Abstract
Constrictive bronchiolitis obliterans is a fibrotic disease of small airways characterized by progressive obliteration of the airway lumen, with resulting obstructive pulmonary physiology. While previous work has demonstrated the collagenous nature of the constrictive fibrotic lesions, elastin expression in the disease has been poorly characterized. Elastin is a critical component of the pulmonary extracellular matrix, and is responsible for the reversible deformability characteristic of the alveoli, pulmonary blood vessels, and airways. Elastin is a long-lived protein with virtually no active protein production occurring after lung development is completed during early childhood. We report a novel case of cryptogenic bronchiolitis obliterans in which elastin gene expression is actively upregulated in affected airways, and accompanied by myofibroblast hyperplasia and disorganized elastic fiber deposition. In addition, deposition of new elastic fibers by myofibroblasts is noted in the alveoli surrounding the affected bronchioles.
Introduction
Constrictive bronchiolitis obliterans (CBO) is a fibrotic lung disease primarily affecting the small (≤3 mm diameter) conducting airways of the lungs (CitationCouture and Colby 2003). “Constrictive” bronchiolitis obliterans was first used as a descriptive term in 1973 (CitationGosink et al 1973), and describes a fibrosing inflammatory process that surrounds, rather than fills, the airway lumen (CitationColby 1998). This process results in extrinsic airway compression and luminal obliteration. CBO is rare in adults, and has been poorly studied. In most cases, the etiologic agent associated with CBO is known, although the evidence underlying some of these associations is tenuous (CitationRyu et al 2003). The term “cryptogenic obliterative bronchiolitis” was first used in 1981 to characterize cases of otherwise unexplained progressive airflow obstruction (CitationTurton et al 1981). A small number of pathologically confirmed cryptogenic cases of CBO have however been documented (CitationKraft et al 1993).
The bronchioles are a physiologically silent area of the lung as a result of their considerable cross-sectional area. The diameter of each successive distal airway generation decreases progressively from the tracheal division onward. However, the resulting total cross-sectional luminal area increases with each successive airway generation, a feature that attains the most significance in the conducting airways distal to the origin of the bronchioles (CitationBaraldo et al 2003). Thus, extensive damage to the bronchioles may result in considerable pathophysiologic consequences. In CBO, where progressive obliteration of bronchioles leads to decreased conducting airway cross-sectional area, concomitant obstructive physiology develops that is complicated by air-trapping and hyperinflation.
Elastic fibers are composite structures containing two primary morphologically distinguishable components, elastin and microfibrils (reviewed in CitationKielty et al (2002)). Elastin, a major elastic fiber component, is known to be essential for lung development and mechanical function (CitationMariani et al 1997), and elastic fibers are distributed throughout the lung, including the alveoli, airways, vessels and pleura. The rate of lung elastin synthesis is greatest during fetal and neonatal development, but is almost undetectable in healthy adult lung (CitationMariani et al 2002). However, in pathological circumstances such as emphysema or pulmonary fibrosis, there may be reactivation of elastin expression in the adult lung leading to excessive or aberrant elastin deposition (CitationSnider et al 1991; CitationLucey et al 1998; CitationHoff et al 1999). Functional fiber formation requires coordinated re-expression of all fiber components in a temporally and spatially regulated sequence. This is assumed to be an inefficient process in adult tissue, as re-initiated elastin deposition in injured adult tissues is often characterized by faulty organization of the newly formed three-dimensional fiber.
CBO predominantly affects small airways, with few or no histologic changes observed in the alveoli surrounding the diseased airways (CitationCouture and Colby 2003). Given that CBO involves both airway injury and a fibrotic response, the finding of increased production of extracellular matrix components in the airways would not be surprising. While previous work has confirmed the presence of extensive collagenous fibrosis in the walls of bronchi with CBO (CitationMarkopoulo et al 2002), elastin gene expression and protein deposition have been poorly characterized. Here, we report a case of cryptogenic bronchiolitis obliterans in which elastin expression and deposition are markedly and pathologically increased, not only in the affected bronchioles, but also in the surrounding alveoli.
Case report
A 63-year-old man with a 5-month history of progressive dyspnea, cough, and scant sputum production was referred for pulmonary evaluation. Prior to the onset of dyspnea he was fully functional without exercise limitation. Onset of his symptomatology was coincident with the development of an upper respiratory infection. Outpatient therapy with antibiotics and bronchodilators had resulted in a minimal but temporary improvement. At presentation, the patient was dyspneic with showering and climbing any stairs. Social history was remarkable for a 5-year history of cigar smoking, which he discontinued 25-years prior to referral. Extensive questioning did not yield any significant history of occupational or environmental exposures, and family history was non-contributory for pulmonary disease. The patient had a history of a thymoma, but testing revealed no evidence of myasthenia gravis or any other systemic manifestations of thymic disease. CXR was significant for bilateral hyperinflation, without evidence of emphysema. CT scan again demonstrated marked hyperinflation with little evidence to suggest emphysema. Bilateral bronchiectasis and bronchiolitis with lower lobe predominance were evident. Extensive workup for causes of secondary bronchiectasis was unremarkable. Spirometry confirmed a partially reversible severe obstructive ventilatory defect, with an FEV1 = 0.67L (19% predicted) an FVC = 2.17L (48% predicted), and an FEV1: FVC ratio of 39%. Lung volumes revealed no hyperinflation with a TLC = 7.84L (109% predicted), but air trapping was significant with an RV = 5.28L (222% predicted). Resting ABG showed pH = 7.38, PaCO2 = 54, and PaO2 = 50 on room air, with a room-air SpO2 of 88%. Six-minute walk testing demonstrated that the patient required supplemental oxygen at 2 L/min at rest and 3 L/min with activity to maintain an SpO2 ≥90%. Despite aggressive therapy with oxygen, bronchodilators, intermittent antibiotics, anti-inflammatories (corticosteroids and macrolides), and rehabilitation, his disease continued to progress. Due to his severe clinical and physiologic impairments the patient was evaluated and listed for lung transplantation. Bilateral sequential lung transplantation was performed 3-months later without complication. At the time of surgery, the lungs were noted to be grossly hyperinflated, and did not deflate after resection and removal from the thorax. An explant wedge biopsy sent for pathologic examination revealed the diagnosis of constrictive bronchiolitis obliterans, which on the basis of his clinical history was considered cryptogenic in etiology. The rest of the lung was placed in storage at −80°C for further study. At the time of writing, the patient continues to do well >15 months after transplantation.
Methods and materials
Lung processing
The explanted diseased lung was kept on ice during sampling, as was a donor lung rejected for transplantation that was used as a tissue control. Portions of peripheral lung 1 cm2 were excised with a scalpel and fixed without inflation in 4% paraformaldehyde overnight at 4 °C before paraffin embedding. Serial 5 μm-thick sections were cut, deparaffinized, and stained with hematoxylin and eosin (H and E). Identical sections were used for Hart’s staining, in-situ hybridization and immunohistologic staining.
Hart’s elastin staining
Peripheral CBO and control lung tissue sections (5 μm) embedded in paraffin were deparaffinized and re-hydrated through graded ethanol to PBS, then soaked in 0.25% potassium permanganate solution for 5 minutes. Slides were cleared in 5% oxalic acid and soaked in resorcin-fuchsin solution (Poly Scientific, Bay Shore, NY, USA) overnight. After washing in water, sections were counterstained with tartrazine (yellow), dehydrated through graded ethanol, cleared in xylene and mounted.
Smooth muscle actin immunohistochemistry
Sections of paraffin embedded peripheral CBO and control lung were deparaffinized in xylene, then re-hydrated in a descending ethanol series and allowed to stand in PBS for 10 minutes. Slides were blocked for 1 hour with 10% fetal horse serum, rinsed in PBS, then incubated overnight at 4 ºC with the primary alpha smooth muscle actin (α-SMA) antibody (1A4 mouse monoclonal, 1:200 dilution, Sigma, St. Louis, MO, USA). After rinsing in PBS, sections were incubated in the dark with secondary antibody (fluorescein-conjugated goat anti-mouse IgG, 1:250 dilution, 1 hour, room temperature, Jackson Immuno Research, Westgrove, PA). Following rinsing in PBS, sections were cover slipped using Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and viewed under fluorescent light for image capture.
In situ hybridization
Digoxygenin-labeled antisense and sense riboprobes were generated from a baboon tropoelastin cDNA that had been linearized for in vitro transcription (Promega, Madison, WI, USA). Sections of paraffin embedded peripheral CBO and control lung were deparaffinized, re-hydrated and digested with proteinase K. The tissue sections were blocked with triethanolamine and acetic anhydride, then hybridized with denatured digoxygenin-labeled riboprobe overnight at 60 °C. Following hybridizations, tissue sections were subjected to a series of washes including digestion with RNase A, treated with blocking agents, and incubated with an antibody conjugated to alkaline phosphatase. After additional washes, slides were incubated with a chromogen substrate for 1–3 days. After color development, specimens were counterstained with nuclear fast red.
Results
Initial analysis of the CBO lung
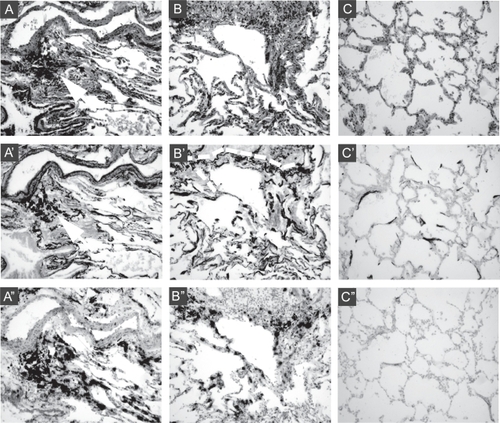
The explanted lung remained inflated after resection and was firm to the touch. When tissue specimens of approximately 1 cm3 were cut from the periphery of the lung, these too remained inflated. Gross examination showed no obvious emphysematous changes within peripheral tissue sections. Staining with hematoxylin and eosin (H and E) showed that the peripheral tissue had few bronchioles, but no evidence of emphysema, confirming the CXR, CT and formal pathologic findings. Inflammatory infiltrates were present adjacent to large airways paralleling intralobar arteries. Some particulate deposits were associated with the inflammatory infiltrates (, arrowheads).
Figure 1 Abnormal morphology, elastic fiber accumulation, and elastin mRNA expression in CBO. Sections of peripheral lung tissue from CBO-affected lung (panels A and B), and control lung (panel C) were fixed without inflation, embedded, and sectioned at 5 μM. Serial sections were stained with H and E (A, B, C), for elastic fibers using Hart’s elastic fiber stain (A′, B′, C′), and hybridized in situ for elastin mRNA (A″, B″, C″). In panel A, a large vessel (top of each panel) is flanked by a large airway (bottom left of each panel) with accumulation of particulates, visible as black deposits, between the vessel and airway (indicated by white arrow in A and A′). A′ shows elastic laminae (stained black) in the intralobar pulmonary artery, and an increased density of elastic fibers beneath the epithelium of the large airway and the surrounding lung parenchyma compared to control (C′). A″ shows that elastin mRNA expression (black signal) is present in the large blood vessel, conducting airway, and particularly in tissue between these structures, and is significantly increased compared to control lung (C″). Panel B shows a large infiltrate (top of panels) flanked by surrounding tissue containing obliterated airways. Elastin staining in B′ indicates the infiltrate likely encompasses an airway, as there is a continuous elastic fiber (outlined by white dashed line) indicating a luminal structure has been present. Again, elastin density is increased compared to control lung (C’). B″ shows ongoing, intense elastin mRNA expression in tissue surrounding the infiltrate that is greater than that seen in control lung (C″).
Excess elastic fiber deposition and mRNA expression
Because the lung exhibited unusual mechanical properties, did not deflate, and had no evidence of emphysematous changes, we next examined elastic fibers by staining with Hart’s elastin stain (, A′ and B′). Assessing and representing the integrity of elastic fibers in single sections is exceptionally difficult. Nonetheless, extensive analysis of a large number of sections revealed a high density of elastic fibers with a disorganized appearance, embedded within the tissue. Many of the elastic fibers displayed a fractured appearance (as represented in , B′). To determine whether elastin synthesis was ongoing despite the accumulation of elastic fibers in the tissue, we performed in situ hybridization for elastin mRNA on serial sections of tissue (, A″ and B″). This showed that elastin expression is active, and localizes to the walls of intralobar arteries, conducting airways, and terminal airways and alveoli adjacent to the inflammatory infiltrates. Within an inflammatory infiltrate encompassing a previously patent vessel, elastin expression localized to the vessel remnants (, B′ and B″), identified on the basis of its elastic lamellae and α-smooth muscle actin (α-SMA) staining.
Increased smooth muscle and myofibroblasts in CBO
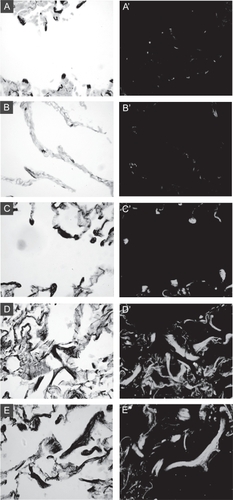
Elastic fibers in the lung are largely the product of smooth muscle cells of blood vessels and conducting airways and myofibroblasts within alveolar walls. Sections of CBO lung, along with normal and emphysematous control lungs, were stained for α-SMA to determine whether altered abundance or distribution of cells expressing a “myo” phenotype may be responsible for the abnormal elastin expression found in this adult lung specimen (). The typical appearance and distribution of elastic fibers and alveolar myofibroblasts in normal adult human lung is shown in , A and A′. Dense elastic fibers localize to alveolar tips, where small alveolar myofibroblasts expressing α-SMA reside. In emphysematous lung specimens, some areas exhibit a loss of elastic fibers associated with enlarged alveolar spaces, and the distribution of alveolar myofibroblasts is altered (, B and B’). In the CBO lung, alveolar regions had dense, enlarged elastic fibers localizing to alveolar tips, where intensely staining myofibroblasts were found (, C and C’). In multiple sections containing areas of extensive remodeling in the CBO specimens, dense accumulations of disorganized elastic fibers were populated by single cells and groups of cells staining intensely for α-SMA (, D/E and D’/E’).
Figure 2 Altered distribution and abundance of cells expressing α-SMA in CBO lung. Serial sections of lung were stained with Hart’s elastin stain (left panels) or for a-SMA (right panels). A: Alveolar section from a control lung specimen showing focal elastic fibers (black stained) at tips of alveolar walls (grey counterstain). A′: Serial section stained for a-SMA shows small alveolar myofibroblasts localizing to alveolar tips. B: Emphysematous lung specimen with diminished complexity, enlarged airspaces, and less elastin staining. B′ shows loss of focal staining for a-SMA. C: Alveolar region from CBO lung shows large focal elastic fibers at alveolar septal tips, and C′ shows enlarged myofibroblasts at the same sites staining intensely for a-SMA. D and E: Remodeled regions of CBO lung showing accumulation of thick elastic fibers. D′ and E′: The elastic fibers are surrounded by cells staining intensely for a-SMA.
Discussion
In the case of cryptogenic CBO described, the root cause of the dyspnea and severe airways obstruction was suspected to be bronchiolitis, but the pathologic diagnosis was not confirmed until after lung transplantation. Upon explantation of the diseased lung, it was observed that the lung did not deflate, and that even sections of lung tissue removed for pathologic examination remained inflated. These unusual findings led to a more detailed analysis of the peripheral lung tissue, which was compared to other specimens from an ongoing study of elastic fiber changes in COPD. In marked contrast to emphysematous lungs, there was no evidence of alveolar septal destruction. In addition, alveolar elastic fibers were greatly increased in number and density compared to normal lung specimens.
The role of elastic fibers in CBO has not been well characterized. Pulmonary elastin is an extremely stable and long-lived polymer (CitationShapiro et al 1991), and the majority of lung elastin expression is limited to the alveolar stage of lung development (CitationParks 1997; CitationMariani et al 2002). Thus, in normal uninjured adult lungs, elastin expression is virtually non-existent. Even in lung pathology associated with extensive remodeling, fibrosis due to excess collagen deposition is much more common than elastosis. Notably, the remodeling of airway walls in CBO is thought to be the result of unrestrained collagenous fibrosis (CitationMarkopoulo et al 2002; CitationCouture and Colby 2003; CitationVisscher and Myers 2006), a phenomenon that has been associated with and shown to be dependent upon increased transforming growth factor-beta (TGF-β) signaling (CitationRamirez et al 2004). A number of other genes have been shown to undergo upregulation in response to TGF-β signalling, including elastin (CitationDavidson et al 1995; CitationDavidson 2002) and alpha smooth muscle actin (α-SMA) (CitationDesmouliere et al 1993; CitationDavidson 2002). Most cell types expressing elastin in lung development, including airway and vascular smooth muscle cells and alveolar myofibroblasts, are positive for α-SMA, and upregulation of α-SMA has been implicated in the transition of cells to a myofibroblast phenotype in adults with idiopathic pulmonary fibrosis (CitationWillis et al 2005). For these reasons, we examined this case to characterize elastin mRNA expression and the abundance of α SMA-positive cells.
The sampled lung showed evidence of severe CBO, with most of the areas demonstrating the phenomenon of ‘disappearing’ bronchioles, an indication of complete bronchiolar obliteration by the constrictive process. Elastic fibers were abundant, dense, thick, and ropy in appearance in the affected bronchioles, and in situ hybridization for elastin mRNA showed abundant elastin expression localized to the collapsed and diseased bronchiolar walls, which were the primary sites of excess elastin accumulation. In addition, a similar upregulation of elastin deposition was noted in the adjacent alveolar areas, and was more extensive than those observed in either normal or emphysematous lung specimens.
The combination of upregulated elastin gene expression along with myofibroblast (α-SMA-positive cell) proliferation, when compared with normal lung, leads us to hypothesize that the elastosis noted in the bronchiolar and alveolar walls is likely the result of recent elastin protein synthesis. The specific signals leading to increased myofibroblast abundance and elastin expression are still unknown in this case.
Acknowledgements
The research reported in this manuscript was supported by National Institutes of Health HL54049 and HL-P50 084922 to R.A.P. and by American Lung Association RT-9702-N to A.S.
References
- BaraldoSSaettaMCosioMG2003Pathophysiology of the small airwaysSemin Respir Crit Care Med244657216088567
- ColbyTV1998Bronchiolitis. Pathologic considerationsAm J Clin Pathol10910199426525
- CoutureCColbyTV2003Histopathology of bronchiolar disordersSemin Respir Crit Care Med244899816088569
- DavidsonJM2002Smad about elastin regulationAm J Respir Cell Mol Biol26164611804864
- DavidsonJMZangMCZoiaO1995Regulation of elastin synthesis in pathological statesCiba Found Symp1928194 discussion 94–98575269
- DesmouliereAGeinozAGabbianiF1993Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblastsJ Cell Biol122103118314838
- GosinkBBFriedmanPJLiebowAA1973Bronchiolitis obliterans. Roentgenologic-pathologic correlationAm J Roentgenol Radium Ther Nucl Med11781632
- HoffCRPerkinsDRDavidsonJM1999Elastin gene expression is upregulated during pulmonary fibrosisConnect Tissue Res401455310761639
- KieltyCMSherrattMJShuttleworthCA2002Elastic fibresJ Cell Sci11528172812082143
- KraftMMortensonRLColbyTV1993Cryptogenic constrictive bronchiolitis. A clinicopathologic studyAm Rev Respir Dis14810931018214931
- LuceyECGoldsteinRHStonePJ1998Remodeling of alveolar walls after elastase treatment of hamsters. Results of elastin and collagen mRNA in situ hybridizationAm J Respir Crit Care Med158555649700135
- MarianiTJReedJJShapiroSD2002Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrixAm J Respir Cell Mol Biol26541811970905
- MarianiTJSandefurSPierceRA1997Elastin in lung developmentExp Lung Res23131459088923
- MarkopouloKDCoolCDElliotTL2002Obliterative bronchiolitis: varying presentations and clinicopathological correlationEur Respir J19203011843321
- ParksWC1997Posttranscriptional regulation of lung elastin productionAm J Respir Cell Mol Biol17129224202
- RamirezAMTakagawaSSekosanM2004Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation modelAm J Pathol16512233215466388
- RyuJHMyersJLSwensenSJ2003Bronchiolar disordersAm J Respir Crit Care Med16812779214644923
- ShapiroSDEndicottSKProvinceMA1991Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbonJ Clin Invest871828342022748
- SniderGLCiccolellaDEMorrisSM1991Putative role of neutrophil elastase in the pathogenesis of emphysemaAnn N Y Acad Sci62445592064248
- TurtonCWWilliamsGGreenM1981Cryptogenic obliterative bronchiolitis in adultsThorax36805107330801
- VisscherDWMyersJL2006Bronchiolitis: the pathologist’s perspectiveProc Am Thorac Soc341716493150
- WillisBCLieblerJMLuby-PhelpsK2005Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosisAm J Pathol16613213215855634