Abstract
Chronic obstructive pulmonary disease (COPD) and heart diseases are considered independent risk factors for mortality and major cardiopulmonary complications after surgery. Coronary artery disease, heart failure and COPD share common risk factors and are often encountered, - isolated or combined -, in many surgical candidates. Perioperative optimization of these high-risk patients deserves a thorough understanding of the patient cardiopulmonary diseases as well as the respiratory consequences of surgery and anesthesia.
In contrast with cardiac risk stratification where the extent of heart disease largely influences postoperative cardiac outcome, surgical-related factors (ie, upper abdominal and intra-thoracic procedures, duration of anesthesia, presence of a nasogastric tube) largely dominate patient’s comorbidities as risk factors for postoperative pulmonary complications.
Although most COPD patients tolerate tracheal intubation under “smooth” anesthetic induction without serious adverse effects, regional anesthetic blockade and application of laryngeal masks or non-invasive positive pressure ventilation should be considered whenever possible, in order to provide optimal pain control and to prevent upper airway injuries as well as lung baro-volotrauma. Minimally-invasive procedures and modern multimodal analgesic regimen are helpful to minimize the surgical stress response, to speed up the physiological recovery process and to shorten the hospital stay. Reflex-induced bronchoconstriction and hyperdynamic inflation during mechanical ventilation could be prevented by using bronchodilating volatile anesthetics and adjusting the ventilatory settings with long expiration times. Intraoperatively, the depth of anesthesia, the circulatory volume and neuromuscular blockade should be assessed with modern physiological monitoring tools to titrate the administration of anesthetic agents, fluids and myorelaxant drugs. The recovery of postoperative lung volume can be facilitated by patient’s education and empowerment, lung recruitment maneuvers, non-invasive pressure support ventilation and early ambulation.
Introduction
COPD and cardiovascular disease in surgical patients
Nowadays, surgeons, anesthesiologists and chest physicians are coping with large numbers of high-risk respiratory patients as a consequence of prolonged life expectancy, increasing prevalence of COPD and greater needs for invasive diagnostic procedures and surgical interventions (CitationHalbert et al 2006). The prevalence of COPD is even higher among surgical candidates compared with aged-matched population groups (eg, 5%–10% of COPD patients in general surgery, 10%–12% in cardiac surgery and 40% in thoracic surgery vs. 5% of COPD patients in the general population) (CitationMcAlister et al 2003; CitationHalbert et al 2006; CitationLicker et al 2006). As common risk factors (ie, smoking, advanced age and sedentarity) are shared by cardiac and pulmonary diseases, a large proportion of COPD patients are afflicted with hypertension (34%), occlusive or aneurismal arterial disease (12%), heart failure (5%), cardiac arrhythmia or conduction blockade (12%) and ischemic heart disease (11%) (CitationSin et al 2005).
Although mortality directly attributable to anesthesia is very low – probably around 1 in every 250,000 anesthetics – the operative mortality risk averages 0.5%–1%, being mainly attributed to myocardial infarct and heart failure, the leading causes of death in Western countries (CitationErgin et al 2004). Not surprisingly, patients with pre-existing organ dysfunction (eg, ischemic heart disease, COPD and renal insufficiency) are more likely to develop an acute coronary syndrome, heart failure, bronchopneumonia or respiratory failure following major interventions (CitationKaafarani et al 2004).
In the past decades, the attention of health care providers was mainly focused on cardiovascular ischemic events and the importance of postoperative pulmonary complications (PPCs) has been largely underestimated. More recent prospective cohort studies have highlighted that the incidence of respiratory failure (1%–3%) and bronchopneumonia (1%–5%) after noncardiac surgery was similar to the incidence of major cardiovascular complications (cardiac failure, 1%–2%; myocardial infarction, 0%–6%) (CitationFleischmann et al 2003). In addition to the short- and long term death toll, these major cardiac and pulmonary complications implicate an enormous economic burden as a result of patient’s admission to intensive care units (ICU), prolonged hospital stay and use of expensive therapeutic treatment (CitationSchweizer et al 2002; CitationFleischmann et al 2003). Not infrequently, cardiac and pulmonary complications concur in the same surgical patients. For instance, severe intra-operative bleeding directly increases the risk of myocardial ischemia/infarct, ventilator-induced pneumonia, sepsis and transfusion-related acute lung injury (ALI).
How to define postoperative pulmonary complications (PPC)
As a prerequisite for perioperative risk assessment, clinicians and scientists should clearly define meaningful criteria of specific disease conditions and surgical outcome endpoints. In the medical literature, the wide range in the incidence of PPCs (from 3% up to 80%) reflects heterogenous population groups, inconsistent diagnostic/outcome definitions and incomplete data derived from retrospective studies (CitationFisher et al 2002). Although the development of respiratory dysfunction may forecast significant PPCs, it may also reflect the “natural” postoperative recovery processes. Transient and self-limiting impairment in spirometric values, respiratory muscle strength and gas exchange should be considered as part of the physiological responses to surgery. For instance, most patients undergoing cardiothoracic or abdominal operations present some degree of hypoxemia and diffuse micro-atelectasis that will barely impact on the postoperative clinical course. In contrast, pleural effusions, sustained bronchospasm or fever, lobar atelectasis or hypoxemia non-responsive to supplemental oxygen may forecast more serious adverse events like bronchopleural fistula, bronchopneumonia, ALI or respiratory failure requiring urgent medical interventions.
In an effort to standardize the reporting of adverse peri-operative events, a group from the University Hospital of Zurich has validated a 5-grade scoring system based on the therapeutic consequences and residual disabilities in relation to surgical operations (CitationDindo et al 2004). Instead of taking into account biased surrogate items such as the length of hospital stay, the new scoring system ranks the severity of postoperative complications by assessing organ dysfunction, long-term disabilities as well as the incremental need for pharmacologic treatment or other supportive/corrective interventions (physiotherapy, endoscopy, drainage, re-interventions) (). Grade I complication entails any deviation from the normal postoperative course with no need for medical interventions, except antiemetics, antipyretics, analgesics, electrolytes, diuretics. Grades II and III involve complications requiring pharmacological treatment, blood transfusions or endoscopic, surgical or radiological interventions. Grade IV includes life-threatening complications as well as single or multiple organ failure requiring ICU admission. Ultimately, perioperative death corresponds to a grade V. Availability of these objective and detailed outcome data is helpful to qualify hospital’s and physician’s performances and to improve quality of care allowing cost-efficient assessment of health care interventions and human resources (CitationBruce et al 2001).
Table 1 Grading of postoperative cardiopulmonary complications
Understanding the physiological response to surgical insults, identification of mortality/morbidity risk factors and gaining control over health care processes are the preliminary steps before testing and implementing medical interventions aimed to improve postoperative outcome. In this review, we will first discuss the risk factors of postoperative cardiopulmonary complications, then we will describe the respiratory consequences of surgery and anesthesia; finally, we will propose guidelines for optimizing perioperative medical care in COPD patients.
Risk factors for postoperative mortality and cardiopulmonary morbidity
Risk stratification
Along with major advances in surgical and anesthetic management, the operative mortality and morbidity rates have been considerably lowered over the past 20 years (CitationBonnet and Marret 2005). For instance, after lung resection and aortic abdominal reconstruction, operative mortality has dropped from 10% in the eighties to the current rates of less than 3% in most reference hospital centers (CitationSpiliopoulos and de Perrot 2000; CitationLicker et al 2002). In such context, patient- and procedure-related risk factors need to be thoroughly re-evaluated concerning their impact on postoperative cardiac and pulmonary outcomes.
Cardiac risk stratification
In 1999, CitationLee et al (1999) analyzed a comprehensive large-scale surgical database and identified 5 independent risk factors for major postoperative cardiac complications: (1) evidence for coronary artery disease, (2) history of cerebrovascular disease (stroke, ischemic attack), (3) moderate-to-severe renal insufficiency (serum creatinine greater than 180 mm/L), (4) diabetes mellitus, and (5) high-risk surgery (chest, abdominal, vascular). After validation in further prospective studies (CitationBoersma et al 2005), these five items have been incorporated in a Revised Cardiac Risk Index (RCRI) which has been endorsed by the American College of Cardiology and American Heart Association (ACC/AHA) (CitationFleisher et al 2006). Noteworthy, patients presenting with one or more of these risk factors (RCRI), particularly those with limited exercise capacity (less than 4 MET; 1 MET = basal metabolic rate) and those undergoing major surgical procedure should be considered for preoperative non-invasive testing in order to exclude poor ventricular function, valvular dysfunction or silent coronary artery disease. Ultimately, preoperative clinical assessment (RCRI) and cardiac testing (thallium scintigraphy, echocardiography, stress test) are most useful to identify patients with ischemic heart diseases who might benefit from preoperative revascularization and perioperative cardiac protective techniques (ie, beta-blockers, anti-platelets, statines) (CitationFleisher et al 2006).
Recent data indicate that the risk-adjusted mortality following noncardiac surgery is much higher in patients with aortic stenosis (10%–28%), or heart failure (11.7%) than among those with coronary artery disease (6.6%) (CitationHernandez et al 2004; CitationKertai et al 2004; CitationZahid et al 2005). When the aortic valvular surface area is less than 0.5 cm2/m2 body surface area, valvular prosthetic replacement should be planned before elective noncardiac surgery or alternate non-surgical options should be considered. Chronic heart failure is chiefly the end stage of most hypertensive, coronary and valvular diseases. Because of the aging population and the prolongation of the lives with modern therapies, the burden of these cardiovascular diseases is also growing among COPD patients. In adults older than 65 years, 2 to 9% suffer from aortic valve stenosis and the prevalence of congestive heart failure lies within 8%–12% (CitationChrist et al 2005; CitationZahid et al 2005).
Pulmonary risk factors
In two prospective studies, a group of Canadian pneumologists have questioned whether preoperative clinical assessment, pulmonary functional testing and intraoperative interventions could predict the occurrence of PPCs, namely pneumonia, atelectasis, respiratory failure, pneumothorax and pleural effusion (CitationMcAlister et al 2003; CitationMcAlister et al 2005). The anesthesiologists referred only 8% of surgical candidates to a specialized consultation and the PPCs were best predicted by the following markers: advanced age (≥ 65years, Odds Ratio [OR] 5.9), a positive cough test (OR 3.8), smoking ≥40 pack-years (OR 1.9), the presence of a nasogastric tube (OR 7.7) and anesthesia duration exceeding 2.5 hours (OR 3.3). Major limitations of this study were related to the small population sample (N = 1,055 and 272 patients, respectively), the low incidence of PPCs (2.8 and 8%), the small proportion of major surgical procedures and the exclusion of high-risk patients, namely those with sleep apnea syndrome, neuromuscular disease and severe COPD requiring a planned admission to the ICU.
In 2001, the first pulmonary risk indices for the occurrence of pneumonia and respiratory failure have been validated by analyzing two large cohorts including more than 160,000 veterans undergoing noncardiac surgery (CitationArozullah et al 2000, Citation2001). In analogy with the RCRI, these pulmonary risk scores were established by assigning points to several predictive factors for pneumonia (14 variables) and respiratory failure (12 variables) (). Overall, the major contributors to PPCs were related to an advanced age (>70 yrs), the type of surgery (vascular, thoracic), the presence of COPD, renal failure or poor nutritional status as well as the occurrence of major intraoperative blood loss (>4 units packed blood cells).
Table 2 Multivariate risk indices for postoperative pneumonia, respiratory failure and cardiac complications
More recently, a systematic review including 145 studies (cohort, case-control, case-series and randomized controlled trials) of whom 27 reported multivariate analysis has provided further critical insight in preoperative pulmonary risk stratification. The overall incidence of PPC was 6.8% (ranging from 2% to 19%) (CitationSmetana et al 2006). Not surprisingly, these clinical studies were heterogenous regarding their sample sizes (median 148 patients per group), study objectives, definitions of PPCs and inclusion criteria. In contrast with cardiac risk stratification where the extent of heart disease largely determined postoperative cardiac outcome, procedure-related factors largely dominated patient’s related comorbidities to predict PPCs. Peripheral and orthopedic procedures were considered at low-risk to develop PPCs whereas surgical interventions involving the thorax, the upper abdomen, as well as the head and neck regions, were more likely to interfere with respiratory function, particularly in the context of extended tissue trauma, large body fluid shifts and multiple transfusion. Among patient-related predictors, the following items have been found to be independently associated with PPCs : 1) advanced age (≥70 yrs), 2) the American Society of Anesthesiologist (ASA) physical status classification (classes ≥2), 3) a history of congestive heart failure, 4) the presence of COPD, 5) a poor general condition and 6) a low blood albumin level (<35 g/L).
Specific risk factors for pulmonary complications
COPD
Exacerbation of bronchial inflammation with airway instrumentation, preoperative bacterial airway colonization, surgery-induced immunosuppression and increased muscular work of breathing may all promote the onset of PPCs. Although results from small series have suggested an acceptable operative risk in COPD patients (CitationKroenke et al 1992), the incidence of PPCs (except atelectasis) most often parallels the severity of respiratory impairment (moderate, if FEV1 50%–80%; severe, if FEV1 < 50%), particularly in patients with abnormal clinical findings (decreased breath sounds, wheezes, ronchi, prolonged expiration) and/or marked alterations of gas exchange (PaCO2 > 7 kPa, hypoxemia requiring supplemental oxygen). The worst prognosis is expected in patients with pulmonary artery hypertension and chronically “fatigued” respiratory muscles given the risk of right ventricular failure with hemodynamic collapse and ventilator-dependency (CitationJaber et al 2005; CitationRamakrishna et al 2005).
In patients undergoing lung resection, a FEV1 below 60% has been shown to carry a two to threefold increased risk for operative mortality and major respiratory complications (CitationWhalen F et al 2006). Besides the severity of COPD, the extent of lung resection, bronchial colonization and male sex have also been identified as predictors of postoperative pneumonia (CitationLicker, de Perrot et al 2003; CitationLicker et al 2006; CitationSchussler et al 2006; CitationTirumalasetty and Grammer 2006).
Asthma
Recent asthma symptoms, current use of anti-asthma drugs and history of tracheal intubation for asthma have all been associated with the development of PPCs. In contrast, other clinical studies have shown that patients with well-controlled asthmatic disease present similar risk for PPCs as those patients without asthma, regardless of the type of anesthesia (CitationTirumalasetty and Grammer 2006). In Warner’s study of over 1,500 asthmatic patients, the PPC rates following general anesthesia and regional anesthesia were similar, refuting the empiric notion that regional anesthetic techniques were safer (CitationWarner et al 1996). Although infrequently associated with major PPCs, sustained or unrecognized bronchospasm induced by airway instrumentation may cause life-threatening hypoxemia whereas overzealous treatment with beta-adrenergic agonists may cause fatal ventricular arrhythmias. In the closed claim study of the American Society of Anesthesiologists, severe bronchospasm resulting in death or irreversible brain damage was implicated in 90% of cases charged because of severe intraoperative respiratory problems (CitationPeterson et al 2005).
General anesthesia (GA)
General anesthetic agents, opiates, myorelaxants as well as mechanical ventilation are known to interfere with the respiratory system. The combined effects of the supine position, GA and thoracic/abdominal incision produce an immediate decline in lung volumes with atelectasis formation in the most dependent parts of the lung (CitationHedenstierna and Edmark 2005). Moreover, residual neuromuscular blockade persisting after anesthesia emergence has been incriminated in deficient coughing, depressed hypoxic ventilatory drive and “silent” inhalation of gastric contents (CitationBerg et al 1997).
General anesthesia exceeding 2.5–4 hours has been identified as a strong predictor of PPCs (CitationArozullah et al 2000, Citation2001). Indeed, prolonged exposure to general anesthetics alters the immune defenses and gas exchange capacity mainly by depressing the alveolar macrophage function, interfering with surfactant production, slowing of the mucociliary clearance and increasing the permeability of the alveolar-capillary barrier. Other deleterious mechanisms involve the enhanced sensitivity of the pulmonary vasculature to neurohumoral mediators, activation of the alveolar nitric-oxide synthase and enhanced release of pro-inflammatory cytokines.
Upper airway instrumentation (eg, tracheal intubation) and inhalation of irritants (eg, desflurane, external disinfectants) may trigger vagally-mediated reflex bronchoconstriction thereby promoting the expiratory collapse of the peripheral airways with incomplete lung alveolar emptying (CitationBerry et al 1999; CitationGoff et al 2000).
Obstructive apnea syndrome (OAS)
A preoperative history of snoring, apnea during sleep and abnormalities of the oropharynx (large tonsils, retrognathia, maxillary hypoplasia) are strongly evocative of an OAS, particularly in obese subjects where difficulties with airway instrumentation should be anticipated (Citationden Herder et al 2004). Even very low concentrations of anesthetics, sedatives and opiates may trigger or worsen OSA by decreasing pharyngeal muscle tone and blunting the ventilatory and arousal responses to hypercarbia and upper airway obstruction. Given their higher prevalence of hypertension, arrhythmias, congestive (right) heart failure and coronary artery disease, patients with OAS are more susceptible to develop postoperative cardiac and cerebral ischemic damages.
Age
After adjustment for concomitant systemic illnesses, advanced age (>70 years) remains an independent predictor of operative mortality, PPCs and cardiac morbidity (CitationPolanczyk et al 2001). The loss of physiological organ reserve and “silent” organ dysfunction make the elderly prone to develop cardiopulmonary and cerebrovascular complications when facing surgical or traumatic insults. For instance, the airway closing capacity increases with age as does the perfusion in lung units with low ventilation/perfusion ratios (VA/Q) that result in increasing venous admixture and, in turn deterioration in blood oxygenation (CitationHedenstierna and Edmark 2005). Surprisingly, atelectasis formation is independent of age, with children and teenagers showing as much atelectasis as elderly patients (CitationGunnarsson, Tokics, Gustavsson et al 1991).
Obesity
Premature collapse of peripheral airways is most likely to occur in obese patients because of the restrictive ventilatory pattern and the elevated closing airway capacity (Citationvon Ungern-Sternberg et al 2004). Although the reduction in functional reserve capacity (FRC) facilitates the formation of atelectasis and aggravates postoperative hypoxemia, clinical investigations have failed to document any association between mild-to-moderate obesity and severe PPCs, except among the subset of patients with OSA. A body mass index exceeding 40 kg/m2 also conveys a higher risk for PPCs. In these morbidly obese patients, the likelihood to develop atelectasis or pneumonia after abdominal surgery was found to be close to 30% with an adjusted odds ratio of 2.8 (95% confidence interval 1.7–4.8) compared with non-obese patients (Citationvon Ungern-Sternberg et al 2004). Besides functional pulmonary impairment, a greater propensity to develop postoperative wound infection and thromboembolic events has been consistently demonstrated in overweight patients.
General status and physical fitness
The 5 grade American Society of Anesthesiology (ASA) classification is applied worldwide by all anesthesiologists. This physical status scoring system was originally designed to estimate the risk of operative mortality. Subsequently, a number of studies have extended its use to predict postoperative outcome. Patients belonging to ASA classes 3 and 4 being at higher risk for cardiopulmonary complications than ASA 1 and 2 patients () (CitationWolters et al 1996).
Table 3 General health status assessment
Similarly, simple information from the patient’s history and clinical examination such as an altered sensorium, the inability to climb at least two flights of stairs and functional dependency (ie, need for assistance in daily life activities) have been shown to be predictive of major postoperative cardiopulmonary complications. More sophisticated physiological assessment such as the determination of the anaerobic threshold during exercise (maximal oxygen consumption, VO2 max) and the six minutes walking test also provides valuable prognostic information in borderline patients (CitationWin et al 2005). Before lung resection, a VO2 max less than 15 ml/kg/min, a walking distance less than 300 m over 6 minutes or the inability to climb at least four flights of stairs are better predictors than spirometric variables to discriminate those patients who will, or will not, develop cardiopulmonary complications. Diminished cardiac and pulmonary physiological reserves should be differentiated from muscular deconditioning due to chronic debilitating illnesses as possible causes of reduced aerobic exercise capacity.
Smoking
Cigarette-smoke contains more than 1,000 components with wide-ranging effects on pulmonary, cardiovascular and immune functions, healing of wounds, hemostasis, drug metabolism and patient mental status, all of which may influence the postoperative outcome (CitationMoller et al 2002; CitationWarner 2006). Smoking status is a consistent univariate risk factor for adverse cardiac events and a variety of pulmonary adverse events such as bronchospasm, laryngospasm, cough and hypoxemia requiring ICU admission. In observational studies using multivariate analysis, current smoking (≥20 units pack year) has been identified as a modest risk factor for serious PPCs (OR 1.26 with 95% CI, 1.01–1.56) and tissue-healing problems but not for cardiac complications, although smoking behavior obviously influences the progression of vascular atheromatosis and long-term survival (CitationSmetana et al 2006). Not only active smoking but also passive exposure to smoking in children and adults have been found to increase the risk of intra- and postoperative respiratory problems and to prolong the effects of neuromuscular blocking agents (CitationDrongowski et al 2003; CitationReisli et al 2004).
Several mechanisms render smokers more susceptible to develop PPCs: excessive production of mucus, increased sensitivity of upper airway reflexes, slowed bronchial mucus clearance, alteration in surfactant synthesis, increase in pulmonary epithelial permeability as well as impaired macrophage and natural killer cytotoxic activity. The number of pack-year cigarettes/cigars and the timing of smoking cessation may influence both the sensitivity and the reactivity of the tracheobronchial tree, mucociliary transport as well as the occurrence of PPCs (CitationWarner et al 1989). Actually, impaired wound/bone healing, susceptibility to bacterial infections and dehiscence of vascular/bowel anastomosis may result from the limited availability of oxygen within healing tissues along with the anti-proliferative effects of nicotine on red blood cells, fibroblasts and macrophages.
Interestingly, the dose-requirements for anesthetic induction and postoperative opiate analgesia are slightly higher in smokers compared with non-smokers whereas the incidence for nausea and vomiting is surprisingly lower (CitationCreekmore et al 2004; CitationLysakowski et al 2006; CitationWhalen F et al 2006). Although increased baseline stress is often reported among smokers, changes in perceived stress over the perioperative period and acute withdrawal symptoms are unrelated to the current smoking status (CitationWarner et al 2004; CitationWarner 2006).
Alcohol
A two-fold increased risk of ALI has been observed among heavy alcohol consumers (more than 60 g of ethanol per day) admitted to the medical ICU and those undergoing lung resection, compared with alcohol-free patients (CitationLicker, de Perrot et al 2003). Experimental data indicate that ethanol causes depletion of pulmonary antioxidant glutathione which in turn leads to decreased surfactant production, impaired alveolar liquid clearance and alterations in epithelial cell permeability (CitationBurnham et al 2004). Moreover, chronic alcoholics have a greater vulnerability to postoperative bleeding and infectious complications consequent to impaired immune status (eg, suppression of interleukin-6 to interleukin-10 ratio), nutritional deficits and bronchoaspiration in the context of autonomic neural dysfunction and alcohol withdrawal syndrome (CitationPaull et al 2004).
Respiratory consequences of anesthesia and surgery
Anesthetic techniques and respiratory function
Respiratory mechanics and general anesthesia
In awake supine subjects, contraction of the inspiratory muscles causes lung expansion along with a downward shift of the diaphragm whereas during passive expiration, the dome-shaped diaphragm moves cranially as a result of the elastic lung recoil and the relaxation of the inspiratory muscles; the hydrostatic pressure gradient due to the abdominal content and the eventual active contraction of the expiratory abdominal wall muscles may further displace the diaphragm in cranial direction (). Following anesthesia induction – with or without muscular paralysis – all respiratory muscles being relaxed, there is a fall in the functional reserve capacity (FRC) with a consistent cephalad shift of the dependent part of the diaphragm while the nondependent diaphragmatic part moves slightly downwards or remains static (CitationWarner 2002). With mechanical ventilation, the positive transpulmonary pressure causes the greatest displacement of the nondependent part, although with large tidal volume, all parts of the diaphragm move equally (“piston-like” motion). These anesthetic-induced changes in diaphragmatic motion have been observed in healthy subjects as well as in COPD patients (CitationGunnarsson, Tokics, Lundquist et al 1991).
Figure 1 Thoracic wall and diaphragmatic motion during breathing in awake (dotted line) and in anesthetized subjects (continuous line).
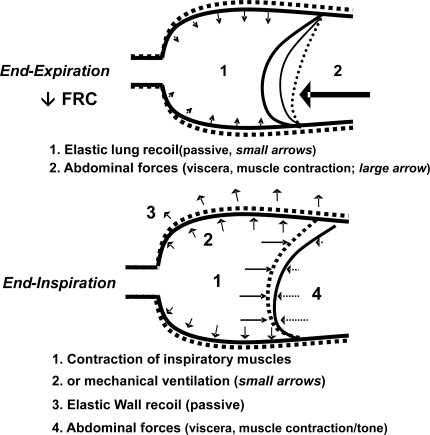
As demonstrated by chest imaging techniques, atelectatic areas develop in almost 90% of anesthetized patients, involving as much as 5%–20% of the total lung volume, predominantly in its dependent parts (CitationHedenstierna and Edmark 2005). By changing the position from upright to supine in healthy adults, the FRC is reduced by 0.8–1.0 L and there is a further decrease by 0.4–0.5 L after the induction of anesthesia. The reduction in FRC (from 3.5 to 2 L, near the residual volume) corresponds to the formation of atelectasis and low ventilation-perfusion areas () that in turn reduce the compliance of the respiratory system (from a mean of 95 to 60 ml/cm H2O). Not surprisingly, the size of the atelectatic areas correlates with the decrease in FRC and the fall in arterial oxygen saturation (CitationLindberg et al 1992).
Some of the collapsed areas persist in the postoperative period and are compounded by limited respiratory motion due to insufficient pain control, injuries of respiratory muscles and/or diaphragmatic dysfunction after manipulation of abdominal viscera or intra-thoracic interventions. Increased “stiffness” of the thoracic/abdominal compartments and increased airway resistance due to lower lung volumes both contribute to increase the work of breathing after major surgical procedures (CitationHedenstierna and Edmark 2005).
General anesthetic agents
General anesthetic agents modulate the respiratory function at 3 key levels: (1) the central medullary command and the neural reflex ventilatory pathways, (2) the airways involving muscular tone and mucociliary clearance, and (3) the hypoxic pulmonary vasoconstriction (HPV) which controls the distribution of blood flow versus ventilation (). Of note, the anesthetic-induced impairment in respiratory function is only transient and the breathing pattern completely normalizes within 2 to 6 hours after anesthesia emergence in volunteers and following peripheral or lower abdominal procedures.
Table 4 Effects of anesthesia on respiratory function
Opiates, benzodiazepines and anesthetic agents attenuate the central and peripheral chemosensitive reflexes with a consequent dose-dependent depression of the hypoxic and hypercapnic ventilatory responses (Citationvan den Elsen et al 1995, Citation1998; CitationDahan et al 2004). Few sedative drugs are devoid of central respiratory depressive effects: droperidol potentiates the hypoxic ventilatory response by blocking the action of endogenously released dopamine and the histamine-antagonist, diphenhydramine, counteracts the alfentanil-induced decrease in the slope of the CO2 ventilatory response (CitationBabenco et al 1998). None of the intravenous agents are thought to interfere with bronchial smooth muscle tone neither with the HPV response.
In contrast, volatile anesthetic agents (halothane, isoflurane, desflurane, sevoflurane) are known to relax directly bronchial and vascular smooth muscles (CitationRooke et al 1997). In isolated lung preparations with lobar atelectasis or hypoxic ventilation, volatile anesthetics given at twice the minimum alveolar concentration (2 MAC) have been shown to decrease the HPV response by 50% if the pulmonary flow was maintained constant. In these experimental conditions, redistribution of pulmonary blood flow towards poorly oxygenated regions contributes to widen the alveolar-arterial oxygen gradient (PA-aO2) and to cause arterial oxygen desaturation by increasing intra-pulmonary shunting (CitationMoudgil et al 2005). In clinical practice, only mild impairment in blood gas exchange is observed under lower concentrations of inhalational anesthetics (1–1.5 MAC), because the direct anesthetic-induced inhibition of the HPV response is partially opposed by the reduced mixed venous oxygen pressure consequent to the anesthetic-induced myocardial depression.
Myorelaxants
As already mentioned, residual neuromuscular blockade is an independent predictor of PPCs and has been incriminated in alveolar hypoventilation and silent gastric regurgitation with bronchoaspiration. Indeed, mild blockade of the muscarinic receptors with myorelaxants has been shown to attenuate the ventilatory response to hypoxia and to impair the contracting capacity of the pharyngo-laryngeal muscles, thereby interfering with the control of the upper airways (CitationEikermann et al 2006). In addition, volatile anesthetic agents and several pharmacologic agents (eg, magnesium, aminoglycosides) are known to enhance the effects of myorelaxants at the neuromuscular endplate by lowering the neural release of acetylcholine, inducing conformational change within the cholinergic receptor and/or by altering intra-muscular signaling (CitationEriksson 1994). Moreover, prolonged neuromuscular blockade should be anticipated in patients with defective metabolic clearance pathways (hepatic or renal insufficiency) and those with neuromuscular disorders (ie, myasthenia, deconditioning, Guillain-Barre syndrome), particularly if long acting myorelaxants are repeatedly or continuously administered.
Regional anesthesia
Spinal and epidural anesthesia at the lumbar level have no significant effects on respiratory function, except in morbidly obese patients where the neuraxial blockade has been shown to produce a 20%–25% fall in expiratory functional volume (FEV1, FVC) that may interfere with the ability to cough and to clear bronchial secretions as a result of blocking the abdominal wall muscles (CitationRegli et al 2006).
With thoracic epidural analgesia (TEA) using high concentrations of local anesthetics (lidocaine 2%, bupivacaine 0.5%), paralysis of the intercostal and abdominal wall muscles are responsible for a −10% to −20% decrease in inspiratory and expiratory capacity without affecting the HPV, the bronchial smooth muscle tone and the ventilatory response to hypoxia and hypercarbia (CitationGroeben et al 1994; CitationSakura et al 1996). Diaphragmatic function remains unimpeded as far as the neuraxial blockade remains below the cervical emergence of phrenic nerves (C3–C5). In addition to blocking the nociceptive afferences, TEA directly suppresses the sympathetic efferent neural outflow (from C8 to T4), it reduces by 20% the hypnotic requirements and confers cardiac protective effects by augmenting coronary blood flow, lowering myocardial oxygen consumption, attenuating platelet hyperaggregability and raising the arrhythmic ventricular and atrial thresholds (CitationGroban et al 1999; CitationHodgson and Liu 2001; CitationLicker, Spiliopoulos et al 2003; CitationLiu et al 2004).
Regarding peripheral nerve blockade, the supraclavicular techniques (ie, interscalene anesthetic block) are contraindicated in patients with severe pulmonary disease since paralysis of the ipsilateral hemidiaphragm occurs in 100% of cases with consequent limitations of lung volumes (mean −32% FEV1, −30% FVC) (CitationUrmey and Gloeggler 1993). Preliminary studies suggest that the infraclavicular techniques – except the modified Raj approach – also produce mild-to-moderate impairment in respiratory function (CitationRettig et al 2005).
Noteworthy, following systemic reabsorption or i.v. administration, local anesthetics exert direct central neural effects manifested by mild enhancement of the ventilatory drive and blunting of the nociceptive inputs.
Blood gas exchange
Despite the administration of 30%–40% oxygen in the gradient increases during general inspired gas, the PA-aO2 anesthesia, regardless of the type of anesthetic agent and the mode of ventilation (spontaneous or mechanically controlled). Breathing of pure oxygen may even further increase PA-aO2 by promoting alveolar collapse in low VA/Q areas (CitationRothen et al 1995).
The increase in PA-aO2 has been mainly associated with increased venous admixture (“shunting” effect) as a result of atelectasis formation, greater mismatch in VA/Q units and attenuation of the HPV response (CitationRothen et al 1998). According to a three-compartment lung model, GA produces a “shrinkage” of the FRC: the lung compartment with normal VA/Q becomes smaller whereas the other two compartments become larger, one with atelectasis (“true shunt” effect) and one with low VA/Q units (“shunt-like” effect) as a consequence of increasing airway closure (CitationHedenstierna and Edmark 2005).
On the second and third day following major surgery, recurrent nocturnal hypoxemic episodes have been attributed to sleep disturbances and abnormal breathing patterns that may be aggravated by the administration of analgesic and sedative drugs (CitationRosenberg et al 1999). Besides abnormal ventilatory patterns, circulatory failure, sepsis and hypermetabolic conditions may also alter oxygen exchanges (CitationFleischmann et al 2003; CitationLicker, de Perrot et al 2003). For instance, elevated capillary hydrostatic pressure due to acute heart failure and inflammatory damages of the alveolar-capillary barrier may produce interstitial lung edema and alveolar floading resulting in poor oxygen diffusion capacity.
Anesthetic and analgesic techniques in COPD patients
In patients with bronchospastic disease, the bronchodilating effects of volatile anesthetics are associated with improved ventilatory mechanics which outweighs the partial inhibition of the HPV and the consequent increase in intra-pulmonary shunting (CitationVolta et al 2005).
Interestingly, perioperative impairment in gas exchange does not parallel the severity of pre-existent lung disease (CitationBardoczky et al 2000). Indeed, blood oxygenation is better maintained within the first 10 minutes of one-lung ventilation in patients with end-staged COPD compared with healthy control subjects (CitationAschkenasy et al 2005). The lack of atelectasis formation and preservation of FRC in COPD patients may be explained by three complementary mechanisms (CitationGunnarsson, Tokics, Lundquist et al 1991; CitationKleinman et al 2002; CitationWarner 2002): (1) chronic airflow obstruction that generates intrinsic positive end-expiratory pressure (PEEPi), (2) loss of lung elastic recoil and the increased stiffness of the chest wall, (3) relaxation of the abdominal muscular tone (facilitated with analgesia) promoting caudal displacement of the diaphragm.
Postoperatively, the risk of respiratory insufficiency requiring mechanical ventilation is mainly attributed to abnormal ventilatory control and/or muscular pump failure (CitationSchweizer et al 2002; CitationLicker, de Perrot et al 2003). A leaky alveolar-capillary barrier associated with sepsis or elevated pulmonary capillary pressure due to fluid overload or heart failure represent alternative mechanisms of acute respiratory failure (CitationLicker et al 2006). As a rule, most COPD patients exhibit weaker HPV and blunted ventilatory responses to CO2 and hypoxia. Flattening and shortening of the diaphragm represent mechanical disadvantages whereas the diaphragmatic pumping efficiency may be impaired by deconditioning, insufficient muscular O2 delivery and enhanced muscle catabolism as a result of bed rest, heart failure as well as the neuroendocrine and inflammatory response to surgery. Nevertheless, the contractile efficiency within the crural and costal parts of the diaphragm is partly maintained by length adaptation processes whereby chronically shortened muscular fibers lose excess sarcomeres and with time, the remaining sarcomeres are restored to the optimal operating length, generating a virtual normal contraction (CitationGunnarsson, Tokics, Lundquist et al 1991; CitationGorman et al 2002).
In contrast with parenteral opiates and sedatives which may worsen OAS and ventilatory depression, TEA represents the ideal analgesic modality following thoracic and upper abdominal surgery. Given the differential sensitivity of motor and sensitive nerves to analgesics, TEA using low concentrations of local anesthetic agents and low doses of opiates effectively suppresses both the afferent nociceptive inputs and the efferent sympathetic output while the respiratory muscular activity and the HPV response are well preserved. Importantly, in severe COPD patients, TEA has been shown to improve ventilatory mechanics resulting in decreased airway resistance, lower work of breathing and better preservation of the inspiratory and expiratory capacities allowing lung recruitment maneuvers and voluntary drainage of bronchial secretions (CitationGroeben, Schafer et al 2002).
Influence of major surgery on respiratory function
In contrast to lower abdominal, orthopedic and superficial surgery, upper abdominal and intra-thoracic procedures produce marked alterations in respiratory function as evidenced by standard pulmonary functional tests, imaging techniques (ultrasound, radiology, CT-scanning) and various physiological measurements such as electromyography, body plethysmography as well as esophageal and gastric pressures (Pga, Pes) (CitationHedenstierna and Rothen 2000; CitationHedenstierna and Edmark 2005).
The pulmonary restrictive syndrome following thoracic and abdominal surgery is characterized by substantial reductions in lung volumes (−30% of FRC and total lung capacity, −40% to 60% of FEV1) with an elevated airway occlusion pressure and a shallow “thoracic” breathing pattern (CitationHedenstierna and Edmark 2005). These respiratory changes peak within the first 24–48 h and normalize over the next 4–10 days. Typically, minute ventilation is maintained by increasing the respiratory rate at smaller tidal volumes whereas the reduction in muscular diaphragmatic activity is associated with the inspiratory expansion of the thorax instead of the normal abdominal excursion (CitationNimmo and Drummond 1996; CitationHedenstierna and Rothen 2000; CitationGroeben, Schafer et al 2002). Following uncomplicated surgical cases, neural pathways and diaphragmatic muscles remain functionally intact as shown by the restoration of the trans-diaphragmatic pressure swings when the phrenic nerves are electrically stimulated at the cervical level or when patients are simply asked to “take a deep breath”.
Overall, postoperative diaphragmatic dysfunction is attributed to 3 different mechanisms: direct phrenic nerve injuries, reflex phrenic nerve inhibition and muscular pump failure.
Direct phrenic nerve injuries occur rather infrequently as a result of nerve elongation due to surgical retractors or neck malposition, surgical dissection, the application of cold-packs or the infusion of cold fluids in the thoracic cavity (CitationLicker et al 1997). In the case of postoperative acute respiratory distress, chest ultrasounds and fluoroscopy are helpful to document an elevated hemidiaphragm with paradoxical upward motion during inspiration or the “sniffing” test.
Reflex inhibition of phrenic nerve output is directly linked to abdominal and thoracic manipulations that have been shown to activate afferent medullary pathways (CitationSprung et al 1992). Moreover, with the increasing central drive to breath, the increased intercostal muscular activity outweighs the diaphragmatic activity resulting in a decreased Pga/Pes ratio and in turn, a reduced diaphragmatic motion. Sustained tonic activity of the abdominal wall muscles which persists during the early phase of inspiration may further prevent caudal displacement of the diaphragm. Conversely, sudden relaxation of the abdominal muscles at the onset of inspiration has been shown to produce paradoxical abdominal motion in some COPD patients.
Finally, clinical studies comparing laparoscopic and traditional “open” surgical approaches lend support to the hypothesis that alterations of indices of diaphragmatic function paralleled the severity of surgical trauma leading to reflex inhibition of phrenic nerve and inspiratory muscles activity. Not surprisingly, diaphragmatic function is less impaired and respiratory volumes are better preserved after minimally-invasive surgical approaches given the lesser inflammatory and neurohumoral responses (CitationCiofolo et al 1990; CitationHasukic et al 2002). Conversely, prolonged surgical stress and septic events activate the hypothalamic-pituitary-adrenal axis leading to progressive atrophy of respiratory muscles via the production of glucocorticoids and the release of cytokines, nitric oxide and oxygen-derived free radicals (CitationLanone et al 2005). In these critically-ill patients, the fall in maximal diaphragmatic force over several days parallels the reduction in muscular mass as a result of oxidative myofibrillar injuries, increased protease activity and downregulation of the insulin-like growth factor-I. In an attempt to limit this ongoing wasting process, the release of beta-endorphin decreases the activation of respiratory muscles, by changing the pattern of breathing which becomes more rapid and shallow. In addition to the neurohumoral and inflammatory interferences, sustained inspiratory overload may promote diaphragmatic muscular “fatigue” whereas electrolyte imbalance (hypokalemia, hypophosphatemia, hypocalcemia), nutritional defects and circulatory failure may further compromise the contracting capacity of the respiratory muscles.
In contrast with parenteral opiates, blocking nociceptive and visceral neural afferences by central neuraxial anesthesia has been shown to reverse the abnormal breathing pattern (CitationPansard et al 1993; CitationVassilakopoulos et al 2000). Indeed, following abdominal surgery, provision of TEA is accompanied by slowing the respiratory rate, increasing tidal volume and restoration of diaphragmatic function (electromyographic activity and transdiaphragmatic pressure). Besides interrupting the afferent inputs originating from the thorax and abdomen, TEA using high concentrations of local anesthetics also blocks the efferent output reaching the abdominal expiratory muscles and intercostal muscles, thereby limiting the ability to cough and to clear bronchial secretions (CitationPolaner et al 1993). Moreover, it has been speculated that abdominal motor blockade would facilitate downward diaphragmatic displacement by suppressing the interference of sustained tonic abdominal muscular activity on the indices of diaphragmatic activity rather than by changing the real diaphragmatic activity.
Taken together, animal and clinical experiments support the concept that abdominal/thoracic manipulations activate afferent medullary pathways leading to a reflex inhibition of the phrenic nerve output whereas inflammatory mediators (eg, cytokines), oxygen free-radical and insufficient O2 delivery may directly impair the contractile capacity of the diaphragmatic myofibrilles.
Principles of perioperative anesthetic care
Among COPD patients undergoing major surgery, PPCs and cardiac complications are equally prevalent and clinically important in terms of mortality/morbidity rates and hospital resource consumption. Care bundles, a package of best evidence-based practices are being introduced into several areas of medicine to speed up the recovery process, shorten the hospital stay and alleviate the costs of health care interventions. The current medical knowledge supports the implementation of perioperative strategies aimed to reduce the risk of cardiac, pulmonary and infectious complications () (CitationBuhre and Rossaint 2003; CitationLawrence et al 2006).
Table 5 Strategies to reduce the risk of major postoperative complications
Preoperative consultation
Patients at risk of serious cardiac and respiratory complications can be identified by combining information gathered from the clinical history (age, comorbidities, presenting disease) as well as the assessment of functional autonomy (social dependency, exercise tolerance) and the severity of the surgical stress. The anesthetic consultation is aimed to answer the following key questions: “Is the patient fit for surgery? Is it possible to improve the patient’s condition? Are there alternate therapeutic modalities? What are the specific risk and benefits related to these surgical treatment options” (CitationAnon 2002).
For cardiac risk stratification, the preoperative workup is focused on screening the known predictors of cardiac complications: severe aortic stenosis, severe heart failure, poor exercise tolerance (<4 MET) as well as the five items of the RCRI (major surgery, coronary artery disease, renal insufficiency, history of stroke, diabetes mellitus) (CitationFleisher et al 2006). To complement clinical and functional assessment, cardiac testing should be selectively ordered to evaluate the extent of ischemic/valvular disease and their consequences on cardiac performances.
Echocardiographic examination is mandated in most patients with a history of acute pulmonary oedema, abnormal clinical signs (heart murmur, fine crackles in basal lung areas, distended neck veins, peripheral oedema) and/or poor exercise tolerance (<4 MET) (CitationLee et al 1999; CitationRohde et al 2001).
For respiratory risk stratification, patients should be specifically asked about the frequency of dyspnea and cough attacks, the intensity of medical treatment, smoking habits, previous hospital admission and recent infection (CitationSmetana et al 2006). Procedure-related factors such as the site of incision, the duration of intervention and transfusion needs should be discussed with the surgeon, taking into account the presenting pathology and lesser invasive therapeutic approaches. Routine chest-X rays and functional laboratory testing are not helpful to further refine pulmonary risk assessment, except when the diagnosis of lung disease is unclear, in the case of clinical deterioration (ie, poorly controlled asthma, recent onset of fever or “greenish-yellow” sputum) and in patients scheduled to undergo lung resection. A large survey conducted in the United States revealed that anesthesiologists ordered preoperative lung function tests in approximately 60% of surgical candidates presenting with chronic lung diseases: 68% of patients with asthma, 80% of patients COPD and 53% of patients with severe scoliosis (CitationAnon 2002).
Patient preparation
The preoperative setting provides a “teaching window” for effective encouragement of exercise training, smoking and alcohol cessation and instructions regarding chest physiotherapy. For instance, education in lung expansion maneuvers reduces pulmonary complications to a greater degree when educative information is provided before, rather than after surgery (CitationCastillo and Haas 1985). Muscular endurance training and re-nutrition may enhance respiratory muscle strength and facilitate weaning from the ventilator after major or prolonged operations. Interestingly, multidisciplinary rehabilitation programs have been shown to be cost-effective in severe COPD patients, compared with lung volume reduction surgery (CitationNici et al 2006).
Preoperative inhaled beta-2 adrenergic agonists (ie, salbutamol) and anticholinergic agents (ie, ipratropium) should be continued up to the day of surgery in all symptomatic asthmatics and in COPD patients with bronchial hyperreactivity. Short-term treatment with systemic or inhaled corticosteroids has been shown to “tune up” the lung function and to decrease the incidence of wheezing following endotracheal intubation without increasing the risk of infection or wound dehiscence (CitationSilvanus et al 2004). Antibiotics should be prescribed only when the character of sputum or bronchoalveolar lavage fluid suggests an infection and, whenever possible, the surgical procedure should be postponed for at least 10 days. Although theophylline is effective to attenuate bronchospasm and to strengthen diaphragmatic contractility, it is no longer a front-line drug to manage acute respiratory failure and it has the potential to produce fatal arrhythmias and convulsive episodes.
To prevent myocardial infarct, implementation of pharmacological cardioprotective regimen is recommended in patients with known coronary artery disease or clinical risk factors (CitationFleisher et al 2006). Anti-adrenergic agents, platelet anti-aggregants and/or statins should be continued or started preoperatively (CitationKarthikeyan and Bhargava 2006). Although beta-adrenergic blockers are contra-indicated in case of moderate-to-severe bronchospastic disorders, a meta-analysis supports the safety of selective beta1-blockers in the majority of COPD patients in whom FEV1 remains stable. In these circumstances, inhaled beta-2 adrenergic agonists are still effective in alleviating acute bronchoconstrictive exacerbations, despite chronic beta-blockade (CitationMcGory et al 2005). Patients with combined bronchospastic and coronary artery diseases might benefit from the administration of alpha-2 adrenergic agonists (ie, clonidine, dexmedetomidine). Indeed, these agents are devoid of any bronchospastic effect and have been shown to prevent myocardial ischemia while reducing anesthetic/analgesic requirements and blunting thermoregulatory shivering during the perioperative period (CitationSalpeter et al 2004).
Given the risk of sudden death and acute heart failure, postponing elective surgery is advisable in patients with recent cardiac symptoms (syncope, angina) and even in asymptomatic patients with high grade stenosis (mean transvalvular gradient >50 mm or valvular area <0.5cm2/m2) (CitationZahid et al 2005). For those with heart failure (NYHA class 3 and 4), lesser invasive procedures should be considered and medical treatment should be carefully optimized by combining several drugs such as angiotensin converting enzyme inhibitors, angiotensin II antagonists, beta-adrenergic blockers and diuretics. In selected cases of heart failure (eg, bundle branch blockade), cardiac re-synchronization with multi-lead electrical stimulation might enhance ventricular performances and improve the short-term perioperative outcome.
Cessation of smoking shortly before cardiothoracic surgery (less than 2 months) has not been associated with significant reduction in PPCs (CitationWarner et al 1989; CitationBarrera et al 2005). A “hang-over” effect comparable to delirium and increased sputum production may actually increase the risk of PPCs, particularly atelectasis and silent bronchoaspiration. Smoking cessation is successful in less than 30% of cases. Fewer tissue-healing problems and PPCs have been observed only after more than 8 weeks of smoking cessation, compared with non-smoking patients (CitationMoller et al 2002; CitationBarrera et al 2005). Overwhelming health benefits in smoking cessation accrue with increasing duration of abstinence. Firstly, carboxyhemoglobin levels decrease and the excitatory effects of nicotine are abolished within 48 hours. Respiratory symptoms improve after 4 to 6 weeks, with FEV1 increasing within the first year. The risks for ischemic myocardial and cerebrovascular events are reduced after 2–5 years and lower risk for cancer are observed after 5–9 years (CitationWarner 2006).
Hospital structures and health care processes
Volume of procedures and structural aspects
Besides specialized health care training, professional expertise and technical skills, the operative outcome is largely influenced by the number of major procedures performed at a single institution, emphasizing the critical importance of the organizational processes and a multidisciplinary coordinated team approach (CitationLicker et al 2001; CitationBirkmeyer and Birkmeyer 2006). In these large-volume hospitals and health networks, better results in terms of patient mortality/morbidity data and cost containment can be achieved by adopting stringent safety practices, implementing organ protective strategies, standardizing the processes of care and defining clinical pathways for homogeneous groups of patients. In several leading American hospitals, health care quality-improvement projects are focused on specific targets such as the perioperative use of beta-blockers, computerization of the medical and nursing files as well as the 24h availability of high-dependency units. Hence, some health authorities and insurance companies recommend the referral of higher-risk patients and complex operations to high-volume hospitals where all necessary supporting facilities are available and where evidence-based and cost-efficient medical interventions and nursing care are more likely to be applied.
Minimally-invasive procedures
Compared with the “open” standard operations, minimally-invasive procedures (eg, endovascular aortic prothesis, laparoscopy) produce lesser tissue damage and in turn, an attenuated neurohumoral and inflammatory response (CitationCiofolo et al 1990; CitationHasukic et al 2002). Consequently, the minimally-invasive approach confers major advantages in terms of shorter hospital stay, earlier ambulation and feeding, with lesser need for analgesic medications and better preservation of lung functional volume (CitationJohnson et al 2005; CitationAziz et al 2006). For COPD patients who require an “open” aortic abdominal reconstruction, the retro-peritoneal approach should be preferred as it produces lesser impairment in respiratory function and fewer PPCs than the classical median laparotomy (CitationCompton et al 2005).
Nasogastric drainage
Routine nasogastric drainage fails to accelerate bowel recovery and may even increase the risk of silent bronchoaspiration. Randomized controlled trials have shown that selective nasogastric drainage resulted in earlier bowel functioning and fewer PPCs compared with routine gastric drainage (CitationNelson et al 2005). Hence, the indications for nasogastric decompression should be limited to emergency situations (acute abdomen, head trauma, drug overdose), symptomatic abdominal distension and recurrent nausea or vomiting.
Anesthetic agents and techniques
General anesthesia
Whenever possible, central neuraxial blockade, peripheral nerve blockade or general anesthesia without tracheal intubation are preferentially considered in COPD patients. The new laryngeal masks offer the advantage of lesser upper airways irritation compared with endotracheal tubes; various modes of spontaneous assisted ventlation, pressure support ventilation or volume-controlled ventilation can be applied depending on the patient’s respiratory status and the planned intervention (CitationBerry et al 1999).
Following endotracheal intubation, wheezing is more likely to occur when barbiturates are used as anesthetic induction agents, compared with propofol, ketamine or volatile anesthetics (CitationBrown and Wagner 1999; CitationGoff et al 2000). To attenuate the bronchoconstrictive reflex due to endotracheal intubation or suctioning, prophylactic treatments with lidocaine (intravenous or inhaled) and/or a beta2-adrenergic agonist are recommended in asthmatic and COPD patients with bronchospastic response (CitationGroeben, Schlicht et al 2002). Intravenous lidocaine doses of 1–2 mg/kg of body weight have been shown to attenuate histamine-induced bronchoconstriction in asthmatic volunteers whereas nebulized lidocaine produces transient airway irritation followed by blunted bronchial reactivity that occurs at lower plasma concentrations than following systemic administration.
As pointed out previously, volatile anesthetics are effective bronchodilating agents and are currently recommended for anesthesia maintenance in COPD patients with hyperreactive airways (CitationRooke et al 1997; CitationVolta et al 2005). With respect to their efficacy in treating intraoperative bronchospasm, volatile anesthetics are equipotent (isoflurane, desflurane, sevoflurane), except desflurane which may even provoke coughing, bronchospasm, laryngospasm and bronchial hypersecretion (CitationDikmen et al 2003). In rare cases where hypoxemia is attributed to the inhibition of HPV response, administration of volatile anesthetics should be interrupted and replaced by a continuous infusion of intravenous propofol or ketamine.
When muscle relaxants are needed, short-acting agents are titrated to maintain an adequate depth of muscular blockade as assessed by neuromuscular testing. Residual neuromuscular blockade should be reversed before tracheal extubation with the administration of cholinesterase inhibitors (prostigmine).
Controlled mechanical ventilation and inspiratory oxygen fraction
In patients with healthy lungs, lung recruitment maneuvers are more effective than application of continuous PEEP to prevent or reverse intra-operative atelectasis (CitationRothen et al 1999; CitationOczenski et al 2004). Re-opening 50%–100% of atelectatic areas requires that a plateau airway pressure of 30 to 40 cm H2O should be maintained for 7 seconds in patients with healthy lungs. Such a large inflation corresponds to the maximal inspiratory volume (vital capacity) which may transiently compromise hemodynamics by influencing preload, afterload and contractility of the ventricules. In morbidly obese patients, Wahlen et al demonstrated the efficacy of alveolar recruitment to improve oxygenation. However, the positive ventilatory effects were short lived and associated with greater requirements for vasopressors (CitationWhalen FX et al 2006).
In COPD patients, intermittent positive pressure ventilation (IPPV) may aggravate dynamic hyperinflation and cause premature closure of small airways leading to further ventilation/perfusion mismatch (CitationAlvisi et al 2003). Accordingly, ventilatory settings should be precisely adjusted to maximize alveolar gas emptying and to avoid dynamic hyperinflation (low frequency rate, inspiratory/expiratory ratio; intrinsic PEEP) ().
Figure 2 Progressive hyperdynamic inflation in COPD patients during mechanical ventilation and its treatment.
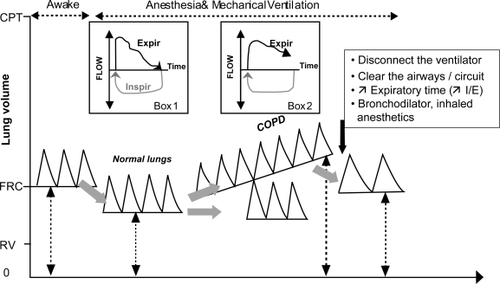
The pressure ventilatory mode (PV) with a decelerating flow has the potential advantage of decreasing the peak airway pressure and providing more homogenous distribution of inspiratory air flow at a lower or similar mean distending pressure (CitationYu et al 2006). The continuous airflow from a bi-level positive airway pressure ventilator (BiPAP: two different levels of inspiratory and expiratory airway pressure) may deliver adaptive pressures to prevent the dynamic airway collapse which tend to occur with standard ventilation in COPD patients (CitationFerrer et al 2002). The flow triggering during BiPAP allows spontaneous breathing throughout the mechanical ventilatory cycle, enabling unrestricted movement of the diaphragm. In postoperative patients and in animal experiments with bronchoconstriction, BiPAP has been shown to improve ventilation-perfusion mismatch and blood gas exchange when compared with conventional IPPV (with or without PEEP).
In patients with ALI, setting PEEP slightly higher than the lower inflection point on the pressure-volume curve and limiting the tidal ventilation volume (5–7 ml/kg) have been shown to prevent cyclic atelectasis, to attenuate the mechanical and biological stress acting at the alveolar-capillary level and to improve postoperative outcome (CitationMoran et al 2005). Similar protective ventilatory settings have been demonstrated to blunt the pulmonary inflammatory responses; accordingly, BiPAP or PV with low tidal volume and lung recruitment maneuvers are currently advocated during major operations, particularly lung resection in COPD patients (CitationLicker, de Perrot et al 2003; CitationFernandez-Perez et al 2006).
Besides i.v. antibiotic prophylaxis and maintenance of normothermia and normoglycemia, the delivery of a high-percentage of inhaled oxygen (80% intraoperatively and supplemental oxygen up to 2 hours after surgery) has been shown to lower the risk of wound infections while it may potentially increase the production of oxygen-derived free radicals (CitationBelda et al 2005). Although formation of resorption atelectasis occurs in all patients breathing pure O2 within 7 min after anesthesia induction, the amount of atelectatic areas is much less in patients ventilated with 80% O2 inspiratory concentration and almost absent in those ventilated with 60%.129 Accordingly, prevention of complete alveolar gas resorption and preservation of bactericidal activity of inflammatory cells can be achieved by setting the inspiratory O2 fraction between 50%–80% and by performing periodic “vital capacity manoeuvers”. These new proposals contrast with the current practice of setting the lowest inspiratory O2 fraction, usually around 30% that theoretically minimizes the risk of oxidative damage.
Regional anesthesia and analgesia
Provision of effective static and dynamic pain relief is a prerequisite for an accelerated postoperative recovery. Regional anesthetic techniques and patient-controlled rather than “on demand” drug regimen are the cornerstone of modern analgesic strategies. By combining different analgesic agents (opioids, paracetamol, non-steroidal anti-inflammatory drugs), the effectiveness of pain control may be further increased while drug-related side-effects are minimized (sedation, respiratory depression, nausea and vomiting) (CitationElia et al 2005; CitationMarret et al 2005).
Several meta-analysis support the concept that regional anesthetic techniques improve surgical outcome not only through better pain control but also through attenuation of the neuroendocrine response to surgery, maintenance of the immune function, improvement of tissue oxygenation and modulation of the catabolic phase. The use of intraoperative central neuraxial techniques (spinal/epidural) has been associated with reduced operative mortality rates and fewer episodes of deep vein thrombosis, pulmonary embolism and stroke as well as a lower need for blood transfusion. Likewise, maintenance of thoracic epidural analgesia (TEA) for several days after major abdominal and thoracic surgery has been shown to reduce the operative mortality rate as well as the incidence of myocardial infarct, arrhythmia, atelectasis, bronchopneumonia and respiratory failure (CitationBeattie et al 2001; CitationRigg et al 2002; CitationLawrence et al 2006; CitationLicker et al 2006). By blocking the afferent nociceptive stimuli and inhibiting the efferent sympathetic outflow, TEA affords cardiac and pulmonary protection as demonstrated by improvement of myocardial oxygen balance, inhibition of platelet hyperaggregability, limitation of myocardial infarct size and preservation of diaphragmatic function (CitationPansard et al 1993; CitationPolaner et al 1993; CitationGroban et al 1999; CitationLiu et al 2004). In addition, the time to extubation is shortened in patients with TEA as lower doses of opiates and anesthetic drugs are administered intraoperatively. Faster recovery of bowel motility allows early resumption of oral intake and blunting of protein catabolism facilitates rapid mobilization, avoiding the consequences of bed rest with muscle wasting, fatigue and venous thrombosis (CitationCarli et al 2002).
Compared with epidural analgesic techniques, a single shot of intrathecal morphine (0.2–1 mg) at the lumbar site is simpler, faster and more easily performed by non-experienced hands. A recent meta-analysis including 668 surgical patients failed to document any benefit in terms of mortality and major cardiopulmonary complications following cardiac surgery (CitationLiu et al 2004). Nevertheless, much lower doses of opiates are required to provide analgesia through the epidural/spinal route. For instance, intrathecal morphine is 100 times more potent than after systemic injection, due to the presence of opioid receptors in the substantia gelatinosa of the spinal cord. Clinical studies have demonstrated the beneficial effects of intra-thecal opiates on several surrogate endpoints: modulating the perioperative stress response, alleviating pain for the first 24 hours after surgery, shortening the extubation time and improvement of the early postoperative respiratory function (CitationLiu et al 2004). Importantly, the dose-related respiratory depressive effect of intrathecal morphine mandates close monitoring within the first 24 hours after the injection of doses exceeding 0.5 mg (CitationBailey et al 1993).
Despite the undisputable advantages of central neuraxial techniques, concerns have been raised regarding two types of catastrophic complications: cardiac arrests due to anesthetic overdose or concurrent sedation (2.5–6.4 per 10,000 spinal anesthesia; 0–2.5 per 10,000 epidural anesthesia) and spinal hematoma with paraplegic syndrome (1/1,150,000 with epidural anesthesia and 1/220,000 with spinal anesthesia) (CitationHorlocker et al 2003). For understandable reasons, fear of spinal hematoma and nerve injuries will continue to discourage some anesthesiologists to perform regional anesthetic techniques in low risk patients in whom various combinations of parenteral opiates, non-steroidal anti-inflammatory drugs and paracetamol might be preferred. In contrast, in patients at higher risk for cardiopulmonary complications, the risk-benefit analysis speaks in favor of central neuraxial blockade. These important issues should be discussed preoperatively with each patient. For instance, surgical candidates undergoing major aortic surgery have an average 3%–5% risk of myocardial infarct and 5%–10% risk of PPCs. Considering the fact that central neuraxial blockade confers a median 30% to 50% relative risk reduction for cardiac and pulmonary complications, then one myocardial infarct and one PPC can be avoided for every 30 to 60 treated patients. Furthermore, for each person suffering a spinal hematoma (1 in 150,000), it may be estimated that TEA may prevent at least 40 to 100 deaths in the high-risk group of COPD patients undergoing major surgery.
Monitoring
Although no single monitoring device has been shown to improve outcome in the operating room and ICU, the combination of ECG, pulse oxymetry, blood pressure monitoring and capnography may identify critical cardio-pulmonary events and trigger corrective measures. Given the lack of benefits and the added risk related to right heart catheterization, there is a trend towards using lesser invasive monitoring tools such as Doppler ultrasound, arterial pulse contour analysis and the simple thermodilution technique, to assess the blood circulating volume, the extravascular lung water content and ventricular contractility indices (CitationRocco et al 2004; CitationCholley and Payen 2005). Future clinical studies are still warranted to demonstrate the outcome benefits of goal-oriented hemodynamic treatments.
As moderate levels of hypothermia (34 °C–35 °C) have been associated with higher incidences of myocardial ischemia and wound infection, body temperature should be measured and kept above 36 °C by preventing heat losses and using warming devices (pulsed air blanket, fluid infusion warmers, warm mattress devices).
Monitoring the neuromuscular function is mandatory to ascertain both the depth of muscular relaxation and the functional recovery before tracheal extubation. The ability to raise the head for 5 seconds, to open the eyes on demand or to take a deep inspiratory breath are unreliable signs of neuromuscular recovery and should be routinely complemented by neuromuscular testing (CitationPino 2006). Different modes of electrical stimulation have been proposed, of which the train-of-four and double-burst methods are widely used. Squeletal muscles vary greatly in their sensitivity to muscle relaxants: the diaphragm being the most resistant whereas the peripheral small muscles of the hands and feet, the upper airway and the pharyngeal muscles are more sensitive (CitationEriksson 1994).
Improved processing of electroencephalographic signals and evoked potentials may be helpful to target an adequate depth of anesthesia. Preliminary evidence indicates that the administration of general anesthetics guided by the bispectral index of the electroencephalogram lowers the risk for intraoperative awareness and might even improve the overall postoperative outcome (CitationAnon 2006). Indeed, two prospective studies including more than 6,000 patients demonstrated that deeper maintenance of anesthetic levels was associated with higher 1-year postoperative death rates in patients aged 40 years or more undergoing major noncardiac surgery (CitationEkman et al 2004; CitationMonk et al 2005). The risk of death within 1-year after surgery increased nearly 20% for every hour that a patient had a bispectral index monitor score of less than 45 (indicating excessive deep hypnotic levels).
Lung expansion maneuvers
To complement the intraoperative vital capacity maneuvers, various techniques of respiratory physiotherapy such as incentive spirometry, deep breathing exercises, diaphragmatic breathing, intermittent positive-pressure breathing, continuous positive airway pressure (CPAP) or non-invasive positive pressure ventilation (NIPPV) are currently applied to prevent the postoperative fall in functional lung volume and/or to re-open atelectatic areas. Although several bias limit the interpretation of most clinical studies (inclusion of low risk groups, poorly defined clinical endpoints, nonstandardized respiratory interventions), a meta-analysis of 14 randomized controlled trials suggests that incentive spirometry and deep breathing exercises each reduces by about 50% the risk of postoperative complications. The combination of these techniques fails to confer additional benefit (CitationThomas and McIntosh 1994).
Patient’s empowerment can be reinforced by an informative preoperative consultation including practical demonstration of prophylactic respiratory maneuvers which are aimed to reverse the diaphragmatic dysfunction consequent to upper abdominal and thoracic surgery (CitationMackay et al 2005). The positive impact of optimal analgesia without undue sedation and early mobilization should also be emphasized.
Prophylactic application of CPAP or NIPPV is equally effective but less cost-efficient, as it requires more sophisticated devices and the availability of trained hospital personnel. The use of CPAP should be restricted for patients unable to perform deep breathing exercises (or incentive spirometry). NIPPV which delivers positive pressure support ventilation plus PEEP via a face-mask, has emerged as a significant advance in the management of OAS, cardiogenic pulmonary edema and respiratory failure, particularly in postoperative patients and those with an acute exacerbation of COPD (CitationJoris et al 1997Joris et al 2005; CitationRam et al 2005; CitationSquadrone et al 2005; CitationFernandez-Perez et al 2006; CitationYu et al 2006). In obese patient following gastroplasty, application of prophylactic NIPPV has been shown to reduce the magnitude of the restrictive lung syndrome (CitationJoris et al 1997). By unloading the inspiratory muscles and improving gas exchanges, NIPPV avoids the need to re-intubate the patients and thereby lowers the risks of nosocomial infections.
Fluid infusion
To date, algorithms guiding perioperative fluid administration are based on the empiric assumptions that preoperative deficits, maintenance fluids, third space losses and blood loss should be replaced by crystalloids or a combination of colloids and crystalloids. For major abdominal surgery, the proposed rule to infuse large quantities of salts and fluids (crystalloids 10–15 ml/kg/h intraoperatively followed by 1.5–2 L per day postoperatively), results in a positive fluid balance of 1–3 L and a body weight gain often exceeding 10% over the first 3 postoperative days (CitationGrocott et al 2005). Excessive intravascular plasma volume leads to extravascular water accumulation and tissue edema that will predispose these patients to respiratory/heart failure, wound infection, anastomotic dehiscence and prolong ileus prohibiting oral feeding. In a large retrospective study, CitationArieff (1999) found that acute pulmonary edema occurred in 7.6% of patients undergoing various surgical procedures (n = 612/8052). Interestingly, overzealous hydration leading to a net fluid retention exceeding 90 ml/kg/day was the sole contributory factor in 2.6% of patients, being free from any cardiopulmonary disease.
Based on prospective controlled studies, two strategies of fluid infusion have been proposed: (1) hemodynamicgoal directed fluid loading aimed at increasing cardiac output and correcting “silent” hypovolemia, and (2) restrictive fluid administration (no compensation for third space losses and combined epidural blockade) continued over 2–3 days and targeting a maximal weight gain of 10% (CitationGrocott et al 2005).
A “dry” fluid regimen has been widely adopted in pulmonary surgery resulting in a decreased risk of ALI. In major abdominal surgery, three randomized controlled trials support the safety and beneficial effects of the restrictive approach: hemodynamics remained stable, renal function was not impaired, fewer patients experience complications, gastrointestinal function recovered earlier and time to hospital discharge was shorter (CitationGrocott et al 2005).
Conclusion
To date, perioperative physicians are confronted with increasing numbers of patients with COPD and cardiovascular diseases who should not be denied appropriate surgical treatments. Besides patient’s comorbidities, procedure-related factors (thoracic and upper abdominal interventions, prolonged surgery) are the main risk factors for PPCs and cardiac adverse events that will markedly impact on long-term patient survival, hospital resource and health care costs.
Strong scientific evidence supports the concept that patient safety and surgical outcome may be improved by preoperative screening of these high risk patients, optimisation of organ function, control of the neuroendocrine and inflammatory response as well as standardization of health care processes.
Several advances in surgery and anesthesia care have been shown to be particularly beneficial in COPD patients:
Minimally-invasive procedures and selective nasogastric drainage
Implementation of cardioprotective strategies (eg, beta-blockers, platelet anti-aggregants, alpha2-agonists, statines, thoracic epidural analgesia)
Regional anesthetic blockade and laryngeal masks to minimize airway instrumentation
Volatile anesthetic agents with bronchodilating properties (eg, sevoflurane)
Physiological monitoring in order to target the adequate circulatory volume, blood gas exchanges, depth of anesthesia and neuromuscular activity
Prevention and treatment of atelectasis by lung recruitment maneuvers while avoiding stress-induced lung injuries (low tidal volume ventilation)
Multimodal analgesic regimen for blocking nociceptive inputs
Fast-track anesthesia and NIPPV to avoid the deleterious effects of tracheal intubation and prolonged mechanical ventilation.
Maintenance of normothermia, tight control of blood glucose and provision of supplemental oxygen to reduce wound infection
Adjustment of fluid infusion (colloids, cristalloids, blood products) to maintain an adequate circulatory volume, blood coagulation and oxygen delivery to all tissues
References
- AlvisiVRomanelloABadetM2003Time course of expiratory flow limitation in COPD patients during acute respiratory failure requiring mechanical ventilationChest12316253212740283
- [Anon]2002Practice advisory for preanesthesia evaluation: a report by the American Society of Anesthesiologists Task Force on Preanesthesia EvaluationAnesthesiology964859611818784
- [Anon]2006Practice advisory for intraoperative awareness and brain function monitoring: a report by the american society of anesthesiologists task force on intraoperative awarenessAnesthesiology1048476416571982
- ArieffAI1999Fatal postoperative pulmonary edema: pathogenesis and literature reviewChest1151371710334155
- ArozullahAMDaleyJHendersonWG2000Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery The National Veterans Administration Surgical Quality Improvement ProgramAnn Surg2322425310903604
- ArozullahAMKhuriSFHendersonWG2001Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgeryAnn Intern Med1358475711712875
- AschkenasySVHoferCKZalunardoMP2005Patterns of changes in arterial PO2 during one-lung ventilation: a comparison between patients with severe pulmonary emphysema and patients with preserved lung functionJ Cardiothorac Vasc Anesth194798416085253
- AzizOConstantinidesVTekkisPP2006Laparoscopic versus open surgery for rectal cancer: a meta-analysisAnn Surg Oncol134132416450220
- BabencoHDBlouinRTConardPF1998Diphenylhydramine increases ventilatory drive during alfentanil infusionAnesthesiology8964279743400
- BaileyPLRhondeauSSchaferPG1993Dose-response pharmacology of intrathecal morphine in human volunteersAnesthesiology7949598342828
- BardoczkyGISzegediLLd’HollanderAA2000Two-lung and one-lung ventilation in patients with chronic obstructive pulmonary disease: the effects of position and F(IO)2Anesth Analg90354110624972
- BarreraRShiWAmarD2005Smoking and timing of cessation: impact on pulmonary complications after thoracotomyChest12719778315947310
- BeattieWSBadnerNHChoiP2001Epidural analgesia reduces postoperative myocardial infarction: a meta-analysisAnesth Analg93853811574345
- BeldaFJAguileraLGarcia de la AsuncionJ2005Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trialJama29420354216249417
- BergHRoedJViby-MogensenJ1997Residual neuromuscular block is a risk factor for postoperative pulmonary complications. A prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuroniumActa Anaesthesiol Scand4110951039366929
- BerryABrimacombeJKellerC1999Pulmonary airway resistance with the endotracheal tube versus laryngeal mask airway in paralyzed anesthetized adult patientsAnesthesiology9039579952143
- BirkmeyerNJBirkmeyerJD2006Strategies for improving surgical quality – should payers reward excellence or effort?N Engl J Med3548647016495401
- BoersmaEKertaiMDSchoutenO2005Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk indexAm J Med11811344116194645
- BonnetFMarretE2005Influence of anaesthetic and analgesic techniques on outcome after surgeryBr J Anaesth9552815579487
- BrownRHWagnerEM1999Mechanisms of bronchoprotection by anesthetic induction agents: propofol versus ketamineAnesthesiology90822810078684
- BruceJRussellEMMollisonJ2001The measurement and monitoring of surgical adverse eventsHealth Technol Assess51194
- BuhreWRossaintR2003Perioperative management and monitoring in anaesthesiaLancet36218394614654324
- BurnhamELMossMHarrisF2004Elevated plasma and lung endothelial selectin levels in patients with acute respiratory distress syndrome and a history of chronic alcohol abuseCrit Care Med32675915090946
- CarliFMayoNKlubienK2002Epidural analgesia enhances functional exercise capacity and health-related quality of life after colonic surgery: results of a randomized trialAnesthesiology97540912218518
- CastilloRHaasA1985Chest physical therapy: comparative efficacy of preoperative and postoperative in the elderlyArch Phys Med Rehabil6637694004535
- CholleyBPPayenD2005Noninvasive techniques for measurements of cardiac outputCurr Opin Crit Care11424916175028
- ChristMSharkovaYGeldnerG2005Preoperative and perioperative care for patients with suspected or established aortic stenosis facing noncardiac surgeryChest12829445316236971
- CiofoloMJClergueFSeebacherJ1990Ventilatory effects of laparoscopy under epidural anesthesiaAnesth Analg70357612138437
- ComptonCNDillavouEDSheehanMK2005Is abdominal aortic aneurysm repair appropriate in oxygen-dependent chronic obstructive pulmonary disease patients?J Vasc Surg42650316242549
- CreekmoreFMLugoRAWeilandKJPostoperative opiate analgesia requirements of smokers and nonsmokersAnn Pharmacother389495315113988
- DahanARombergRTeppemaL2004Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusionAnesthesiology1011201915505457
- den HerderCSchmeckJAppelboomDJ2004Risks of general anaesthesia in people with obstructive sleep apneaBmj329955915499112
- DikmenYEminogluESalihogluZ2003Pulmonary mechanics during isoflurane, sevoflurane and desflurane anaesthesiaAnaesthesia58745812859465
- DindoDDemartinesNClavienPA2004Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a surveyAnn Surg2402051315273542
- DrongowskiRALeeDReynoldsPI2003Increased respiratory symptoms following surgery in children exposed to environmental tobacco smokePaediatr Anaesth133041012753442
- EikermannMBlobnerMGroebenH2006Postoperative upper airway obstruction after recovery of the train of four ratio of the adductor pollicis muscle from neuromuscular blockadeAnesth Analg1029374216492855
- EkmanALindholmMLLennmarkenC2004Reduction in the incidence of awareness using BIS monitoringActa Anaesthesiol Scand4820614674969
- EliaNLysakowskiCTramerMR2005Does multimodal analgesia with acetaminophen, nonsteroidal antiinflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Meta-analyses of randomized trialsAnesthesiology103129630416306743
- ErginAMuntnerPSherwinR2004Secular trends in cardiovascular disease mortality, incidence, and case fatality rates in adults in the United StatesAm J Med1172192715308430
- ErikssonLI1994Ventilation and neuromuscular blocking drugsActa Anaesthesiol Scand Suppl1021157976158
- Fernandez-PerezERKeeganMTBrownDR2006Intraoperative tidal volume as a risk factor for respiratory failure after pneumonectomyAnesthesiology10514816809989
- FerrerMIglesiaRRocaJ2002Pulmonary gas exchange response to weaning with pressure-support ventilation in exacerbated chronic obstructive pulmonary disease patientsIntensive Care Med281595912415446
- FisherBWMajumdarSRMcAlisterFA2002Predicting pulmonary complications after nonthoracic surgery: a systematic review of blinded studiesAm J Med1122192511893349
- FleischmannKEGoldmanLYoungB2003Association between cardiac and noncardiac complications in patients undergoing non-cardiac surgery: outcomes and effects on length of stayAm J Med1155152014599629
- FleisherLABeckmanJABrownKA2006ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice GuidelinesCirculation11326627416754815
- GoffMJArainSRFickeDJ2000Absence of bronchodilation during desflurane anesthesia: a comparison to sevoflurane and thiopentalAnesthesiology93404810910489
- GormanRBMcKenzieDKPrideNB2002Diaphragm length during tidal breathing in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med1661461912406839
- GrobanLZvaraDADealDD1999Thoracic epidural anesthesia reduces infarct size in a canine model of myocardial ischemia and reperfusion injuryJ Cardiothorac Vasc Anesth135798510527228
- GrocottMPMythenMGGanTJ2005Perioperative fluid management and clinical outcomes in adultsAnesth Analg100109310615781528
- GroebenHSchaferBPavlakovicG2002Lung function under high thoracic segmental epidural anesthesia with ropivacaine or bupivacaine in patients with severe obstructive pulmonary disease undergoing breast surgeryAnesthesiology965364111873024
- GroebenHSchlichtMStieglitzS2002Both local anesthetics and salbutamol pretreatment affect reflex bronchoconstriction in volunteers with asthma undergoing awake fiberoptic intubationAnesthesiology9714455012459670
- GroebenHSchwalenAIrsfeldS1994High thoracic epidural anesthesia does not alter airway resistance and attenuates the response to an inhalational provocation test in patients with bronchial hyperreactivityAnesthesiology81868747943838
- GunnarssonLTokicsLGustavssonH1991Influence of age on atelectasis formation and gas exchange impairment during general anaesthesiaBr J Anaesth66423322025468
- GunnarssonLTokicsLLundquistH1991Chronic obstructive pulmonary disease and anaesthesia: formation of atelectasis and gas exchange impairmentEur Respir J41106161756845
- HalbertRJNatoliJLGanoA2006Global burden of COPD: systematic review and meta-analysisEur Respir J285233216611654
- HasukicSMesicDDizdarevicE2002Pulmonary function after laparoscopic and open cholecystectomySurg Endosc16163511961630
- HedenstiernaGEdmarkL2005The effects of anesthesia and muscle paralysis on the respiratory systemIntensive Care Med3113273516132894
- HedenstiernaGRothenHU2000Atelectasis formation during anesthesia: causes and measures to prevent itJ Clin Monit Comput163293512580216
- HernandezAFWhellanDJStroudS2004Outcomes in heart failure patients after major noncardiac surgeryJ Am Coll Cardiol4414465315464326
- HodgsonPSLiuSS2001Epidural lidocaine decreases sevoflurane requirement for adequate depth of anesthesia as measured by the Bispectral Index monitorAnesthesiology9479980311388531
- HorlockerTTWedelDJBenzonH2003Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation)Reg Anesth Pain Med281729712772135
- JaberSDelayJMChanquesG2005Outcomes of patients with acute respiratory failure after abdominal surgery treated with noninvasive positive pressure ventilationChest12826889516236943
- JohnsonNBarlowDLethabyA2005Methods of hysterectomy: systematic review and meta-analysis of randomised controlled trialsBmj330147815976422
- JorisJLSottiauxTMChicheJD1997Effect of bi-level positive airway pressure (BiPAP) nasal ventilation on the postoperative pulmonary restrictive syndrome in obese patients undergoing gastroplastyChest111665709118706
- KaafaraniHMItaniKMThornbyJ2004Thirty-day and one-year predictors of death in noncardiac major surgical proceduresAm J Surg188495915546557
- KarthikeyanGBhargavaB2006Managing patients undergoing non-cardiac surgery: need to shift emphasis from risk stratification to risk modificationHeart92172016370040
- KertaiMDBountioukosMBoersmaE2004Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgeryAm J Med11681314706659
- KleinmanBSFreyKVanDrunenM2002Motion of the diaphragm in patients with chronic obstructive pulmonary disease while spontaneously breathing versus during positive pressure breathing after anesthesia and neuromuscular blockadeAnesthesiology9729830512151916
- KroenkeKLawrenceVATherouxJF1992Operative risk in patients with severe obstructive pulmonary diseaseArch Intern Med152967711580723
- LanoneSTailleCBoczkowskiJ2005Diaphragmatic fatigue during sepsis and septic shockIntensive Care Med311611716189683
- LawrenceVACornellJESmetanaGW2006Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of PhysiciansAnn Intern Med14459660816618957
- LeeTHMarcantonioERMangioneCM1999Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgeryCirculation1001043910477528
- LickerMde PerrotMSpiliopoulosA2003Risk factors for acute lung injury after thoracic surgery for lung cancerAnesth Analg9715586514633519
- LickerMKhatchatourianGSchweizerA2002The impact of a cardioprotective protocol on the incidence of cardiac complications after aortic abdominal surgeryAnesth Analg95152533table of contents12456411
- LickerMSchweizerAHohnL1997Unexpected postoperative respiratory failure due to diaphragmatic paralysisAnesthesiology8712399366477
- LickerMSpiliopoulosAFreyJG2001Management and outcome of patients undergoing thoracic surgery in a regional chest medical centreEur J Anaesthesiol18540711473561
- LickerMSpiliopoulosATschoppJM2003Influence of thoracic epidural analgesia on cardiovascular autonomic control after thoracic surgeryBr J Anaesth915253114504154
- LickerMJWidikkerIRobertJ2006Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trendsAnn Thorac Surg811830716631680
- LindbergPGunnarssonLTokicsL1992Atelectasis and lung function in the postoperative periodActa Anaesthesiol Scand36546531514340
- LiuSSBlockBMWuCL2004Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta-analysisAnesthesiology1011536115220785
- LysakowskiCDumontLCzarnetzkiC2006The effect of cigarette smoking on the hypnotic efficacy of propofolAnaesthesia618263116922747
- MackayMREllisEJohnstonC2005Randomised clinical trial of physiotherapy after open abdominal surgery in high risk patientsAust J Physiother51151916137240
- MarretEKurdiOZuffereyP2005Effects of nonsteroidal anti-inflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trialsAnesthesiology10212496015915040
- McAlisterFABertschKManJ2005Incidence of and risk factors for pulmonary complications after nonthoracic surgeryAm J Respir Crit Care Med171514715563632
- McAlisterFAKhanNAStrausSE2003Accuracy of the preoperative assessment in predicting pulmonary risk after nonthoracic surgeryAm J Respir Crit Care Med167741412598217
- McGoryMLMaggardMAKoCY2005A meta-analysis of perioperative beta blockade: what is the actual risk reduction?Surgery138171916153424
- MollerAMVillebroNPedersenT2002Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trialLancet359114711809253
- MonkTGSainiVWeldonBC2005Anesthetic management and one-year mortality after noncardiac surgeryAnesth Analg10041015616043
- MoranJLBerstenADSolomonPJ2005Meta-analysis of controlled trials of ventilator therapy in acute lung injury and acute respiratory distress syndrome: an alternative perspectiveIntensive Care Med312273515678318
- MoudgilRMichelakisEDArcherSL2005Hypoxic pulmonary vasoconstrictionJ Appl Physiol9839040315591309
- NelsonREdwardsSTseB2005Prophylactic nasogastric decompression after abdominal surgeryCochrane Database Syst RevCD00492915674971
- NiciLDonnerCWoutersE2006American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitationAm J Respir Crit Care Med173139041316760357
- NimmoAFDrummondGB1996Respiratory mechanics after abdominal surgery measured with continuous analysis of pressure, flow and volume signalsBr J Anaesth77317268949802
- OczenskiWSchwarzSFitzgeraldRD2004Vital capacity manoeuvre in general anaesthesia: useful or useless?Eur J Anaesthesiol21253915109186
- PansardJLMankikianBBertrandM1993Effects of thoracic extradural block on diaphragmatic electrical activity and contractility after upper abdominal surgeryAnesthesiology7863718424574
- PaullDEUpdykeGMDavisCA2004Complications and long-term survival for alcoholic patients with resectable lung cancerAm J Surg188553915546569
- PetersonGNDominoKBCaplanRA2005Management of the difficult airway: a closed claims analysisAnesthesiology10333915983454
- PinoRM2006Residual neuromuscular blockade: a persistent clinical problemInt Anesthesiol Clin44779016849957
- PolanczykCAMarcantonioEGoldmanL2001Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgeryAnn Intern Med1346374311304103
- PolanerDMKimballWRFratacciMD1993Thoracic epidural anesthesia increases diaphragmatic shortening after thoracotomy in the awake lambAnesthesiology79808168214761
- RamFSWellingtonSRoweBH2005Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthmaCochrane Database Syst RevCD004360
- RamakrishnaGSprungJRaviBS2005Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortalityJ Am Coll Cardiol451691915893189
- RegliAvon Ungern-SternbergBSReberA2006Impact of spinal anaesthesia on peri-operative lung volumes in obese and morbidly obese female patientsAnaesthesia612152116480344
- ReisliRApilliogullariSReisliI2004The effect of environmental tobacco smoke on the dose requirements of rocuronium in childrenPaediatr Anaesth142475014996264
- RettigHCGielenMJBoersmaE2005Vertical infraclavicular block of the brachial plexus: effects on hemidiaphragmatic movement and ventilatory functionReg Anesth Pain Med305293516326337
- RiggJRJamrozikKMylesPS2002Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trialLancet35912768211965272
- RoccoMSpadettaGMorelliA2004A comparative evaluation of thermodilution and partial CO2 rebreathing techniques for cardiac output assessment in critically ill patients during assisted ventilationIntensive Care Med3082714652718
- RohdeLEPolanczykCAGoldmanL2001Usefulness of transthoracic echocardiography as a tool for risk stratification of patients undergoing major noncardiac surgeryAm J Cardiol87505911230829
- RookeGAChoiJHBishopMJ1997The effect of isoflurane, halothane, sevoflurane, and thiopental/nitrous oxide on respiratory system resistance after tracheal intubationAnesthesiology86129499197298
- RosenbergJRasmussenGIWojdemannKR1999Ventilatory pattern and associated episodic hypoxaemia in the late postoperative period in the general surgical wardAnaesthesia54323810455829
- RothenHUNeumannPBerglundJE1999Dynamics of re-expansion of atelectasis during general anaesthesiaBr J Anaesth82551610472221
- RothenHUSporreBEngbergG1995Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesiaAnesthesiology82832427717553
- RothenHUSporreBEngbergG1998Airway closure, atelectasis and gas exchange during general anaesthesiaBr J Anaesth81681610193276
- SakuraSSaitoYKosakaY1996The effects of epidural anesthesia on ventilatory response to hypercapnia and hypoxia in elderly patientsAnesth Analg82306118561332
- SalpeterSROrmistonTMSalpeterEE2004Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysisChest12523092115189956
- SchusslerOAlifanoMDermineH2006Postoperative pneumonia after major lung resectionAm J Respir Crit Care Med1731161916474029
- SchweizerAKhatchatourianGHohnL2002Opening of a new postanesthesia care unit: impact on critical care utilization and complications following major vascular and thoracic surgeryJ Clin Anesth144869312477582
- SilvanusMTGroebenHPetersJ2004Corticosteroids and inhaled salbutamol in patients with reversible airway obstruction markedly decrease the incidence of bronchospasm after tracheal intubationAnesthesiology1001052715114199
- SinDDWuLManSF2005The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literatureChest1271952915947307
- SmetanaGWLawrenceVACornellJE2006Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of PhysiciansAnn Intern Med1445819516618956
- SpiliopoulosAde PerrotM2000Four decades of surgery for bronchogenic carcinoma in one centreEur Respir J15543610759450
- SprungJBarnasGMChengEY1992Changes in functional residual capacity and regional diaphragm lengths after upper abdominal surgery in anesthetized dogsAnesth Analg75977821443717
- SquadroneVCohaMCeruttiE2005Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trialJama2935899515687314
- ThomasJAMcIntoshJM1994Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta-analysisPhys Ther743108265725
- TirumalasettyJGrammerLC2006Asthma, surgery, and general anesthesia: a reviewJ Asthma43251416809236
- UrmeyWFGloegglerPJ1993Pulmonary function changes during interscalene brachial plexus block: effects of decreasing local anesthetic injection volumeReg Anesth1824498398959
- van den ElsenMDahanADeGoedeJ1995Influences of subanesthetic isoflurane on ventilatory control in humansAnesthesiology83478907661348
- van den ElsenMSartonETeppemaL1998Influence of 0.1 minimum alveolar concentration of sevoflurane, desflurane and isoflurane on dynamic ventilatory response to hypercapnia in humansBr J Anaesth80174829602581
- VassilakopoulosTMastoraZKatsaounouP2000Contribution of pain to inspiratory muscle dysfunction after upper abdominal surgery: A randomized controlled trialAm J Respir Crit Care Med1611372510764336
- VoltaCAAlvisiVPetriniS2005The effect of volatile anesthetics on respiratory system resistance in patients with chronic obstructive pulmonary diseaseAnesth Analg1003485315673854
- von Ungern-SternbergBSRegliASchneiderMC2004Effect of obesity and site of surgery on perioperative lung volumesBr J Anaesth92202714722169
- WarnerDO2002Diaphragm function during anesthesia: still crazy after all these yearsAnesthesiology97295712151915
- WarnerDO2006Perioperative abstinence from cigarettes: physiologic and clinical consequencesAnesthesiology1043566716436857
- WarnerDOPattenCAAmesSC2004Smoking behavior and perceived stress in cigarette smokers undergoing elective surgeryAnesthesiology10011253715114209
- WarnerDOWarnerMABarnesRD1996Perioperative respiratory complications in patients with asthmaAnesthesiology8546078853074
- WarnerMAOffordKPWarnerME1989Role of preoperative cessation of smoking and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patientsMayo Clin Proc64609162787456
- WhalenFSprungJBurkleCM2006Recent smoking behavior and postoperative nausea and vomitingAnesth Analg10370516790629
- WhalenFXGajicOThompsonGB2006The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgeryAnesth Analg10229830516368847
- WinTJacksonASharplesL2005Cardiopulmonary exercise tests and lung cancer surgical outcomeChest12711596515821190
- WoltersUWolfTStutzerH1996ASA classification and perioperative variables as predictors of postoperative outcomeBr J Anaesth77217228881629
- YuGYangKBakerAB2006The effect of bi-level positive airway pressure mechanical ventilation on gas exchange during general anaesthesiaBr J Anaesth965223216500951
- ZahidMSonelAFSabaS2005Perioperative risk of noncardiac surgery associated with aortic stenosisAm J Cardiol96436816054477