Abstract
Chronic obstructive pulmonary disease (COPD) is associated with significant morbidity and mortality. Its possible association with obstructive sleep apnea is a major cause of concern for clinicians. As the prevalence of both COPD and sleep apnea continues to rise, further investigation of this interaction is needed. In addition, COPD patients are at risk for hypoventilation during sleep due to the underlying respiratory dysfunction. In this study, 13 COPD subjects and 13 non-COPD control subjects were compared for the presence and severity of obstructive sleep apnea and nocturnal hypoventilation. All 26 subjects had presented to a sleep clinic and showed no signs of daytime hypoxemia. After matching for BMI and age, COPD subjects had a similar prevalence of sleep apnea with a lower degree of severity compared to the control subjects. However, less severe events, such as RERA, occurred at similar rates between the two groups. There was no significant difference between groups in the magnitude of oxyhemoglobin desaturation during sleep. Interestingly, severity and presence of nocturnal hypoxemia correlated with that of sleep apnea in the control group, but not in the COPD subjects. In conclusion, COPD without daytime hypoxemia was not a risk factor for sleep apnea or nocturnal hypoventilation in this study.
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of mortality in many countries. It is currently the fourth leading cause of death in the United States and is expected to increase to the third leading cause by the year 2020 (CitationBarnes 2000; CitationHurd 2000). Sleep related respiratory disturbances such as obstructive sleep apnea (OSA) may be present in such patients. As upper airway resistance has been shown to be increased in COPD patients during sleep when compared to wakefulness (CitationBallard 1995), the overlapping with OSA is of clinical concern due to its high prevalence in the general population (24% of adult males and 9% of adult women) (CitationYoung et al 1993; CitationNieto et al 2000). There has been conflicting data in the literature regarding the prevalence of sleep disorder breathing in patients with COPD (CitationChaouat 1995; CitationGuilleminault 1980; CitationFlenley 1985). A possible source of this controversy may relate to the technical difficulties involved in obtaining accurate data from monitoring sleep systems. One recent, large scale study evaluating data from the Sleep Heart Health Study (SHHS) found no association between mild obstructive airway disease and OSA (CitationSanders et al 2003). However, most of the data published in the literature was acquired using a thermistor or a thermocouple f or monitoring respiratory flow. Recent studies have demonstrated that these techniques may miss 25%–50% of respiratory events when compared to gold standard techniques, such as esophageal manometry (CitationNorman 1997). Therefore, studies using less accurate respiratory monitoring techniques may miss or underreport respiratory events. Given the underlying pathophysiology of obstructive airway disease and the increased upper airway resistance seen during sleep, COPD patients are at risk for developing obstructive respiratory disturbance during sleep. Likewise, more subtle respiratory abnormalities, such as respiratory-effort related arousal (RERA) are associated with nocturnal cortical arousals and sleep fragmentation leading to subsequent daytime fatigue and somnolence. These events have not previously been evaluated in COPD patients. Because of the lack of an adequate non-invasive technique to monitor respiratory flow during sleep, the prevalence of respiratory disturbance associated with COPD is still uncertain.
Furthermore, COPD patients frequently complain of poor sleep quality and inadequate sleep (CitationCalverley 1982; CitationCormick 1986). Some of the factors leading to sleep disruption in COPD patients may also be related to the worsening of respiratory function during sleep, which may result from nocturnal hypoventilation, ventilation-perfusion mismatch, and decreased functional residual capacity. Though decreases in nocturnal oxyhemoglobin saturation (SaO2) may occur during sleep in COPD patients, the episodes tend to be more pronounced during rapid eye movement (REM) stages of sleep due to loss of peripheral muscle tone and a functional dependence on an impaired flattened diaphragm (CitationDouglas 1979; CitationStradling 1983; CitationGeorge 1987).
The nasal cannula/pressure transducer system has been considered the best non-invasive method to detect minimal changes in airflow during nocturnal polysomnogram (CitationAyappa 2000; CitationEpstein et al 2000a, 2004; CitationMontserrat et al 1997; CitationThurnheer et al 2001) and has also been validated to detect RERA events (CitationAyappa 2000). In this study, we used the nasal cannula/pressure transducer system to evaluate respiratory events and oxyhemoglobin desaturation in COPD patients without evidence of daytime hypoxemia referred for evaluation of sleep-disordered breathing. Our study compared a group of patients with COPD without daytime hypoxemia (PaO2 > 60 mmHg) to a non-COPD control group, matched for BMI and age, during an attended overnight sleep study.
Methods
Twenty-eight subjects were recruited during a one-year period from the Manhattan Veterans Administration Sleep Clinic. Participation in this study was offered to all subjects with a known history of COPD and absence of daytime hypoxemia who were referred to the clinic for evaluation of snoring. For each COPD subject, a control subject without COPD, matched for BMI and age was selected from the clinic pool of patients that were evaluated within the 6-week period that followed inclusion of each COPD subject. COPD was defined using the Global Initiative for Obstructive Lung Disease (GOLD) criteria (CitationPauwels et al 2001). All COPD patients had evidence of adequate oxygenation (PaO2 > 60 mmHg) during wakefulness. Exclusion criteria were: (1) current or past use of continuous positive airway pressure, bi-level or supplemental oxygen; (2) respiratory failure requiring hospitalization within the previous 8-weeks; and (3) diagnosis of anemia. For each control subject, pulmonary function test was performed to rule out obstructive airway pathology.
All patients underwent spirometry while in sitting position within 6-months of the study inclusion date. An arterial blood gas sampling was performed in all COPD subjects during wakefulness, at rest in sitting position to verify adequate oxygenation. The room air oxyhemoglobin saturation (RA) SaO2), FEV1 and FEV1/FVC were recorded during spirometry. Subsequently, all patients were administered the Epworth Sleepiness Scale (ESS) in order to assess daytime sleepiness (CitationJohns 1991). Twenty six subjects (13 with COPD and 13 non-COPD controls) completed the sleep study. An attended nocturnal polysomnography was performed in all patients at their approximate routine sleeping hours. Recorded channels included electroencephalogram (C3/A2, C4/A1, O1/A2, 02/A1), bilateral electrooculogram (EOG), submental and anterior tibialis electromyogram (EMG), EKG, rib cage and abdominal motion by inductive plethysmography, body position, nasal cannula/pressure transducer system for respiratory monitoring (Protech®, Woodenville, WA) and oximetry with digital signal extraction (Masimo®, Irvine, CA). This oximetry technology was chosen because it is based on real time acquisition and processing of SaO2 data and because it has improved detection in states of low perfusion, motion, and weak signal. Sleep data was recorded via Bio-logic® (Mundelein, IL). Standard Rechtschaffen and Kales criteria were used to score sleep; arousals and respiratory events were scored using the CitationAmerican Academy of Sleep Medicine criteria (1999). Respiratory events consisted of apneas, defined as a reduction in air flow = 90% associated with sustained chest and abdominal effort, hypopneas, defined as a reduction in air flow = 50% associated with sustained chest and abdominal effort, and RERA events, defined as inspiratory flow limitation events followed by an EEG arousal which was not associated with any other sleep disturbance. The apnea-hypopnea index (AHI) included obstructive apneas and hypopneas linked to oxyhemoglobin drop = 4%. The respiratory disturbance index (RDI) consisted of the addition of the RERA hourly index to the AHI. All subjects signed an informed consent form and the study was approved by the IRB and GCRC.
Statistical analysis
All respiratory events were tabulated (apnea, hypopnea, and RERA) and calculated to a final hourly index of events. Respiratory events were tabulated as both AHI and RDI. Other sleep variables included total sleep time (TST) and sleep efficiency (SE). Decreases in oxyhemoglobin saturation were tabulated as follows: (1) percentage of sleep time at SaO2 < 90% (TST% < 90); (2) total sleep time (min) at SaO2 < 90% (TSTmin <90); and (3) lowest SaO2 level recorded during wake, and REM and non-REM stages of sleep.
The variables were examined for deviations from normality, and appropriate transformations were performed prior to analyses to satisfy model assumptions, whenever applicable. The sleep data were analyzed using SPSS® software. Paired t-tests and Pearson’s correlation coefficients were used for descriptive analyses. Confidence intervals were calculated for each study variable for COPD and control subjects and for the pairwise differences between groups. Linear regression analysis was used to build prediction models and p-values of 0.05 (two-sided) were used for statistical significance (with no adjustments for multiplicity).
Results
Twenty-eight subjects were recruited from the Manhattan Veterans Affairs Sleep Clinic to participate in this study. Two subjects who did not complete the overnight sleep study were excluded from analyses. A total of 13 subjects with a known history of COPD and absence of daytime hypoxemia and 13 control subjects without COPD were included in the analyses. All subjects were male.
Sleep apnea
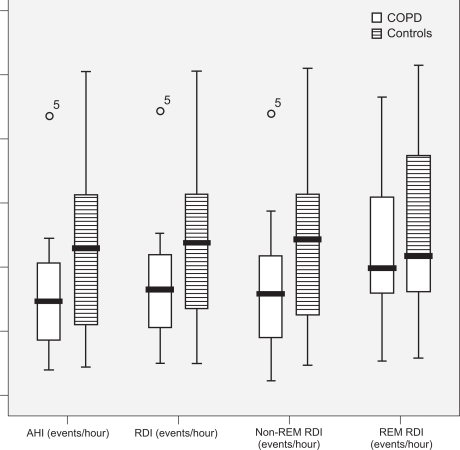
summarizes the study variables and the pairwise differences between COPD subjects and control subjects. Nocturnal attended polysomnography detected OSA as defined by AHI >5 events/hour in all subjects and controls. The AHI in COPD subjects was lower by an average of 13 events/hour when compared to control subjects, despite their similar age and BMI (). This difference was not statistically significant. The mean AHI of the COPD subjects was 38.2 events/hour (95% CI: 23.7–54.5) vs 51.7 events/hour (95% CI: 33.4–70.8) in the control subjects (p-value not significant).
Table 1 Baseline characteristics of all subjects by group
The RDI in COPD subjects was lower by an average of 13.5 events/hour as compared to control subjects during non-REM sleep and by an average of 6.3 events/hour as compared to control subjects during REM sleep. The mean non-REM RDI in COPD subjects was 40.2 events/hour (95% CI: 25.5–57.4) vs 53.6 events/hour (95% CI: 36.7–71.7) in control subjects. The mean REM RDI in COPD subjects was 44.9 events/hour (95% CI: 32.5–58.4) vs 51.3 events/hour (95% CI: 35.8–65.9) in control subjects. However, these differences were not statistically significant. In addition, RERA events did not differ significantly between the two groups. The mean RERA index in COPD subjects was 2.69 events/hour (95% CI: 1.33–4.23) vs 2.18 events/hour (95% CI: 0.48–4.85) in control subjects.
Nocturnal hypoventilation
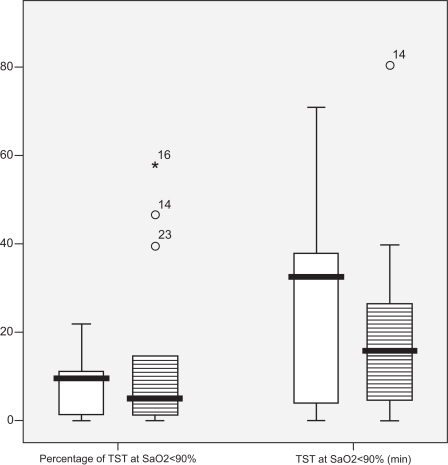
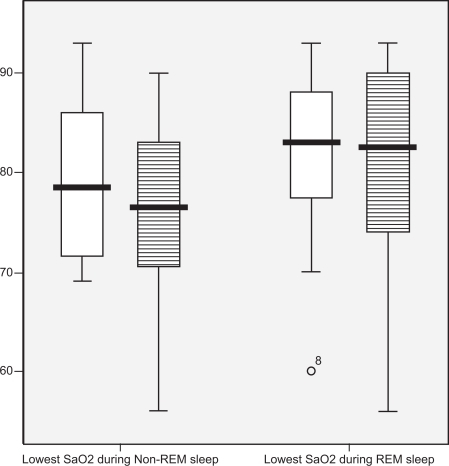
Hypoxemia during sleep, as defined by the percentage of sleep time spent at SaO2 levels below 90%, was similar between groups as seen in . Additional measurements of nocturnal hypoxemia and oxyhemoglobin saturation during sleep and wakefulness were also not statistically significant between groups, as shown in .
The severity of sleep apnea as measured by AHI was a significant predictor of the TST% < 90 in the control group (r = 0.61, p < 0.05). However, the association between TST% < 90 and sleep apnea was not significant in the COPD group (r = −0.15, p = ns). In the linear regression model, after controlling for BMI and FVC that could potentially confound the association of AHI and TST% < 90, AHI was still a significant predictor of TST% < 90 in the control group. After backward elimination, AHI and COPD status were the only variables remaining in the model and this model was significant (r = 0.66, p < 0.05).
Other sleep variables
The mean sleep duration was increased in the COPD group by 38 minutes (95% CI for the difference: −100, 18.5), with a tendency towards a higher sleep efficiency (by 8%, 95% CI: −21.9, 4.80), when compared to the control subjects. However, those differences were not statistically significant. Sleep efficiency was negatively associated with (RA) SaO2 (r = −0.70, p = 0.01) in the control group. The arousal index, as measured by total number of EEG arousals divided by the TST in hours was similar between groups.
The assessment of daytime somnolence by the ESS demonstrated a tendency to slightly higher scores in control subjects when compared to COPD subjects (difference: 2.8 points [−0.62, 6.38]). However, this difference was not statistically significant. Of note, the ESS score was not predictive of the severity of sleep apnea in either group. All subjects had scores above 7 (range 7–23), which is lower than the cutoff commonly used for inclusion in sleep apnea research studies (ESS = 11).
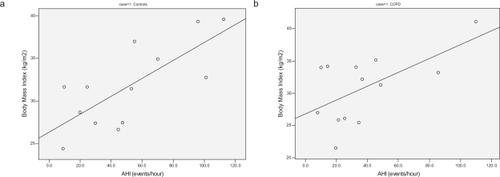
Body mass index (BMI) was a significant predictor of the severity of OSA in all subjects as shown in (r = 0.60 and 0.75, for COPD and control patients respectively, p < 0.05).
Figure 4 Severity of sleep apnea (AHI) plotted against BMI in both groups shows a better correlation in the control group (r = 0.75) (a) than COPD (r = 0.60) (b).
There was no significant association between BMI and lowest saturation in NREM sleep in either COPD patients or control subjects. The lowest saturation in REM sleep was negatively correlated with REM RDI in the control group (r = −0.67, p = 0.02). However, this association was not seen in the COPD subjects (r = −0.12), implying that other mechanisms controlling oxygen saturation levels may be present during REM sleep in COPD patients.
There was an inverse relationship in all subjects between severity of COPD by FEV/FVC ratio and BMI (r = −0.66, p < 0.02), which was clinically confirmed by findings of muscle wasting in patients with more severe lung disease. Nonetheless, the severity of sleep apnea in COPD subjects was strongly associated with the severity of lung disease, measured by the FEV/FVC ratio (r = −0.85, p < 0.001).
Discussion
The objective of this study was to compare the magnitude and specific features of OSA and nocturnal hypoventilation in an unselected sample of COPD patients without daytime hypoxemia presenting to a sleep clinic for complaints of possible sleep apnea. A control group of age and BMI-matched subjects without COPD was used for comparison. Our data demonstrated that COPD without daytime hypoxemia is not associated with increased severity of sleep apnea or nocturnal hypoxemia. In fact, most COPD subjects without daytime hypoxemia had similar to lower degrees of sleep apnea than control subjects without COPD. However, the patients with more severe COPD also had severe sleep apnea. The rate of less severe events, such as RERA was the same in both groups. This is concurrent to findings described by other investigators using different respiratory monitoring techniques (CitationSanders et al 2003; CitationAoki 2005). In the present study, the mean AHI of COPD subjects was 38.2 events/hour vs 51.7 events/hour in the control subjects. This result is similar to that published by Sanders et al who demonstrated a lower index of sleep apnea severity in COPD subjects that could not be explained by changes in BMI when compared to control subjects (CitationSanders et al 2003).
Interestingly, the effects of obesity on sleep apnea in COPD patients seem to be different than in control subjects. In fact, COPD patients may be protected against the effects of obesity on upper airway function, when compared to non-COPD patients. In this study, BMI was a much stronger predictor of sleep apnea severity in control subjects compared to COPD subjects. REM-related events and oxygen desaturation during sleep were not dependent on AHI or obesity in COPD subjects. These findings imply that other mechanisms may be the driving factors leading to respiratory disturbance and hypoxemia during sleep in COPD patients. These mechanisms may correlate to changes in ventilation/perfusion and altered mechanisms of respiratory control, which are difficult to document with standard clinical testing. As compared to control subjects, COPD subjects showed decreases in oxygen saturation during sleep that were independent of sleep apnea.
Subjects with COPD demonstrated adequate sleep duration and efficiency. The levels of daytime sleepiness were similar in both groups, as all subjects tested positive for sleep apnea. Interestingly, the ESS score was not predictive of sleep apnea or lung disease severity, with all subjects scoring above 7 on the scale. Some of the limitations of this study included the small sample size and the absence of females, which will limit our conclusions about COPD to subjects in settings that are similar to this VA-based sleep clinic population.
Conclusion
This study’s findings demonstrate that mild COPD is not associated with increased severity of sleep apnea or nocturnal hypoxemia. Due to the fact that those factors in COPD do not appear to correlate with obesity, other mechanisms might be involved. The present findings provide no basis for managing patients with COPD without daytime hypoxemia any differently than patients without lung disease in respect to sleep apnea evaluation.
COPD does not appear to be a risk factor that magnifies the severity of sleep apnea in COPD patients without daytime hypoxemia, regardless of the COPD severity. These findings along with other published data in the literature set the stage for a more in depth future study of the incidence of OSA and other respiratory disturbances during sleep in COPD patients across the full spectrum of disease severity in patients with chronic lung disease.
Notes
Notes
Support provided by a NIH M01 RR00096 grant. None of the authors has any conflict of interest to disclose.
References
- The Report of an American Academy of Sleep Medicine Task Force1999Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical researchSleep226678910450601
- BahammamA2004Comparison of Nasal prong pressure and thermistor measurements for detecting respiratory events during sleepRespiration713859015316213
- AokiTAkinoriEYogoY2005Sleep disordered breathing in patients with chronic obstructive pulmonary diseaseCOPD22435217136951
- AyappaINormanRGKriegerAC2000Non-Invasive detection of respiratory effort-related arousals (REras) by a nasal cannula/pressure transducer systemSleep237637111007443
- BallardRDCloverCWSuhBY1995Influence of sleep on respiratory function in emphysemaAm J Respir Crit Care Med151945517697271
- BARNESPJ2000Chronic obstructive pulmonary diseaseN Engl J Med3432698010911010
- CalverleyPMBrezinovaVDouglasNJ1982The effect of oxygenation on sleep quality in chronic bronchitis and emphysemaAm Rev Respir Dis126206107103244
- ChaouatAWeitzenblumEKriegerJ1995Association of chronic obstructive pulmonary disease and sleep apnea syndromeAm J Respir Crit Care Med1518267812577
- CormickWOlsonLGHensleyMJ1986Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung diseaseThorax41846543824271
- DouglasNJCalverleyPMLeggettRJ1979Transient hypoxaemia during sleep in chronic bronchitis and emphysemaLancet11483461
- EpsteinMDChicoineSAHanumaraRC2000Detection of upper airway resistance syndrome using a nasal cannula/pressure transducerChest1171073710767242
- FlenleyD1985Sleep in chronic obstructive lung diseaseClin Chest Med6651612935359
- GeorgeCFWestPKrygerMH1987Oxygenation and breathing pattern during phasic and tonic REM in patients with chronic obstructive pulmonary diseaseSleep10234433629085
- GuilleminaultCCummiskeyJMottaJ1980Chronic obstructive airflow disease and sleep studiesAm Rev Respir Dis1223974067416615
- HurdS2000The impact of COPD on lung health worldwide: epidemiology and incidenceChest1171S410673465
- JohnsM1991A new method for measuring daytime sleepiness: the Epworth sleepiness scaleSleep1454051798888
- MontserratJMFarreRBallesterE1997Evaluation of nasal prongs for estimating nasal flowAm J Respir Crit Care Med155211159001314
- NietoFJYoungTBLindBK2000Association of Sleep-Disordered Breathing, Sleep apnea, and hypertension in a large community-based studyJAMA28318293610770144
- NormanRGAhmedMMWalslebenJA1997Detection of respiratory events during NPSG: nasal cannula/pressure sensor versus thermistorSleep201175849493929
- PauwelsRABuistASCalverleyPMA2001Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop SummaryAm J Respir Crit Care Med16312567611316667
- SandersMHNewmanABHaggertyCL2003Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway diseaseAm J Respir Crit Care Med16771412502472
- StradlingLDJr1983Nocturnal hypoxaemia in chronic obstructive pulmonary diseaseClin Sci (Lond)64213226681599
- ThurnheerRXieXBlochKE2001Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleepAm J Respir Crit Care Med16419141911734446
- YoungTPaltaMDempseyJ1993The occurrence of sleep-disordered breathing among middle-aged adultsN Engl J Med328123058464434