Abstract
Tiotropium is a potent, long-acting, selective anticholinergic bronchodilator. Treatment with tiotropium produces sustained improvements in lung function, particularly FEV1 (peak, trough, average, and area under the curve) compared with either placebo or ipratropium in patients with moderate to severe COPD. Preliminary evidence suggests that treatment with tiotropium may slow the rate of decline in FEV1, but this finding awaits confirmation. Tiotropium reduces lung hyperinflation, with associated improvements in exercise capacity. Tiotropium, compared with either placebo or ipratropium, improves a variety of patient-centered outcomes, including subjective dyspnea ratings and HRQL scores. Tiotropium reduces the frequency of COPD exacerbations and of hospitalizations due to exacerbations, but has not been shown to reduce all-cause mortality. Compared with the long-acting bronchodilators, tiotropium provides incrementally better bronchodilation, but it is not clearly superior in terms of patient-centered outcomes. Tiotropium has a good safety profile; however patients with severe cardiac disease, bladder outlet obstruction, or narrow angle glaucoma were excluded from all studies. Medico economic analyses suggest that treatment with tiotropium may also be cost-effective, primarily by reducing costs associated with hospitalizations.
Introduction
It is estimated that COPD afflicts from 4% to 10% of adults and is the fourth leading cause of death worldwide (CitationSullivan et al 2000; CitationWorld Health Organization 2000; CitationMichaud et al 2001; CitationHalbert et al 2003). The true global burden of COPD may be even greater as the condition is often under-diagnosed (CitationHalbert et al 2003). Cigarette smoking is the primary cause of COPD, but environmental and genetic factors also contribute to the disease burden. The primary objective of COPD management is disease prevention through promoting smoking cessation and improving air quality. Other important goals of COPD management include efforts to slow progression of the disease, improve symptoms and overall health status, treat and prevent exacerbations, and reduce mortality.
Inhaled bronchodilators play an important role for improving chronic symptoms and management of exacerbations in COPD, but they have not been shown to reduce mortality or slow the accelerated decline in lung function over time that typifies this disease. The two major classes of inhaled bronchodilators commonly used for COPD are beta adrenergic agonists and anticholinergics. In recent years longer-acting bronchodilators of both drug classes have been widely introduced into clinical practice, either complementing or replacing shorter-acting drugs. This article focuses on tiotropium (Spiriva®, Boehringer Ingelheim), a newly developed, long-acting, potent anticholinergic agent that was approved for use in Europe in 2002 and in the United States and Canada in 2004. We will review what has been learned about the clinical benefits and safety of this drug, relying primarily on results from peer reviewed multi-dose, randomized, controlled trials.
Mechanism of action
Atropine, as well as the short-acting inhaled bronchodilator ipratropium, and the long-acting inhaled bronchodilator tiotropium, are muscarinic receptor antagonists. Unlike atropine, tiotropium and ipratropium (Atrovent®, Boehringer Ingelheim) are quaternary ammonium cation compounds that are poorly absorbed across cell membranes, thus limiting their effects mainly to the airways after inhalation. Three types of muscarinic receptors have been identified in human airways. Two of these receptors, M1 located in parasympathetic ganglia, and M3 located on airway smooth muscle, mediate bronchoconstriction via the discharge of acetylcholine from vagal nerve endings (CitationBelmonte 2005). M2 receptors are located on postganglionic cholinergic nerve endings and on smooth muscle and provide negative feedback so as to counteract smooth muscle contraction. Thus, antagonism of the M1 and M3 receptors reduce smooth muscle tone and cause beneficial bronchodilation while inhibition of the M2 receptor has the opposite effect. Ipratropium and atropine nonselectively block all three receptors, but tiotropium is more selective for the M1 and M3 receptors, from which it dissociates much more slowly (CitationBarnes 2000). Consequently, tiotropium is a somewhat more potent bronchodilator than is ipratropium, and has a much longer duration of action. A single dose of inhaled tiotropium produces bronchodilation that is sustained for 24 hours or more (CitationBarnes 2000).
Cholinergic-mediated pathways also modulate mucus production, vascular tone, and possibly a variety of immune pathways in the airways, raising the question as to whether any of the clinical benefits of anticholinergic therapy in COPD might be mediated via mechanisms other than bronchodilation (CitationKoyama et al 1992; CitationNomura et al 2003; CitationBelmonte 2005). The evidence for this is inconclusive. Excess mucus production is thought to be clinically important in COPD. However, efforts to show that inhaled anticholinergics reduce the volume of sputum production in patients with obstructive lung disease have yielded mixed results (CitationLopez-Vidriero et al 1975; CitationTamaoki et al 1994).
Characteristics of study populations
All tiotropium trials conducted to date enrolled relatively homogeneous populations of COPD patients (Table ). Most patients had moderate to severe disease by spirometric criteria (forced expiratory volume in one second/forced vital capacity [FEV1/FVC] < 0.70 and FEV1 < 65% of predicted) (CitationNational Heart, Lung and Blood Institute 2005). Patients with predominant asthma were excluded by clinical criteria. The majority of participants were men, and the average age of patients in most trials was typically in a range of 60–70 years. All trials banned concomitant use of ipratropium, and most prohibited the use of long-acting beta agonists, but inhaled corticosteroids and unlimited albuterol rescue therapy were generally permitted. Most trials also excluded patients with unstable heart disease, severe or inadequately treated urinary outflow obstruction, narrow angle glaucoma, or moderate to severe azotemia.
Table 1 Characteristics of trials and their study populations
Table 2 Effect of tiotropium on spirometry
Table 3 Summary of tiotropium effects
Delivery of tiotropium in all studies was by inhalation via a single-dose dry powder delivery device (Handihaler®, Boehringer Ingelheim) (CitationChodosh et al 2001). The dose of tiotropium was 18 μg/day, as dose-ranging studies indicated that higher doses increase the incidence of adverse effects without providing significant additional improvement in pulmonary function (CitationLittner et al 2000). Tiotropium was usually given in the morning, although the time of administration has little effect on lung function over a 24-hour period (CitationCalverley et al 2003).
Effects on lung function and exercise
Spirometry
Treatment-related bronchodilator effects, as assessed by spirometry, were evaluated in all tiotropium trials. Spirometry is a useful surrogate for clinical outcomes because airflow limitation is a defining feature of COPD and because spirometric variables such as the FEV1 correlate with the severity of respiratory symptoms, with exercise capacity, and with mortality, albeit in an imperfect fashion (CitationAnthonisen et al 1986). The FEV1 is easily measured, and because it is more reproducible than most other efficacy outcomes in COPD, statistically significant changes can be detected with relatively modest sample sizes. Trough FEV1, measured in the morning 23–24 hours after the last dose of study drug, was the primary outcome in a number of tiotropium studies. Other spirometric assessments included FVC, peak and area under the curve for both FEV1 and FVC, and inspiratory capacity (IC). The direction and relative magnitude of the changes seen with these measurements were generally similar to those observed with trough FEV1.
Several short-term (4–12 weeks) studies demonstrated that tiotropium increases mean peak FEV1 by between 210 mL and 265 mL and mean trough FEV1 by between 120 mL and 184 mL (CitationCelli et al 2003; CitationO’Donnell et al 2004; CitationCovelli et al 2005; CitationVerkindre et al 2006). In a larger 12-week study, Beeh and associates showed that tiotropium increased mean trough FEV1 by an average of 79 mL, compared with placebo (CitationBeeh et al 2006). They found larger improvements in average trough FEV1 (113 mL) in the subgroup of patients with milder COPD (FEV1 50%–70% predicted) compared with the group as a whole. In one trial, mean steady state trough FEV1 occurred within 48 hours of treatment initiation, but mean FVC (trough and peak) continued to increase over the first week of continuous treatment (Citationvan Noord et al 2002).
Longer-term studies demonstrated similar sustained improvements in lung function with tiotropium. At the end of one 13-week study in patients with severe COPD (mean baseline FEV1 38% predicted), treatment with tiotropium increased peak FEV1 by an average of 220 mL and trough FEV1 by an average of 150 mL compared with placebo (CitationCasaburi et al 2000). In a 1-year study of 921 patients with severe COPD (mean baseline FEV1 38%–39% predicted), treatment with tiotropium, compared with placebo, improved mean trough FEV1 by between 120 and 150 mL on the various assessment days (CitationCasaburi et al 2002). Similar results were reported in other 6-month and 12-month studies (CitationBrusasco et al 2003; CitationNiewoehner et al 2005). The MISTRAL study, performed in patients with somewhat milder COPD (mean FEV1 48% predicted), showed similar effects from tiotropium on both peak and trough FEV1 (CitationDusser et al 2006). Rodrigo and associates calculated that tiotropium, compared with placebo, conferred weighted mean improvements of 120 mL in the trough FEV1 and 210 mL in the peak FEV1 among 4214 patients entered in eight trials (CitationRodrigo et al 2006).
Overall, the correlation of FEV1 changes with clinically relevant outcomes, such as dyspnea and health-related quality of life (HRQL), is relatively weak in COPD patients and no general consensus exists as to what constitutes a clinically meaningful improvement. Anchoring of FEV1 changes to clinical outcomes, such as dyspnea and exacerbation, suggests that increases on the order of 100 mL are clinically noticeable in certain settings (CitationRedelmeier et al 1996; CitationNiewoehner et al 2000; CitationDonohue 2005). Thus, it is expected that the bronchodilator effects with tiotropium should be sufficiently large to provide some meaningful impact on clinical outcomes.
Acute bronchodilator responses as predictors of long-term tiotropium responses
Although asthma patients were excluded by clinical criteria in all of the tiotropium trials, none were excluded because the acute bronchodilator response exceeded some arbitrary upper limit. Consequently, individual bronchodilator responses to a short-acting bronchodilator varied over a fairly wide range in those studies where that information is provided (CitationCazzola et al 2004a, Citationb; CitationBeeh et al 2006; Citationvan Noord et al 2006). None of the aforementioned studies reported whether initial responses to short-acting bronchodilators predicted long-term bronchodilator responses to tiotropium. In a secondary analysis of two 1-year trials, patients who had larger responses (≥12% increase and ≥ 200 mL in FEV1) to tiotropium on study day 1 were compared with patients with poorer responses to tiotropium (CitationTashkin et al 2003). Responsive patients, compared with less responsiveness patients, exhibited greater improvement in mean trough FEV1 at the end of the 1-year follow-up (212 ± 17 mL vs 94 ± 17 mL, respectively), but differences in various clinical outcomes tended to be small and mostly statistically insignificant. Neither the initial response to a short-acting bronchodilator nor the initial response to tiotropium has been shown to provide useful information about long-term clinical outcomes with tiotropium. This is consistent with other evidence demonstrating the futility of using acute bronchodilator responses as a guide to therapy in COPD (CitationCalverley et al 2003).
Disease modification – FEV1 decline over time
Accelerated age-related decreases in the FEV1 typify the natural history of COPD. Though the FEV1 is a surrogate out-come, it is widely accepted that slowing the decline in FEV1 over time can be viewed as “disease modifying”, as there is the expectation of eventual reductions in morbidity and mortality. To date, smoking cessation is the only intervention that is known to slow the annual rate of decline in FEV1 in patients with COPD (CitationAnthonisen et al 2002a). Pharmacologic interventions have been mostly unsuccessful. Several large randomized trials failed to show a statistically significant effect of high dose inhaled corticosteroids on FEV1 decline over 3 years in patients with mild to moderate COPD, though summary estimates do not exclude a small effect (CitationHighland et al 2003; CitationSutherland et al 2003). Similarly, the Lung Health Study found that regular treatment with ipratropium over a 5-year period had no effect on the rate of decline in FEV1 in patients with mild COPD (CitationAnthonisen et al 1994).
Preliminary evidence suggests that treatment with tiotropium might alter the progression of COPD. A post-hoc analysis of data from a previously published study (CitationCasaburi et al 2002) found that treatment with tiotropium between study days 8 and 344 statistically significantly reduced the mean rate of decline in trough FEV1 by 46 mL/year compared with placebo (CitationAnzueto et al 2005). In a systematic review, Barr and colleagues confirmed that result and also reported a similar effect from tiotropium when it was compared with ipratropium (CitationBarr et al 2006a; CitationVincken et al 2002). A mean effect size of 46 mL/year is substantially larger than that reported in the ICS trials (5–8 mL/year) and would be viewed as clinically meaningful, if confirmed. These preliminary results formed the basis of the Understanding Potential Long-term Impacts on Function with Tiotropium (UPLIFT) trial. The study has completed enrollment of 6000 patients with COPD and the primary objective is to determine whether tiotropium slows the decline in lung function (as measured by FEV1) over a 4-year period (CitationBoehringer Ingelheim 2004). Results of the study are expected in 2008.
Dynamic hyperinflation and exercise performance
Along with airflow limitation, hyperinflation is thought to play an important role in the genesis of exertional dyspnea, the symptom that is so characteristic of more advanced COPD (CitationCalverley et al 2006). In contrast to normal subjects, end-expiratory lung volume (EELV) increases during exercise in patients with severe COPD. Hyperinflation can be assessed indirectly without resorting to plethysmography by measuring the reduction in IC (CitationO’Donnell et al 1998). Hyperinflation is essential for maintaining the higher expiratory flow and minute ventilation rates that allow the COPD patient to meet the increased metabolic demands of exercise (CitationCalverley et al 2006). However, dynamic lung hyperinflation also carries a penalty because the respiratory system becomes progressively less compliant at larger lung volumes. It is thought that the resultant increase in the elastic component of respiratory muscle work has an important bearing on the sensation of breathlessness.
CitationO’Donnell et al (2004) compared tiotropium with placebo for 6 weeks and found that tiotropium increased resting and end-exercise IC and VC, decreased resting residual volume and functional residual volume, and improved mean exercise endurance time by an average of 105 sec (21%). In a 4-week study CitationCelli et al (2003) showed that treatment with tiotropium, compared with placebo, improved resting trough IC (by an average of 220 mL), and reduced trough thoracic gas volume (by an average of 540 mL). Similar effects on lung hyperinflation and exercise have been reported in other trials (Maltais et al 2005; CitationBeeh et al 2006; CitationVerkindre et al 2006).
Studies of dynamic hyperinflation during exercise provide interesting new insights into the mechanism of dyspnea relief from bronchodilators in COPD patients. It has been suggested that bronchodilator-induced increases in IC and EELV may be better predictors of improvement in exertional dyspnea and exercise times than changes in FEV1, though this claim has not been fully substantiated (CitationBelman et al 1996; CitationO’Donnell et al 1998). IC measurements are substantially less reproducible than more conventional measurements, such as the FEV1 (CitationATS/ERS 2005), and their capability for distinguishing small bronchodilator effects is correspondingly weaker. It also bears emphasizing that the capability for reducing dynamic hyperinflation, though best studied with tiotropium, is most likely a generic effect common to all bronchodilators (CitationBelman et al 1996).
Tiotropium may also provide a useful adjunct to pulmonary rehabilitation. CitationCasaburi et al (2005) demonstrated that tiotropium amplified the benefits of endurance training in patients with severe COPD (FEV1 35% predicted). Over an 8-week program, patients receiving tiotropium improved their mean constant work rate endurance time by 80% as compared with only 57% in patients receiving placebo. This difference was well maintained over a subsequent 12-week period when all patients continued study drug but received no further formal endurance training.
Arterial blood gases and sleep-related oxygen desaturation
The effect of tiotropium on resting awake arterial blood gases has not been well studied, although tiotropium does improve sleep-related oxygen desaturation (SaO2) in patients with COPD. After 1 month of treatment with tiotropium, administered once a day in the evening, mean SaO2 during REM was modestly (3.1%) but statistically greater than placebo, with a similar trend for patients who took tiotropium in the morning (CitationMcNicholas et al 2004). The improvement in SaO2 was most strongly correlated with increases in end-of-treatment FEV1. Tiotropium had no effect on the SaO2 while subjects were awake, the amount of time they spent in REM, or self-reports of sleep quality or daytime sleepiness.
Patient-centered outcomes
Direct measurement of patient-centered outcomes, such as health related quality of life, respiratory symptoms, exacerbations, mortality, adverse drug events, and costs, are increasingly recognized as the most important components in COPD trials (CitationTashkin 2006). Tiotropium has been shown to improve many of these outcomes.
Health-related quality of life (HRQL)
The St. Georges Respiratory Questionnaire (SGRQ) is the most widely used and arguably the best validated instrument for assessing health status in COPD patients. An improvement of at least 4 points in the SGRQ score is judged to be the clinically meaningful difference (CitationJones et al 1997). In a summary analysis of 3 controlled trials, CitationBarr et al (2006b) reported that tiotropium, compared with placebo, improved total SGRQ score by a weighted mean difference of −3.3 units (95% CI, −5.6 to −1.0). While the mean change fell short of the 4-unit change that is considered clinically noticeable, treatment with tiotropium, compared with placebo, did significantly increase the likelihood that an individual patient would achieve an improvement of 4 units or more (odds ratio, 1.9; 95% CI, 1.4–2.7).
Dyspnea
Dyspnea plays a central role in the disability of patients with advanced COPD (CitationRies 2006). Dyspnea evaluation is a component of the SGRQ, but the Transitional Dyspnea Index (TDI) provides a more specific assessment of functional impairment due to breathlessness (CitationMahler et al 1984). The clinically meaningful difference for the TDI score has been reported to be a 1 unit change (CitationWitek et al 2003). Compared with placebo, tiotropium affected statistically significant mean improvements of 1.1 units in a 6-month trial and 0.8–1.1 units at various time points in a 1-year trial (CitationCasaburi et al 2002; CitationBrusasco et al 2003).
Exacerbations
Results from multiple trials consistently show that tiotropium, compared with placebo, reduces the frequency and severity of COPD exacerbations (CitationCasaburi et al 2002; CitationBrusasco et al 2003; CitationNiewoehner et al 2005; Verkindre et al 2005; CitationBeeh et al 2006; CitationDusser et al 2006). Most trials were not clearly designed with exacerbation as the primary outcome and much of the information about exacerbations was collected through adverse event reporting. The single trial that was designed specifically to evaluate exacerbation frequency was also the largest of the trials and it fully confirmed the results obtained in other studies (CitationNiewoehner et al 2005). Though there was some variation from trial to trial, most used an “event-based” definition of exacerbation that required the occurrence of typical symptom complexes coupled with some form of medical intervention, such as antibiotic or systemic corticosteroid use.
Several meta-analyses summarized the results from available trials (CitationSin et al 2003; CitationWilt et al 2005; CitationBarr et al 2006a, Citationb; CitationRodrigo et al 2006). A recent systematic review included 6 placebo-controlled trials of greater than 12 weeks duration and involving 6301 patients (CitationBarr et al 2006b). The summary odds ratio of having one or more exacerbations for tiotropium, compared with placebo, was 0.74 (95% CI, 0.66–0.83) (Figure ). No subgroups have been clearly identified to whom treatment is more effective or less effective.
Figure 1 Summary effects of tiotropium on (A) COPD exacerbations, (B) hospitalizations, and (C) all-cause mortality. Reproduced with permission from Barr RG, Bourbeau J, Camargo CA, et al. 2006. Tiotropium for stable chronic obstructive pulmonary disease: a meta-analysis. Thorax, 61: 854–62. Copyright © 2006 BMJ Publishing Group Ltd. and the British Thoracic Society.
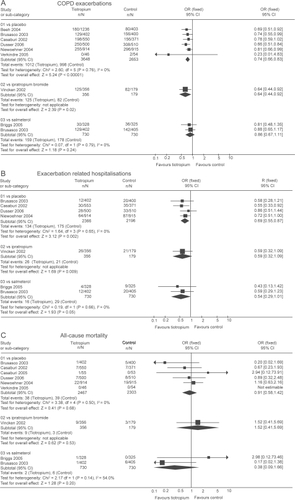
There is also solid evidence that tiotropium reduces the frequency of hospitalizations among those patients who suffer a COPD exacerbation. Hospitalization is very important in human terms, because of its morbidity and increased risk of mortality, and in economic terms because of the inordinately high costs. In the United States, hospital admissions are the single largest source of medical expenditures for COPD, accounting for up to 70% of the total costs of medical care for this disease (CitationHilleman et al 2000).
Information about COPD hospitalization rates were provided in four large placebo-controlled trials (CitationCasaburi et al 2002; CitationBrusasco et al 2003; CitationNiewoehner et al 2005; CitationDusser et al 2006). All studies are consistent in showing fewer hospitalizations among subjects who received tiotropium with the relative reduction in individual trials ranging between 13% and 42%. CitationBarr et al (2006b) calculated the summary odds ratio for tiotropium relative to placebo in these 4 trials as being 0.69 (95% CI, 0.55–0.87) (Figure ).
Since most COPD exacerbations are thought to be caused by infections, the mechanism by which tiotropium reduces the frequency and severity of COPD exacerbations is uncertain. Improvements in lung function and dyspnea could reduce patients’ perception of the severity of exacerbations, thereby decreasing the likelihood that they would seek medical attention. That event would not be counted as an exacerbation, as exacerbation has been defined in most of the tiotropium trials. Other possible mechanisms might include more efficient cough and clearance of respiratory secretions due to better bronchodilation, protection against lung injury due to hyperinflation, or specific anticholinergic effects on mucus secretion or bronchial inflammation (CitationNiewoehner et al 2005; CitationTurino 2005).
Mortality
Salpeter and associates reported that inhaled anticholinergics (tiotropium and ipratropium trials combined), compared with placebo, reduced the risk of respiratory deaths (RR 0.27, 95% CI 0.09–0.81) based on a meta-analysis of 5 trials with 7881 participants (CitationSalpeter et al 2006). However, the total number of respiratory deaths were few and ascertainment of disease-specific mortality is fraught with uncertainties. In 6 trials involving 4770 patients, Barr and colleagues found that treatment with tiotropium, compared with placebo, did not significantly reduce all-cause mortality (OR, 0.91; 95% CI, 0.58–1.42) (CitationBarr et al 2006b) (Figure ). Because of its large size and long duration, the UPLIFT study should provide more definitive information about the effect of tiotropium on mortality.
Comparisons with active controls
One randomized trial compared tiotropium to inhaled ipratropium (40 μg qid) as an active control over a 1-year period in 535 patients (CitationVincken et al 2002). Results were closely similar to those trials in which tiotropium was compared with placebo. At the end of 1 year, trough FEV1 was significantly increased by a mean of 120 mL in the tiotropium arm, compared with ipratropium. Additionally, tiotropium, compared with ipratropium, improved the average TDI score by nearly 1 unit, improved average total SGRQ score by 3.3 units, and reduced the proportion of patients who experienced one or more exacerbations (35% vs 46%), all differences being statistically significant (p < 0.05). There was no significant effect on mortality (Figure ). Dry mouth was the only adverse event that was significantly increased in the tiotropium arm (12% vs 6%).
Two trials, one of 12 weeks’ duration and involving 653 patients and the other of 6 months’ duration and involving 807 patients, compared tiotropium with inhaled salmeterol (50 μg bid) (CitationBrusasco et al 2003; CitationBriggs et al 2005). Summary analyses of these two trials indicate that tiotropium was superior to salmeterol in terms of trough FEV1 at the conclusion of the trials (weighted mean difference, 29 ml; 95% CI, 6–51 mL) (CitationBarr et al 2006b). Though trends favored tiotropium in certain other outcomes, SGRQ scores, TDI scores, exacerbation rates, and mortality did not differ statistically between the two treatment arms (Figure ). As in other trials, there were more reports of dry mouth with tiotropium compared salmeterol.
Combination therapy with long-acting beta agonists
International guidelines for COPD treatment recommended long-acting bronchodilators, either a beta agonist or an anticholinergic, for patients with moderately severe COPD or respiratory symptoms that persist despite the administration of short acting bronchodilators (CitationCanada Thoracic Society 2003; ATS/ERS 2004; National Clinical Guideline UK 2004; CitationNational Heart, Lung and Blood Institute 2005). As with tiotropium, long-acting beta agonists increase the FEV1, improve HRQL and dyspnea scores, and reduce exacerbation rates (CitationWilt et al 2005). The guidelines state no preference of one class of long-acting bronchodilator over the other, nor do they recommend combination treatment with these agents.
There is limited information about the clinical benefits of combining tiotropium with a long-acting beta agonist. Small, single-dose trials demonstrated additive FEV1 improvements when tiotropium is combined with either salmeterol or formoterol (CitationCazzola et al 2004a, Citationb). CitationVan Noord et al (2005) showed in a 6-week study that, compared with baseline, average daytime FEV1 improvements over 12 hours with tiotropium alone, formoterol (12 μg) twice daily, and tiotropium plus once-daily formoterol were 127 mL, 86 mL, and 234 mL, respectively. Average 12 hour night-time FEV1 improvements were 43 mL, 38 mL, and 86 mL, respectively. In another study of tiotropium alone or in combination with formoterol, the authors found that, compared with baseline, average FEV1 improvements over 24 hours with tiotropium alone, tiotropium plus once-daily formoterol, and tiotropium plus twice-daily formoterol were 80 mL, 162 mL, and 198 mL, respectively (Citationvan Noord et al 2006). There was also a significant reduction in albuterol rescue medication in the patients receiving both tiotropium and formoterol, but the trial was not powered to show differences for most relevant clinical outcomes. In contrast with the two previous trials, investigators in another study (Citationvan Noord et al 2006) found that, after 7 days of treatment, the difference in average FEV1 improvement 2 hours after a dose of formoterol was 124 mL greater than with tiotropium; at 12 hours there was no significant difference.
The Canadian Optimal Therapy of COPD trial is a 3-arm study in 449 patients that compared tiotropium alone, tiotropium plus salmeterol, and tiotropium plus salmeterol plus fluticasone for 52 weeks (CitationAaron et al 2007). Although there was no significant difference in the primary outcome of the proportion of patients with exacerbations that required treatment with systemic steroids or antibiotics, other outcomes including lung function, disease-specific quality of life, hospitalizations for COPD, and all-cause hospitalization favored treatment with tiotropium plus salmeterol plus fluticasone compared with tiotropium plus placebo. There was no significant differences in these outcomes, comparing tiotropium plus salmeterol with tiotropium alone.
Safety and adverse effects
Tiotropium has a quaternary ammonium structure so that it is poorly absorbed across cell membranes, thus limiting its activity to local bronchodilating effects and minimizing anticholinergic effects due to systemic absorption. Dry mouth is the most commonly reported adverse drug reaction from tiotropium (CitationKesten et al 2006). Approximately 4%–16% of patients experience this side-effect, although it tends to improve over time and it rarely (<1%) necessitates discontinuation of the drug (CitationTashkin et al 2003; CitationDusser et al 2006). In an analysis of pooled studies including 4435 tiotropium patients and 3384 placebo patients with 2159 and 1662 patient years of exposure, respectively, CitationKesten et al (2006) reported the relative risk for dry mouth in tiotropium patients was 3.60 (95% CI, 2.56–5.05).
Only miniscule amounts of tiotropium are absorbed systemically, but as a drug class, anticholinergics have a potential for causing adverse cardiac effects, particularly tachyarrhythmias. One published article reported a small excess number of tachyarrhythmias among patients with mild COPD who received ipratropium for several years (CitationAnthonisen et al 2002). However, an excess number of serious cardiac side-effects in patients receiving tiotropium has not been reported in any of the published trials. CitationBarr et al (2006b) found no significant difference in the incidence of chest pain, myocardial infarction, congestive heart failure, arrhythmias, or atrial fibrillation with tiotropium compared with controls in their meta-analysis. Caution should be taken before applying these results to all patients with COPD, however, as many studies excluded patients with serious cardiac arrhythmias, recent myocardial infarctions, or hospitalizations for congestive heart failure.
Two studies provide more detailed information regarding potential adverse cardiac effects. One study found no evidence of cardiac toxicity after 6 weeks of tiotropium, as measured by 24-hour electrocardiographic (ECG) monitoring (CitationCalverley et al 2003). A 12-week study found no significant differences in heart rate, conduction abnormalities, rhythm, or QTc duration on 24-hour ECG monitoring over a 12-week period in patients receiving tiotropium, compared with placebo (CitationCovelli et al 2005).
Adverse effects of tiotropium on urinary, renal, gastrointestinal, or ocular function appear to be infrequent. A small but statistically significant increase in urinary tract infections with tiotropium was reported by CitationBarr et al (2006b) in their meta-analysis, although there was no significant increase in cases of urinary retention. In the analysis by CitationKesten et al (2006) the relative risk of urinary retention was 10.93 (95% CI, 1.26–94.88). Again, these results may not be generalizable to all patients with COPD, however, as patients with symptoms of moderately severe prostatic hypertrophy or bladder neck outlet obstruction were excluded from participation.
Patients with severe renal impairment were also excluded from most studies. An exception was one study in which tiotropium (4.8 μg) was administered intravenously over 15 minutes to patients with levels of renal insufficiency ranging from mild to severe (creatinine clearance of <30 mL/min) (CitationTurck et al 2004). Blood levels of tiotropium, which is secreted mainly by the kidney in unchanged form, doubled in patients with severe renal impairment compared with those with normal renal function, but no adverse clinical effects were observed.
A substantial proportion of inhaled tiotropium is ingested and could in theory cause gastrointestinal dysmotility. In their meta-analysis, CitationBarr et al (2006b) found no increase in reports of tiotropium-associated constipation. One case of postoperative paralytic ileus attributed to tiotropium has been reported (CitationPraetorius et al 2005).
Due to its anticholinergic effects, tiotropium might also worsen the signs and symptoms of narrow-angle glaucoma, if drug were inadvertently deposited in the eye. One such case has been reported after self application directly in the eye, but exacerbation of glaucoma has not been reported when tiotropium is taken by inhalation as directed (CitationOksuz et al 2006). Nonetheless, tiotropium should be used with caution in patients with narrow angle glaucoma, since they were excluded from all trials.
Cost effectiveness
The cost effectiveness of tiotropium has been formally evaluated based on data from studies performed in Europe and the United States. An economic analysis by CitationOostenbrink et al (2004) performed alongside a clinical trial in the Netherlands (CitationVincken et al 2002) showed that substituting tiotropium for ipratropium increased mean annual healthcare costs (including acquisition costs for tiotropium) by the equivalent of €180; the additional cost to prevent one COPD exacerbation was €667, and the cost to improve the SGRQ by at least 4 units was €1084. In a retrospective analysis of 2 studies performed in the United States (CitationCasaburi et al 2002), CitationFriedman et al (2004) found that treatment with tiotropium reduced average total annual healthcare costs by $US1043 (95% CI, –$US2136 to $US48), although this analysis excluded the costs of anticholinergic drug acquisition (CitationFriedman et al 2004). The reduction in healthcare costs with tiotropium in this analysis was entirely due a reduction in the costs of hospitalizations. In a recent systematic review of the pharmacoeconomic evidence of maintenance treatment for COPD, D’Souza et al (2006) concluded that treatment with tiotropium is cost-effective relative to ipratropium. These authors also found that treatment with inhaled corticosteroids is cost effective in patients with moderate-to-severe COPD, but data are lacking regarding the cost-effectiveness of long-acting beta agonists.
Clinical implications
Tiotropium should be considered for maintenance therapy in patients with moderate to severe COPD. Improvements in pulmonary function with tiotropium are accompanied by improvements in dyspnea, exercise capacity, and HRQL, and a reduction in exacerbations. Although tiotropium has an excellent safety profile in selected patients, the risks and benefits should be carefully weighed in patients with closed angle glaucoma, bladder outlet obstruction or severe cardiac disease. Limited data support the superiority of tiotropium compared with ipratropium; there is insufficient clinical evidence to support the choice of tiotropium over long-acting beta agonists. Tiotropium may reduce the economic burden of COPD by reducing the frequency of COPD exacerbations and COPD-related hospitalizations.
References
- AaronSDVandemheenKFergusson FitzgeraldM2007Tiotropium in Combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized TrialAnn Intern Med2007219[Epub ahead of print]
- American Thoracic Society/European Respiratory Society Task Force2004[updated 2005 September 8]. Standards for the Diagnosis and Management of Patients with COPD [Internet]. Version 1.2. New York: American Thoracic Society; Available from: http://www-test.thoracic.org/copd/
- [ATS/ERS] American Thoracic Society/European Respiratory Society Task Force2005Standardisation of Lung Function TestingEur Respir J2631933816055882
- AnthonisenNRConnettJEEnrightPL2002aHospitalizations and mortality in the Lung Health StudyAm J Respir Crit Care Med166333912153966
- AnthonisenNRConnettJEKileyJP1994Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: The Lung Health StudyJAMA272149715057966841
- AnthonisenNRConnettJEMurrayRP2002bSmoking and lung function of Lung Health Study participants after 11 yearsAm J Respir Crit Care Med166675912204864
- AnthonisenNRWrightECHodgkinJEthe IPPB Trial Group1986Prognosis in chronic obstructive pulmonary diseaseAm Rev Respir Dis13314203510578
- AnzuetoATashkinDMenjogeS2005One-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropiumPulm Pharmacol & Ther18758115649848
- BarnesPJ2000The pharmacological properties of tiotropiumChest11763s6s10673478
- BarrRGBourbeauJCamargoCA2006aInhaled tiotropium for stable chronic obstructive pulmonary diseaseCochrane Database of Systematic Reviews2CD002876
- BarrRGBourbeauJCamargoCA2006bTiotropium for stable chronic obstructive pulmonary disease: a meta-analysisThorax618546216844726
- BeehKMBeierJBuhlR2006Efficacy of tiotropium bromide (Spiriva®) in patients with chronic-obstructive pulmonary disease (COPD) of different severitiesPneumologie60341616761228
- BelmanMJBotnickWCShinJW1996Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med153967758630581
- BelmonteKE2005Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary diseaseProc Am Thorac Soc22973016267352
- Boehringer Ingelheim [online]Accessed 9 Nov 2006. URL: http://www.boehringer-ingelheim.ca/news_releases/2004/2004-03-09.asp
- BriggsDDJrCovelliHLapidusR2005Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPDPulm Pharmacol Ther1839740416179215
- BrusascoVHodderRMiravitllesM2003Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax5839940412728159
- CalverleyPM2006Dynamic hyperinflation: is it worth measuring?Proc Am Thorac Soc32394416636092
- CalverleyPMALeeATowseLJ2003Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary diseaseThorax588556014514937
- Canadian Thoracic Society2003Canadian Thoracic Society Recommendations for Management of Chronic Obstructive Pulmonary DiseaseCan Respir J104A65A
- CasaburiRBriggsDDJrDonohueJF2000The spirometric efficacy of once-daily dosing with tiotropium in stable COPD: A 13-week multicenter trialChest118129430211083677
- CasaburiRKukafkaDCooperCB2005Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPDChest1278091715764761
- CasaburiRMahlerDAJonesPW2002A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J192172411866001
- CazzolaMCentanniSSantusP2004aThe functional impact of adding salmeterol and tiotropium in patients with stable COPDRespir Med9812142115588043
- CazzolaMDi MarcoFSantusP2004bThe pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPDPulm Pharmacol Ther1735914643169
- CelliBZuWallackRWangS2003Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumesChest12417434814605043
- ChodoshSFlandersJSerbyCW2001Effective use of Handihaler dry powder inhalation system over a range of COPD severityJ Aerosol Med143091511693842
- CovelliHBhattacharyaSCassinoC2005Absence of electrocardiographic findings and improved function with once-daily tiotropium in patients with chronic obstructive pulmonary diseasePharmacotherapy2517081816305289
- DonohueJF2005Minimal clinically important differences in COPD lung functionJ COPD211124
- D’SouzaAOSmithMJMillerLAAn appraisal of pharmacoeconomic evidence of maintenance therapy for COPDChest129169370816778291
- DusserDBravoM-LIaconoPon behalf the MISTRAL study group2006The effect of tiotropium on exacerbations and airflow in patients with COPDEur Respir J275475516507855
- FriedmanMMenjogeSSAntonSF2004Healthcare costs with tiotropium plus usual care versus usual care alone following 1 year of treatment in patients with chronic obstructive pulmonary disorder (COPD)Pharmacoeconomics22741915250751
- HalbertRJIsonakaSGeorgeD2003Interpreting COPD prevalence estimates: what is the true burden of disease?Chest12316849212740290
- HighlandKBStrangeCHeffnerJE2003Long term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease. A meta-analysisAnn Intern Med1389697312809453
- HillemanDEDewanNMaleskerM2000Pharmacoeconomic evaluation of COPDChest11812788511083675
- JonesPWBoshTK1997Quality of life changes in COPD patients treated with salmeterolAm J Respir Crit Care Med155128399105068
- KestenSJaraMWentworthC2006Pooled clinical trial analysis of tiotropium safetyChest1301695117166984
- KoyamaSRennardSIRobbinsRA1992Acetylcholine stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activityAm J Physiol262L466711566862
- LittnerMRIlowiteJSTashkinDP2000Long-acting bronchodilation with once-daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary diseaseAm J Respir Crit Care Med16111364210764302
- Lopez-VidrieroMTCostelloJClarkTJ1975Effect of atropine on sputum productionThorax3054371198394
- MahlerDAWeinbergDHWellsCK1984The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexesChest8575186723384
- MaltaisFHamiltonAMarciniukDImprovements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPDChest12811687816162703
- McNicholasWTCalverleyPMLeeA2004Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPDEur Respir J238253115218993
- MichaudCMChristopherJLMurrayCJL2001Burden of disease - implications for future researchJAMA285535911176854
- National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care2004Thorax593913014694246
- National Heart, Lung, and Blood Institute. Global Initiative for Chronic Obstructive Lung Disease. Updated 2005. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Accessed 3 Nov 2006. URL: http://www.goldcopd.org
- NiewoehnerDECollinsDErblandMLfor the Department of Veterans Affairs Cooperative Study Group2000Relation of FEV1 to clinical outcomes during exacerbations of chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1611201510764312
- NiewoehnerDERiceKCoteC2005Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trialAnn Intern Med1433172616144890
- NomuraJHosoiTOkumaY2003The presence and functions of muscarinic receptors in human T-cells: the involvement in IL-2 and IL-2 receptor systemLife Sci722121612628467
- O’DonnellDEFlugeTGerkenF2004Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPDEur Respir J238324015218994
- O’DonnellDELamMWebbKA1998Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1581557659817708
- OksuzHTamerCAkogluS2006Acute angle-closure glaucoma precipitated by local tiotropium absorptionPulm Pharmacol Ther[Epub ahead of print] doi:10.1016/j.pupt.2006.07.002.
- OostenbrinkJBRutten-van MolkenMPVanMJ2004One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary diseaseEur Respir J23241914979498
- PraetoriusGM2005[Postoperative intestinal paralysis caused by the muscarinic receptor blocker tiotropium bromide]. [German]Urologe44393515672237
- RedelmeierDAGoldsteinRSMinST1996Spirometry and dyspnea in patients with COPDChest109116388625661
- RiesAL2006Impact of chronic obstructive pulmonary disease on quality of life: the role of dyspneaAm J Med119S1220
- RodrigoGJNanniniLJ2006Tiotropium for the treatment of stable chronic obstructive pulmonary disease: a systematic review with meta-analysis [online]Pulm Pharmacol Therdoi:10.1016/j.pupt.2006.02.003.
- SalpeterSRBuckleyNSSalpeterEE2006Meta-analysis: anticholinergics, but not beta-agonists, reduce severe exacerbations and respiratory mortality in COPDJ Gen Intern Med2110111916970553
- SinDDMcAlisterFAManSF2003Contemporary management of chronic obstructive pulmonary disease: scientific reviewJAMA29023011214600189
- SullivanSDRamseySDLeeTA2000The economic burden of COPDChest1175910631188
- SutherlandERAllmersHAyasNT2003Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysisThorax589374114586043
- TamaokiJChiyotaniATagayaE1994Effect of long term treatment with oxitropium bromide on airway secretion in chronic bronchitis and diffuse panbronchiolitisThorax495458016790
- TashkinDP2006The role of patient-centered outcomes in the course of chronic obstructive pulmonary disease: how long-term studies contribute to our understandingAm J Med11910 Suppl 1637216996901
- TashkinDKestenS2003Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responsesChest1231441912740259
- TurckDWeberWSigmundR2004Pharmacokinetics of intravenous, single-dose tiotropium in subjects with different degrees of renal impairmentJ Clin Pharmacol441637214747425
- TurinoGM2005Therapeutic gains of prolonged bronchial dilatation in chronic obstructive pulmonary diseaseAnn Intern Med143386716144897
- van NoordJAAumannJLJanssensE2005Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPDEur Respir J262142216055868
- van NoordJAAumannJLJanssensE2006Effects of tiotropium with and without formoterol on airflow obstruction and resting hyper-inflation in patients with COPDChest1295091716537846
- van NoordJASmeetsJJCustersFLKorducki2002Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary diseaseEur Respir J196394411998992
- VerkindreCBartFAguilaniuB2006The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary diseaseRespiration73420716484769
- VinckenWvan NoordJAGreefhorstAPM2002Improved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J192091611871363
- WiltTJNiewoehnerDKimC-B2005Use of spirometry for case finding, diagnosis, and management of chronic obstructive pulmonary disease (COPD). Evidence Report/Technology Assessment No. 121 (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290–02–0009.) AHRQ Publication No. 05-E017–2. Rockville, MD. Agency for Healthcare Research and Quality. September 2005.
- WitekTJJrMahlerDA2003Meaningful effect size and patterns of response of the transition dyspnea indexJ Clin Epidemiol62485512725879
- World Health Organization2000World health report [online]. Geneva: World Health Organization. URL: URL: http://www.who.int/whr/2000/en/statistics.htm