Abstract
Background
Reticular basement membrane (RBM) thickening has been variably associated with asthma and chronic obstructive pulmonary disease (COPD). Even if RBM thickness is similar in both diseases, its composition might still differ.
Objective
To assess whether RBM thickness and composition differ between asthma and COPD.
Methods
We investigated 24 allergic asthmatics (forced expiratory volume in one second [FEV1] 92% predicted), and 17 nonallergic COPD patients (FEV1 60% predicted), and for each group a control group of similar age and smoking habits (12 and 10 persons, respectively). Snap-frozen sections of bronchial biopsies were stained with hematoxylin/eosin and for collagen I, III, IV, V, laminin and tenascin. RBM thickening was assessed by digital image analysis. Relative staining intensity of each matrix component was determined.
Results
Mean (SD) RBM thickness was not significantly different between asthma and COPD 5.5 (1.3) vs 6.0 (1.8) μm, but significantly larger than in their healthy counterparts, ie, 4.7 (0.9) and 4.8 (1.2) μm, respectively. Collagen I and laminin stained significantly stronger in asthma than in COPD. Tenascin stained stronger in asthma than in healthy controls of similar age, and stronger in COPD controls than in asthma controls (p < 0.05).
Conclusion
RBM thickening occurs both in asthma and COPD. We provide supportive evidence that its composition differs in asthma and COPD.
Introduction
One of the hallmarks of airway remodeling is a thickened airway reticular basement membrane (RBM) as a result of extracellular matrix deposition directly apposing the lamina reticularis.Citation1,Citation2 In an earlier study, Kasahara and colleagues compared the thickness of the RBM (biopsies) and of the whole airway wall thickness as assessed by high-resolution computed tomography (HRCT) in patients with mild-to-moderate asthma and healthy controls matched for age and sex.Citation3 They showed that RBM thickness in asthmatic patients was larger than that of healthy controls and that it correlated strongly with whole airway wall thickness and forced expiratory volume in one second (FEV1). Furthermore, James and colleagues showed similar findings comparing the reticular basement membrane thickness of central airways assessed by endobronchial biopsy and airway wall dimensions in cartilaginous airways in surgical specimens.Citation4 Although disputed by others,Citation5 these findings support earlier studies suggesting that RBM thickness may be used as an index of remodeling of the airway wall in asthma.
In contrast to asthma, the literature is not unanimous whether airway RBM is thickened in patients with chronic obstructive pulmonary disease (COPD). Some studies find a normal RBM,Citation6–Citation8 while others report it to be thickened,Citation9–Citation13 but generally to a smaller extent than in asthma.Citation9,Citation10,Citation12,Citation14,Citation15 The differences in observations may be due to the fact that some studies have included only a few COPD patientsCitation7 or COPD patients with only mildCitation10 or more severeCitation9 airway obstruction. In addition, some studies included healthy controls who were either never smokersCitation11 or smokers not being matched for pack-years smoking.Citation8 Airway inflammation is of a chronic nature in both asthma and COPD, likely resulting in a long-lasting increase of fibrogenic growth factors like transforming growth factor-β in both asthma and COPD,Citation10,Citation16 vascular endothelial growth factor in asthma,Citation17 and epidermal growth factors in COPD.Citation10 Thus it might be anticipated that the RBM is thickened in both asthma and COPD.
Collagen IV, laminin, and proteoglycans are the major components of the true basement membrane. Beneath this thin layer, collagen I, III, V, and tenascin amongst others form the lamina reticularis, which is thickened in asthma patients. Both layers are strongly connected by strands of collagen VII. Laminin and tenascin have binding sites for the other matrix proteins, as well as for mesenchymal and epithelial cells. Together with proteoglycans, these matrix proteins form a firm and complex network that regulates processes like cell-migration and cell-adhesion.
We hypothesized that extracellular matrix deposition, resulting in RBM thickening, occurs in both asthma and COPD. Since the inflammatory profile differs between asthma and COPD, we anticipated that the extracellular matrix composition of RBM differs in terms of the relative contribution of each ECM component. We analyzed bronchial biopsies of patients with asthma and COPD with respect to the relationship between RBM thickness and composition on one hand, and FEV1, bronchial hyperresponsiveness on the other hand. Because the RBM thickness and composition may change during aging, we compared each group with age- and pack-years smoking-matched healthy controls.
Methods
Subjects
Subjects were recruited from our outpatient clinic of the University Medical Center Groningen or by advertisements in local newspapers. The local medical ethics committee approved the protocols and all subjects gave their written informed consent.
Asthma patients and healthy controls
Twenty-four subjects with mild-to-moderate severe asthma, 18–45 years old, were included. Their results of inflammatory composition of airway wall biopsies have been reported previously.Citation18 Asthmatics were selected on: history consistent with asthma; positive intracutaneous tests against house dust mite or two other aeroallergens (18 common aeroallergens of ALK, Groningen, the Netherlands), FEV1 > 60% predicted; provocative concentration of methacholine causing a 20% fall in FEV1 (PC20methacholine) ≤9.8 mg/ml; and provocative concentration of adenosine-5-monophosphate (AMP) causing a 20% fall in FEV1 (PC20AMP) ≤80 mg/ml. Twelve healthy volunteers of similar age were selected on: no history of lung disease; FEV1 > 85% predicted, no atopy and no airways hyperresponsiveness to methacholine or AMP. Asthmatic and healthy subjects who had been smoking in the past two years were excluded.
COPD patients and healthy controls
Seventeen COPD subjects over 45 years of age were included, according to American Thoracic Society (ATS) criteria.Citation19 All subjects had a negative history of atopy, negative skin tests to 18 common aeroallergens (ALK, Groningen, the Netherlands) and negative specific IgE for 11 common aeroallergens (phadiatop®). Subjects with COPD had an FEV1 and FEV1/vital capacity (VC) <predicted value −1.64 residual standard deviationsCitation20 and an increase in FEV1 < 10% predicted after inhalation of 1 mg terbutaline per Turbuhaler®. Three subjects were never-smokers, all other participants were ex-smokers, ie they quitted smoking at least one year before the start of the study. Ten healthy ex-smoking volunteers were included without a history of pulmonary disease, with normal lung function, and similar age and pack-years smoking.
All patients stopped inhaled corticosteroids at least four weeks prior to bronchoscopy. Exclusion criteria were treatment with oral prednisolone and/or antibiotics, or a respiratory infection within four weeks prior to the study. Because of safety reasons, all patients had an FEV1 > 1.5 L.
Lung function
FEV1 was performed according to standardized guidelines of the European Respiratory Society.Citation20 Reversibility was tested 30 minutes after inhalation with 400 μg salbutamol in asthma and with 1 mg terbutaline in COPD. Subjects were not allowed to use short-acting bronchodilators within 12 hours or long-acting bronchodilators within 24 hours.
Bronchoscopy and processing of biopsies
Bronchoscopy was performed using an Olympus B1 IT10 flexible fiberoptic bronchoscope (Olympus Optical, Tokyo, Japan) according to American Thoracic Society (ATS) criteria.Citation21 At least five biopsies were taken from the subsegmental carinas from the left or right lower lobe using a fenestrated cup forceps (Olympus BF-21-C, Tokyo Japan).
Biopsies were mounted in Tissue Tek® embedding compound and snap-frozen by immersion in isopentane (−80 °C). Frozen biopsies were cut in serial sections at 4-μm thickness. Selection of morphologically optimal tissue was based on every twentieth section, stained with Mayer’s hematoxylin and eosin. Slides were stored at −20 °C.
Measuring of reticular basement membrane thickness
Sections from all biopsies from each patient were stained with Mayer’s hematoxylin and eosin. The morphologically most optimal section was selected by including sections with optimal integrity of the RBM and excluding tangentially cut tissue. RBM thickness was assessed by digital analysis (Leica Quantimed, Zeist, The Netherlands). Sections were coded and measured in random order at a magnification of 400x. One technician (MZ) performed all measurements. Fifteen measurements were performed over a cumulative length of basement membrane of 750 μm in each section. These measurements were equally distributed over the total length of the basement membrane. For each measurement, an area of the RBM was marked over a length of 50 μm. Thereafter, the exact surface area of basement membrane and apposed matrix was assessed. Dividing the surface (calculated by the computer) by its exact length resulted in the mean RBM thickness of the marked surface area. The mean RBM thickness of an individual section was the mean of 15 measurements.
Immunostaining
Immunohistochemical staining was performed on 4 μm sections of snap-frozen biopsies, dried at room temperature and fixed for 10 minutes in acetone. Slides were rinsed three times in phosphate-buffered saline (PBS) for five minutes between each step. Sections were stained for collagen I (goat polyclonal antibody; Southern Biotechnology Associates, Birmingham, AL, USA, see ), collagen III (mouse monoclonal antibody; Heyl, Berlin, Germany), collagen IV (rabbit polyclonal antibody; Cappel/ICN, Aurora, OH, USA), collagen V (goat polyclonal antibody; Southern Biotechnology Associates, Birmingham, AL, USA), laminin (rabbit polyclonal antibody; Telios, San Diego, CA, USA), and tenascin (mouse monoclonal antibody; Telios). Subsequently, the sections were put in PBS with 0.075% H2O2 for 30 minutes to block endogenous peroxidase activity. Finally, sections were treated with species-specific peroxidase-conjugated immunoglobulins (DAKO; Glostrup, Denmark) for 30 minutes and peroxidase staining was performed using 3-amino-ethylcarbazole (AEC; Sigma Chemical Company, St. Louis, MO, USA) as a substrate, providing a reddish-brown reaction product.
Examination of (reticular) basement membrane components
Semi-quantitative examination was performed by one investigator (MZ) in a blinded fashion using a light microscope at 100x magnification. Before semi-quantitative scoring of individual sections, all sections immuno-stained for a specific antigen were examined in order to have an impression of the range of immunopositivity. Thereafter, sections were scored on a three-point scale for staining intensity of the reticular basement membrane (1, low; 2, moderate; 3, high intensity). Intra-observer agreements (kappa, standard error) for collagen I, III, IV, V, laminin, and tenascin were: 0.74 (0.14), 0.81 (0.13), 0.80 (0.13), 0.51 (0.19), 0.84 (0.11), and 0.73 (0.14), respectively.
Data analysis
Analyses were performed with the SPSS (version 10.0; SPSS Inc., Chicago, IL, USA) software package. Normality of distribution was assessed with the Kolmogorov–Smirnov test. Differences in RBM thickness were evaluated by ANOVA followed by t-test, differences in staining activity of matrix components by Mann–Whitney U Test. Correlations between RBM thickness and FEV1 were evaluated by Pearson tests, correlations between RBM thickness and composition on the one hand, and PC20AMP on the other hand with Spearman tests. P-values ≤ 0.05 were considered to be significant.
Results
Subjects
Clinical characteristics of the patients with asthma and COPD, and their healthy controls are listed in . After analyzing RBM thickness, two subjects with asthma and one subject with COPD did not yield sufficient tissue to evaluate the RBM composition.
Table 1 Baseline characteristics
Reticular basement membrane thickness
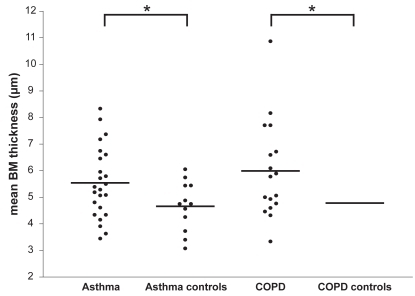
Values of the RBM thickness showed a normal distribution in all groups. The variability in RBM thickness was not significantly different between asthma and COPD. Mean (standard deviation) RBM thickness in asthma and COPD was significantly greater than that of healthy controls of similar age: 5.5 (1.3) μm vs 4.7 (0.9) μm in asthma, and 6.0 (1.8) vs 4.8 (1.2) μm in COPD ().
Figure 1 Mean basement membrane thickness for asthma, COPD, and healthy controls of similar age. Reticular basement membrane thickness in central airway wall biopsies from 24 patients with asthma, 12 age-matched healthy controls of asthma, 17 patients with COPD and 10 age- and pack year-matched healthy controls of COPD.
Abbreviation: COPD, chronic obstructive pulmonary disease.
Composition of the reticular basement membrane
Assessment of the RBM staining with collagen I, III, IV, V, tenascin, and laminin showed that collagen I () and laminin staining of the RBM were significantly more intense in asthma than in COPD (p < 0.05, ). In contrast, collagen IV showed a trend towards more intense staining in COPD than in asthma (p = 0.084, ). Tenascin staining of the RBM was significantly more intense in asthmatics than in healthy controls (p < 0.05, ), but not significantly different from COPD. Tenascin staining was significantly more intense in the COPD than in the asthma control group (). No further significant differences were found between the four study groups.
Figure 2 Reticular basement membrane composition. Relative staining density of collagen I, laminin, collagen IV, and tenascin in the reticular basement membrane of airway wall biopsies in asthma and COPD patients, and their healthy matched control subject. Results of collagen III and V are not shown as there were no significant differences between any of the groups.
Abbreviation: COPD, chronic obstructive pulmonary disease.
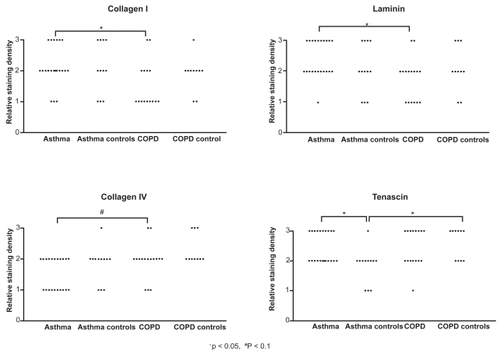
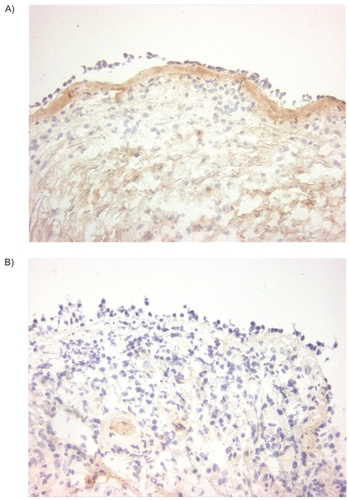
Figure 3 Collagen I staining. Immunohistochemical staining of bronchial biopsies for collagen I (immunoperoxidase, original magnification × 200) showed, a uniform red-brown-stained band beneath the epithelial layer in asthma (A left panel). In contrast to asthma, this is significantly less intense in COPD (p < 0.05, B right panel).
Correlations of basement membrane and clinical characteristics
There were no significant correlations between RBM thickness or extracellular matrix components (Collagen I, III, IV, V, tenascin, and laminin) on one hand and FEV1% pred, PC20AMP, and PC20methacholine (in asthma only) on the other hand, neither in asthmatics, nor in COPD patients, nor in healthy subjects.
Discussion
This study shows that the reticular airway basement membrane is thickened both in patients with mild-to-moderate asthma and COPD, as compared to healthy subjects of similar age and smoking history. An interesting observation is that the extracellular matrix composition of the reticular basement membrane differs between asthma and COPD, the latter containing significantly less collagen I and laminin. The reticular basement membrane thickness and its extracellular matrix composition did not significantly correlate with parameters of lung function.
To our knowledge this is the first study comparing RBM thickness in asthma and COPD patients and their healthy controls of similar age and smoking history. An important finding of this study is that the airway RBM in central airway biopsies is thickened with a similar magnitude in asthma and COPD. Other reports give contrasting results.Citation7,Citation9,Citation10,Citation12,Citation14,Citation15,Citation22 There are several explanations for this difference. Two studies compared asthma patients with a mixed group of COPD and chronic bronchitis patients (thus individuals with and without airway obstruction) finding a thicker RBM in asthma than in the mixed group.Citation10,Citation22 Two other studies reported a thicker RBM in asthma than in COPD,Citation7,Citation9 however these studies did not report their data in a quantitative way. Our study has the advantage that a more homogeneous group of COPD patients was selected, and additionally that RBM was measured using a computerized morphometry system, providing quantitative and non-selective data. In line with our results, Bourdin and colleagues demonstrated RBM thickness of a comparable magnitude as our findings in (mild) asthma and COPD and reported that this is not a useful histological marker to differentiate between these diseases.Citation13,Citation23 In the latter study, a measurement method similar to ours was used which has been validated by Wilson and colleagues.Citation24 We also demonstrate that the mean SBM thickness in asthma and COPD patients is larger than in healthy controls, though with a large overlap. This overlap is in line with earlier studies, despite the use of different methods and use of healthy controls with different age and/or smoking status.Citation1,Citation5,Citation7,Citation9–Citation13,Citation15,Citation17,Citation22,Citation24–Citation44 Taken together, we conclude that an increased RBM thickness indicates the presence of asthma or COPD, irrespective of age and that it is not possible to differentiate mild-to-moderate asthma from COPD purely based on RBM thickness. In addition, a normal RBM thickness does not exclude obstructive airway disease.
One could postulate that the age difference between our asthma and COPD patients, or asthma severity is responsible for the lack of difference in RBM thickness. Earlier studies have shown that RBM thickness is not associated with asthma duration,Citation25,Citation26,Citation45–Citation47 age,Citation20,Citation27 or asthma severity.Citation12,Citation26,Citation27 Notwithstanding this, Bourdin and colleagues recently provided evidence that particularly patients with severe asthma have a larger thickness of the RBM than asthmatics with mild disease. The latter two groups had similar duration of disease, confirming that it is not duration but rather severity of disease that affects RBM thickness. Our data for the first time show that age and aging may not affect RBM thickness, but they do affect the composition of the RBM, since tenascin was significantly more present in COPD controls than in the asthma control group, which by design of the study was younger in age. Alternatively, this may be the result of long-time smoke exposure, because COPD controls were ex-smokers and asthma controls were never-smokers.
The mean RBM thickness in our study is somewhat smaller in both asthma and COPD than in most other studies. This can be the result of using snap-frozen biopsies instead of embedding biopsies in resin or paraffin, or differences in fixation techniques. The manner of measuring RBM thickness is to our opinion decisive for the observed differences. First, we used area measurements, which Bourdin and colleagues showed to result in lower values than when using line measurements.Citation13 By using area measurements, we evaluated a significantly larger part of the RBM to assess thickness; for example we used 15 areas of 50 μm length while Sullivan used 40 line measurements.Citation48 Second, we measured completely random, large areas of RBM, while line measurements are more prone to selection bias. Thus our method has advantage over previous publications in that we used a more unbiased measuring method.Citation12,Citation14,Citation15
This study is the first to demonstrate that the extracellular matrix composition of the RBM differs between asthma and COPD, yet with large overlap in staining pattern. Previous studies have used staining of different collagens, but did not asses its further composition.Citation24,Citation30,Citation33–Citation36,Citation40 The observed differences between asthma and COPD in the extracellular matrix composition may be due to different types of irritation, epithelial damage, epithelial repair, and underlying submucosal airway inflammation, both in a quantitative and qualitative way. Collagen IV is a component of the “true” basement membrane which is not thickened in asthma. Compared to asthma, collagen IV showed a trend for a higher staining intensity in COPD.
The above differences in composition underline differences in pathophysiology of both diseases. Tenascin stained stronger in asthma than in healthy controls of similar age and also stronger in (older) COPD controls than in (younger) asthma controls. Kranenburg and colleagues compared the staining intensity of the RBM in patients with COPD and healthy subjects in surgical resection specimens using a similar scoring system as in our study.Citation49 Their results showed enhanced expression of total collagen, collagen I, and –III, but not of collagen IV, fibronectin, and laminin. Differences with our findings are likely caused by the use of surgical resection specimens whereas we investigated large airway bronchoscopic biopsies.
A thickened RBM and a change in its composition are both features of airway remodelling, which is supposed to contribute to airflow limitation in asthma and COPD. This study shows no significant correlations of RBM thickness and composition with FEV1% predicted, in line with someCitation25,Citation30,Citation47 but not all studies.Citation13,Citation18,Citation27,Citation34 Obviously, different results may be explained by differences in study populations and morphometric methods. Furthermore, we could not demonstrate a significant correlation between PC20AMP and RBM thickness. With respect to PC20methacholine several other studies have shown a negative correlation with RBM thickness in asthma.Citation25,Citation29,Citation34,Citation50 This would suggest that PC20methacholine is more closely related to markers of airway remodeling than PC20AMP, yet our data did not confirm this. Whether a thickened basement membrane is beneficial or harmful for the natural course of asthma or COPD, eg, by protecting against allergen or smoke exposure, is not supported by long-term follow-up studies.
In summary, this study shows that the reticular basement membrane is thickened in both asthma and COPD, yet has a different composition. More studies are needed to elucidate the exact relationship between the process of ongoing airway inflammation and airway remodeling in these diseases.
Acknowledgments
Jeroen Liesker has received an unrestricted research salary grant of AstraZeneca, Benelux. Studies in asthma and COPD were supported by the Dutch Asthma Foundation, Stichting Astma Bestrijding, and AstraZeneca, Benelux.
Disclosure
None of the authors has any financial interest, or any actual or potential conflict of interest in the subjects discussed in this manuscript.
References
- DunnillMSMassarellaGRAndersonJAA comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis, and in emphysemaThorax19692421761795821620
- BousquetJJefferyPKBusseWWJohnsonMVignolaAMAsthma. From bronchoconstriction to airways inflammation and remodelingAm J Respir Crit Care Med200016151720174510806180
- KasaharaKShibaKOzawaTOkudaKAdachiMCorrelation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthmaThorax200257324224611867829
- JamesALMaxwellPSPearce-PintoGElliotJGCarrollNGThe relationship of reticular basement membrane thickness to airway wall remodeling in asthmaAm J Respir Crit Care Med200216612 Pt 11590159512471074
- JefferyPKWardlawAJNelsonFCCollinsJVKayABBronchial biopsies in asthma. An ultrastructural, quantitative study and correlation with hyperreactivityAm Rev Respir Dis19891406174517532690708
- ChanezPVignolaAMO’ShaugnessyTCorticosteroid reversibility in COPD is related to features of asthmaAm J Respir Crit Care Med19971555152915349154853
- OllerenshawSLWoolcockAJCharacteristics of the inflammation in biopsies from large airways of subjects with asthma and subjects with chronic airflow limitationAm Rev Respir Dis19921454 Pt 19229271348169
- VachierIVignolaAMChiapparaGInflammatory features of nasal mucosa in smokers with and without COPDThorax200459430330715047949
- LacosteJYBousquetJChanezPVan VyveTSimony-LafontaineJLequeuNEosinophilic and neutrophilic inflammation in asthma, chronic bronchitis, and chronic obstructive pulmonary diseaseJ Allergy Clin Immunol19939245375488409114
- VignolaAMChanezPChiapparaGTransforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitisAm J Respir Crit Care Med19971562 Pt 15915999279245
- PesciAMajoriMCuomoANeutrophils infiltrating bronchial epithelium in chronic obstructive pulmonary diseaseRespir Med19989268638709850371
- BenayounLDruilheADombretMCAubierMPretolaniMAirway structural alterations selectively associated with severe asthmaAm J Respir Crit Care Med2003167101360136812531777
- BourdinANeveuDVachierIPaganinFGodardPChanezPSpecificity of basement membrane thickening in severe asthmaJ Allergy Clin Immunol200711961367137417481707
- FabbriLMRomagnoliMCorbettaLDifferences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary diseaseAm J Respir Crit Care Med2003167341842412426229
- MilaneseMCrimiEScordamagliaAOn the functional consequences of bronchial basement membrane thickeningJ Appl Physiol20019131035104011509495
- TakizawaHTanakaMTakamiKIncreased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD)Am J Respir Crit Care Med200116361476148311371421
- ChettaAZaniniAForesiAVascular endothelial growth factor up-regulation and bronchial wall remodelling in asthmaClin Exp Allergy200535111437144216297139
- Ten HackenNHTimensWSmithMDrokGKraanJPostmaDSIncreased peak expiratory flow variation in asthma: severe persistent increase but not nocturnal worsening of airway inflammationEur Respir J19981235465509762777
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary diseaseAmerican Thoracic SocietyAm J Respir Crit Care Med19951525 Pt 2S77S1217582322
- QuanjerPHTammelingGJCotesJEPedersenOFPeslinRYernaultJCLung volumes and forced ventilatory flows. Work Group on Standardization of Respiratory Function Tests. European Community for Coal and Steel. Official position of the European Respiratory SocietyRev Mal Respir199411Suppl 35407973051
- Summary and recommendations of a workshop on the investigative use of fiberoptic bronchoscopy and bronchoalveolar lavage in asthmaticsAm Rev Respir Dis198513211801824014864
- O’ShaughnessyTCAnsariTWBarnesNCJefferyPKReticular basement membrane thickness in moderately severe asthma and smokers with and without airflow obstructionAm J Respir Crit Care Med1996153A879
- BourdinASerreIFlammeHCan endobronchial biopsy analysis be recommended to discriminate between asthma and COPD in routine practice?Thorax200459648849315170031
- WilsonJWLiXThe measurement of reticular basement membrane and submucosal collagen in the asthmatic airwayClin Exp Allergy19972743633719146928
- HoshinoMNakamuraYSimJJExpression of growth factors and remodelling of the airway wall in bronchial asthmaThorax199853121279577517
- PayneDNRogersAVAdelrothEEarly thickening of the reticular basement membrane in children with difficult asthmaAm J Respir Crit Care Med20031671788212502479
- ShibaKKasaharaKNakajimaHAdachiMStructural changes of the airway wall impair respiratory function, even in mild asthmaChest200212251622162612426262
- BouletLPLavioletteMTurcotteHBronchial subepithelial fibrosis correlates with airway responsiveness to methacholineChest199711245529228356
- ChoSHSeoJYChoiDCPathological changes according to the severity of asthmaClin Exp Allergy199626121012198911709
- ChuHWHallidayJLMartinRJLeungDYSzeflerSJWenzelSECollagen deposition in large airways may not differentiate severe asthma from milder forms of the diseaseAm J Respir Crit Care Med19981586193619449847289
- RocheWRBeasleyRWilliamsJHHolgateSTSubepithelial fibrosis in the bronchi of asthmaticsLancet1989186375205242466184
- JefferyPKGodfreyRWAdelrothENelsonFRogersAJohanssonSAEffects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic studyAm Rev Respir Dis19921454 Pt 18908991554218
- WenzelSESchwartzLBLangmackELEvidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristicsAm J Respir Crit Care Med199916031001100810471631
- HoshinoMNakamuraYSimJShimojoJIsogaiSBronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammationJ Allergy Clin Immunol199810257837889819295
- LaitinenLALaitinenAAltrajaABronchial biopsy findings in intermittent or “early” asthmaJ Allergy Clin Immunol1996985 Pt 2S3S68939170
- ChakirJLavioletteMBoutetMLaliberteRDubeJBouletLPLower airways remodeling in nonasthmatic subjects with allergic rhinitisLab Invest19967557357448941218
- BrewsterCEHowarthPHDjukanovicRWilsonJHolgateSTRocheWRMyofibroblasts and subepithelial fibrosis in bronchial asthmaAm J Respir Cell Mol Biol1990355075112223105
- ChanezPVignolaAMVicPComparison between nasal and bronchial inflammation in asthmatic and control subjectsAm J Respir Crit Care Med199915925885959927377
- BraunstahlGJFokkensWJOverbeekSEKleinjanAHoogstedenHCPrinsJBMucosal and systemic inflammatory changes in allergic rhinitis and asthma: a comparison between upper and lower airwaysClin Exp Allergy200333557958712752585
- WardCJohnsDPBishRReduced airway distensibility, fixed airflow limitation, and airway wall remodeling in asthmaAm J Respir Crit Care Med200116491718172111719315
- BrightlingCESymonFABirringSSBraddingPWardlawAJPavordIDComparison of airway immunopathology of eosinophilic bronchitis and asthmaThorax200358652853212775868
- BarbatoATuratoGBaraldoSEpithelial damage and angiogenesis in the airways of children with asthmaAm J Respir Crit Care Med2006174997598116917118
- ChettaAZaniniAForesiAVascular component of airway remodeling in asthma is reduced by high dose of fluticasoneAm J Respir Crit Care Med2003167575175712468439
- SiddiquiSMistryVDoeCAirway hyperresponsiveness is dissociated from airway wall structural remodelingJ Allergy Clin Immunol2008122233534118572228
- BaiTRCooperJKoelmeyerTParePDWeirTDThe effect of age and duration of disease on airway structure in fatal asthmaAm J Respir Crit Care Med20001622 Pt 166366910934103
- BouletLPTurcotteHLavioletteMAirway hyperresponsiveness, inflammation, and subepithelial collagen deposition in recently diagnosed versus long-standing mild asthma. Influence of inhaled corticosteroidsAm J Respir Crit Care Med20001624 Pt 11308131311029336
- Tillie-LeblondIdeBJJaubertFWallaertBScheinmannPGossetPAirway remodeling is correlated with obstruction in children with severe asthmaAllergy200863553354118394127
- SullivanPStephensDAnsariTCostelloJJefferyPVariation in the measurements of basement membrane thickness and inflammatory cell number in bronchial biopsiesEur Respir J19981248118159817150
- KranenburgARWillems-WidyastutiAMooriWJEnhanced bronchial expression of extracellular matrix proteins in chronic obstructive pulmonary diseaseAm J Clin Pathol2006126572573517111536
- WardCPaisMBishRAirway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthmaThorax200257430931611923548