphysemaAm Rev Respir Dis13467273767123
- WilsonDORogersRMWrightEC1989Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing TrialAm Rev Respir Dis139143582658702
- WoodcockAJohnsonMGeddesD1982Breathlessness, alcohol, and opiatesN Engl J Med306136347070466
- WoodcockAAGrossERGeddesDM1981aDrug treatment of breathlessness: contrasting effects of diazepam and promethazine in pink puffersBMJ28334366788319
- WoodcockAAGrossERGeddesDM1981bOxygen relieves breathlessness in “pink puffers”Lancet190796112324
- WoodcockAAGrossERGellertA1981cEffects of dihydrocodeine, alcohol, and caffeine on breathlessness and exercise tolerance in patients with chronic obstructive lung disease and normal blood gasesN Engl J Med3051611166796885
- YoungIHDaviskasEKeenaVA1989Effect of low dose nebulised morphine on exercise endurance in patients with chronic lung diseaseThorax44387902669222