Abstract
An excess amount of glucocorticoids represents the primary and most frequent etiological factor influencing secondary osteoporosis. Patients receiving glucocorticoids, but also those with the endogenous form of hypercorticism, are at high risk for the loss of bone density, with the subsequent occurrence of pathological fractures. In this review, we summarize the currently available methods of prevention and the treatment of glucocorticoid-induced osteoporosis. We also include a proposal for both a prophylactic and therapeutic approach that takes into account the risk factors typical for long-term users of glucocorticoids.
Introduction
Glucocorticoid-induced osteoporosis (GIOP) is the most frequent and severe form of secondary osteoporosis.Citation1 The prevalence of oral glucocorticoid use is 0.9% of the total adult population rising to 2.5% at age over 70 years.Citation2 The most frequent indications for oral glucocorticoid therapy are respiratory, musculosceletal and cutaneous diseases.
Figure 1 Effects of glucocorticoids on bone. Derived from.Citation20,Citation85
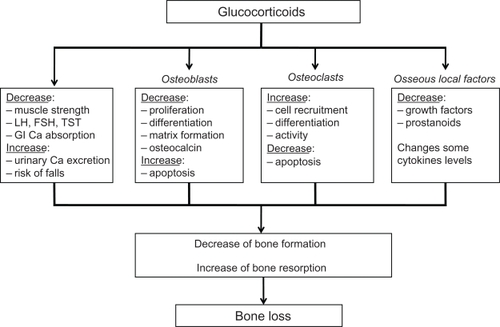
Glucocorticoid therapy results in a rapid loss of bone mineral density (BMD) within the first weeks of treatment. The rate of loss is greatest in the first year of therapy and incidence of osteoporotic fractures may be as high as 20% in endogenous hypercorticism and 30% to 50% in patients with long-term glucocorticoid oral use. First osteoporotic fractures occur as early as the first 3 to 6 months of hypercorticism.
Histomorphometric analyses of biopsies from glucocorticoid-treated individuals have demonstrated a reduction in bone formation at the cellular and tissue level, resulting in reduced bone volume and trabecular thickness. Higher doses and long-term use of glucocorticoids, however, also may be associated with an increase in bone resorption, leading to greater bone loss and disruption of cancellous bone architecture.Citation3–Citation5
According to several studies, it is estimated that a cumulative dose is 30 g of oral prednisolone per year or 5 mg (and more) of oral prednisolone per day for more than 3 months.Citation6,Citation7
GIOP is most common in children, postmenopausal women, young men and patients with long-term immobilization.Citation1
The association between osteoporosis and glucocorticoid therapy was made shortly after the first use of these drugs in humans in the 1950s, particularly in patients treated for asthma.Citation8 The first population-based study of limb fractures was by Hooyman et al,Citation9 who reported that the relative risk of hip, distal forearm and proximal humoral fracture was doubled in a group of patients with rheumatoid arthritis treated with glucocorticoids, compared with patients with rheumatoid arthritis alone. A subsequent British case-control study confirmed that use of glucocorticoids approximately doubles hip fracture risk.Citation10
Long-term glucocorticoid use is one of the most important osteoporosis risk factors. It is also an indication for densitometry.
A fracture risk assessment tool (FRAX) has been developed by World Health Organization to evaluate fracture risk of patients. It is based on individual patient models that integrate the risks associated with clinical risk factors as well as BMD at the femoral neck. The FRAX models have been developed from studying population-based cohorts from Europe, North America, Asia and Australia. The FRAX algorithms give the 10-year probability of fracture. Glucocorticoid use is considered as severe risk factor.
Mechanisms of bone loss in GIOP
Glucocorticoids may exert they actions on the skeleton and related tissues in many ways. Their overall effects depend on a number of factors including the dose, the duration, the steroid type, and the species tested. There are direct effects on bone that result in diminished bone formation and unchanged or enhanced bone resorption. The most important effect of glucocorticoids is suppression of bone formation, which probably involves several mechanisms. First, glucocorticoids effect the differentiation and activity of many cell types, including those of osteoblast lineage and other cells within bone. Second, glucocorticoids modulate the transcription of many of the genes responsible for the synthesis of matrix constituents by osteoblasts, such as type 1 collagen and osteocalcin. Third, glucocorticoids influence the synthesis and activity of many local acting factors that affect osteoblasts, including cytokines (eg, interleukin 1 and 6), and growth factors, especially the insulin-like growth factors (IGF-I and IGF-II) and several of the IGF-binding proteins (IGFBP-3, -4 and -5). The latter effects may contribute in particular to the stunting of growth and retarded skeletal development in children treated with glucocorticoids. Recently, the role of apoptosis has gained prominence.Citation13 Glucocorticoids shorten the lifespan of osteoblasts and osteocytes, the latter also being involved in the pathogenesis in glucocorticoid-induced osteonecrosis;Citation14 in addition, glucocorticoids may promote osteoclast survival.Citation15 Interestingly, bisphosphonates can reverse the pro-apoptotic effects of glucocorticoids on osteoblasts and osteocytes, which may contribute to their efficacy in preventing glucocorticoid-induced bone loss.Citation16 In addition to these direct effects on bone cells, other mechanisms may also contribute to bone loss. Thus reduced intestinal calcium absorption and increased renal calcium excretion have been reported after the administration of glucocorticoids;Citation17 whether these changes are associated with secondary hyperparathyroidism is controversial, most studies showing no increase in serum parathyroid hormone levels in glucocorticoid-treated individuals. Low serum testosterone levels have been reported in glucocorticoid-treated men and are believed to be due both to direct effects on the testis and indirect effects on testosterone production mediated via suppression of gonadotropin hormone secretion. Hypogonadism leads also to muscle decrease and increased risk of falls.Citation18,Citation19
Table 1 Risk factors – indications for densitometry
The diagnosis consists of the patient’s history, clinical examination, densitometry and biochemical analysis (bone turnover – typically there is decreased osteocalcin).Citation1 In the context of detecting asymptomatic vertebral fractures, radiographym, eg, vertebral fracture assessment is also very important.
Table 2 FRAX™ Model – clinical risk factors (CRF)
Prevention and treatment of GIOP
Although there are very few data on the effects of lifestyle interventions or modifications in GIOP, it seems reasonable to recommend certain measures that may reduce bone loss and fracture risk. These include reducing the dose of glucocorticoids to a minimum, the use of alternative routes of administration (eg, inhaled or topical) or formulations (eg, budesonide), and prescription of alternative immunosuppressive agents. Adequate levels of dietary calcium intake should be encouraged and good nutrition and normal body weight should be maintained where possible. In addition, individuals taking glucocorticoids should be advised not to smoke and to avoid alcohol abuse. Within the limits imposed by the underlying disease, physical exercise should be encouraged. Falls risk assessment and advice should be performed in those at increased risk of falling.Citation21,Citation22
Many studies report of inadequate prevention and also treatment of GIOP. According to the GPRD study in the United Kingdom,Citation22 prophylaxis for GIOP was prescribed only to 14% of patients, but in the San Francisco General Hospital it was prescribed to only 8% of 215 patients.Citation23
An adequate intake of calcium and vitamin D is essential in all preventive and also therapeutic recommendations.Citation24 The recommended daily intake of calcium for an adult is 1200 to 1500 mg per day.Citation25 In glucocorticoid-treated patients, combined intake of calcium and vitamin D is more effective. The recommended dose of vitamin D is 800 IU per day. The administration of vitamin D increase calcium absorption and reduce bone resorption. Calcitriol also has a direct effect on osteoblasts, opposing the effects of glucocorticoids on osteocalcin-gene expression, and calcitriol may reverse glucocorticoid-indused suppression of serum osteocalcin concentrations.Citation18,Citation25
Bisphosphonates are currently the major class of drugs used for GIOP, in addition to their primary role in the treatment of postmenopausal osteoporosis. They are synthetic analogues of pyrophosphates that are resistant to the action of endogenous pyrophosphates. They have a high affinity for the bone mineral of the skeletal surface and exert an inhibitory effect on several catalytic enzymes, of which the most important is farnesyl diphosphate synthetase, thus interfering with the mevalonate pathway of cholesterol formation. An insufficient prenylation results with a consequent dysfunction leading to apoptosis of osteoclasts. Osteoabsorption slows and, depending on the type of bisphosphonate, leads to disturbed remodeling, and prolongation of secondary mineralization. The following are bisphosponates used in the treatment of osteoporosis: alendronate, ibandronate, risedronate, and zoledronate, with pamidronate, clodronate and etidronate used in some countries.Citation26 First effect can be seen in 6 months, which means an increase in bone density.Citation27 In most countries risedronate and alendronate are used for treatment of GIOP. Also intravenous ibandronate significantly reduced vertebral fracture risk in patients with GIOP in small study.Citation28 Reid recently reported the safety and efficacy of intravenous zoledronic acid compared with oral risedronate in the prevention and treatment of GIOP.Citation29,Citation30 A single 5-mg infusion of zoledronate and daily oral risedronate, 5 mg, were compared in a 1-year randomized, double-blind, double-dummy study of patients with less than 3 months’ exposure of glucocorticoids and those in treatment for longer than 3 months. After 12 months, lumbar spine BMD increased significantly more with zoledronate than risedronate in the two subpopulations. Zolendronate was also more effective than risedronate in terms of increasing BMD of femoral neck, trochanter and total hip. This superior effect was apparent at 6 months. Currently, bisphosphonates are considered to be the gold standard for the prevention and treatment in GIOP. But, unfortunately, no study has focused on the effect of bisphosphonates on reduction of vertebral fractures like in postmenopausal osteoporosis.Citation31 Importantly, long-term treatment of GIOP with bisphosphonates is very safe.
The effectiveness of bisphosphonate treatment depends on patients adhering to the therapeutic regimen. According to the VIVA study the vast majority of patients prefer a once-monthly regimen – the three most popular reasons are the need to take fewer pills, convenience and simplicity of treatment.Citation32 Poor compliance to osteoporosis treatment is related to several different factors, including the asymptomatic nature of the disease (if fractures are not present) and the gastrointestinal complications of bisphosphonates.Citation32 Therapeutic regimens with longer periods between applications are now available (ibandronate and risedronate once monthly by mouth, ibandronate 4 times yearly intravenous or intravenous zolendronate once a year) and seem to be an interesting way of increasing adherence.Citation33
A very rare complication following administration of bisphosphonates is osteonecrosis of the jaw. It occurs most commonly in high doses parenterally, in the setting of poor dental hygiene with or without dental procedures. The risk with oral treatment is low. It presents with infection and necrosis of bone in the mandible or maxilla. It is important to check for good dental hygiene before administration of bisphosphonates, as treatment is difficult. Conservative management with limited debridement, antibiotics and mouth rinses assist healing.Citation34 The vast majority of these cases with osteonecrosis of the jaw are seen in the oncology population receiving high doses of monthly intravenous zoledronic acid or pamidronate and chemotherapy.Citation35
There are plausible scientific reasons to be vigilant about the potential for long-term negative effects of bisphosphonates on bone strenght, bone turnover and bone quality. In the past 4 years, several cases of unusual femoral fragility fractures – subtrochanteric and diaphyseal fractures – among patients using oral bisphosphonates (usually alendronate) have been reported. In general, patients experiencing such fractures have had bone turnover markers in the low normal range and bone biopsies showed markedly reduced bone turnover.Citation36
The most common safety issues revolve around the effects of bisphosphonates on upper gastrointestinal mucosa. There is no doubt that the amino bisphosphonates may induce esophagitis and that this problem can develop any time during the use of the bisphosphonate, not just at the initiation. This effect can be mitigated by carefully instructing the patient on the proper dosing of the oral bisphosphonate.Citation35 There have been also reports of esophageal cancer in patients who had been taking oral bisphosphonates. Twenty-three cases including 8 fatalities have been reported in the United States, all of them associated with alendronate. In Europe and Japan, 31 cases of esophageal cancer have been reported, with alendronate as the suspected drug in 21 cases. Risedronate, ibandronate and etidronate were the suspected drugs in 6 and a concomitant drug in another 4.Citation37 If patients take the oral bisphosphonate according to the dosing instructions, the incidence of these side effects is small.Citation38 Patients with long-term glucocorticoid use are at high risk of developing various side effects, so the intravenous form may be more appropriate.
We have only partial evidence in terms of other therapeutic modalities – calcitonin, estrogens, testosterone (in men), raloxifen and stroncium ranelate. According to various studies, there is no evidence for a reduction in vertebral fracture rates, so these drugs cannot to be commended.Citation38,Citation39 Some authors suggest the administation of hydrochlorothiazide to reduce hypercalciuria in GIOP.Citation40
Studies of the effect of intranasal or subcutaneous calcitonin on glucocorticoid-induced bone loss have produced conflicting results. Five studies performed in patients undergoing organ transplantation failed to show a significant treatment benefit on BMD.Citation41–Citation45 In the study of Valero et al, in which there was no control group, patients treated with calcitonin or cyclic etidronate showed a significant increase in lumbar spine BMD after 1 year of treatment (mean 6.4% and 8.2%, respectively).Citation46 In several studies performed in other patient groups, a significant treatment benefit has been demonstrated in lumbar spine BMD compared to controls.Citation47,Citation48 However, no effects on spine BMD was demonstrated in three studies,Citation49–Citation52 although in one studyCitation40 a significant gain in proximal femur BMD were demonstrated. In two of the studies in which a significant effects on spine BMD were demonstrated, bone loss was however not completely prevented by calcitonin.Citation38,Citation49
Patients receiving prolonged glucocorticoid therapy may develop hypogonadism due to inhibition of secretion of luteinizing hormone and follicle-stimulating hormone from the pituitary gland, as well as direct effects on hormone production by the ovary and testes. All patients receiving long-term glucocorticoid treatment should be assessed for hypogonadism, and when present, this should be corrected if possible. In a trial of postmenopausal women with rheumatoid arthritis who were taking prednisone and were randomized to receive either hormonal replacement therapy (HRT) or placebo, those who received HRT had a significant (3%–4%) increase in their lumbar spine BMD compared with controls, while there was no significant change in femoral neck BMD in either group.Citation51 In a randomized controlled trial of injectable parathyroid hormone, postmenopausal women receiving long-term low-dose glucocorticoid therapy and HRT in the control group showed no change in BMD at the lumbar spine, hip, or distal radius over the course of 1 year.Citation53
These data suggest that HRT is adequate therapy to prevent bone loss in postmenopausal women receiving prolonged low-to-moderate-dose glucocorticoid therapy. Currently, however, there are no published reports on the efficacy of HRT in preventing bone loss at the initiation of glucocorticoid treatment, or the degree of the protective effect of HRT when moderate-to-high doses of glucocorticoids are used for long-term treatment.
Observational studies in premenopausal female athletes with menstrual irregularities suggest that women who receive oral contraceptives have higher adjusted bone mineral content and BMD than do women who do not take oral contraceptives.Citation54,Citation55 Therefore, premenopausal women who experience menstrual irregularities (oligo- or amenorrhea) while taking glucocorticoids should be offered oral contraceptives or cyclic estrogen and progesterone if contraindications are not present. At this time, no data are available on the efficacy of selective estrogen receptor modulators (SERMs) in the prevention or treatment of glucocorticoid-induced bone loss. The SERM raloxifene is available for the prevention and treatment of postmenopausal osteoporosis.Citation56,Citation57 A SERM could theoretically be used to prevent glucocorticoid-induced bone loss in selected postmenopausal glucocorticoid-treated women who either have contraindications to or do not wish to take HRT or other antiresorptive medications.
There is less information available about men with hypogonadism secondary to glucocorticoid treatment. A randomized crossover trial demonstrated the effectiveness of testosterone replacement therapy in 15 men with glucocorticoid-treated asthma.Citation58 All of the men had low serum testosterone levels prior to therapy. Lumbar spine BMD, but not hip BMD, was significantly increased (nearly 4%) after 12 months of monthly intramuscular testosterone injections; in addition, there was an increase in lean body mass and a reduction in fat mass. Thus, men with low serum levels of testosterone who are receiving glucocorticoids should receive replacement therapy. Based on recommendations published by the American Association of Clinical Endocrinologists and the American College of Endocrinology, men with serum testosterone levels below the physiologic range (<300 ng/mL) should receive replacement therapy.Citation59 Multiple different testosterone preparations are available, including short- and long-acting intramuscular injections and transdermal patches and gels. The goal of testosterone replacement therapy is to provide physiologic-range serum testosterone levels. It is important to emphasize that if testosterone replacement therapy is to be used in a hypogonadal man, the patient should be adequately assessed for the possibility of prostate cancer, with a digital rectal examination and measurement of prostate-specific antigen at baseline and annually thereafter. Prostate cancer is an absolute contraindication to testosterone replacement therapy.
Teriparatide is a recombinant aminoterminal fragment (1–34) of the human parathyroid hormone, which has a predominantly stimulating effect on bone formation. Human parathyroid hormone is a single chain of polypeptide with 84 amino acids and a molecular weight of 9425 Da and the N-terminal region (1–34) is biologically active and sufficient for regulation of mineral ion homeostasis.Citation60 Teriparatide affects the metabolism of calcium and phosphates in several ways – stimulation of the release of calcium and phosphate from the bone, stimulation of the reabsorption of calcium from the glomerular filtrate and loss of phosphate to urine, and stimulation of the renal synthesis of 1, 25-(OH)2-vitamin D3 – and therefore the absorption of calcium and phosphate from the gastrointestinal tract. Patients with large deficits in BMD are at high risk for fracture and might preferentially benefit from such anabolic therapy.Citation61–Citation63 The effectiveness of the treatment with teriparatide consists of fracture reduction in females with severe osteoporosis and patient with GIOP.
Daily dose of 20 μg of teriparatide stimulates the production of growth factors IGF-1 and TGF-β in osteoblasts without reduction of osteoprotegerin.
Neer and colleagues documented in a group of postmenopausal females with prevalent vertebral fracture a decrease in risk of vertebral and non-vertebral fractures in an 18-month prospective study.Citation64 The treatment was associated with significant improvement in quality of life.
Saag have reported the results of the first 18 months of a 36-month prospective trial and later also results of all 36 months.Citation65,Citation66 In this randomized, double-blind clinical trial, the primary outcome was the change from baseline to 36 months in BMD at the lumbar spine associated with the administration of daily teriparatide (at a dose of 20 μg), compared with that of daily alendronate (at a dose of 10 mg), in patients with established GIOP. Prespecified secondary outcomes included changes in BMD at the total hip and markers of bone turnover, the time to changes in BMD at the lumbar spine and total hip, the incidence of vertebral and non-vertebral fractures, and adverse events. Ambulatory patients were eligible for enrollment if they met the following criteria: an age of 21 years or more, a history of sustained glucocorticoid therapy, and a T-score for BMD density at the lumbar spine or total hip of either −2.0 or less or −1.0 or less in addition to at least one fragility fracture during treatment with glucocorticoids. Sustained glucocorticoid therapy was defined as a mean daily dose of 5 mg or more of prednisone or its equivalent for 3 or more consecutive months immediately preceding the screening visit. Patients were randomly assigned to receive either injectable teriparatide (Forteo®; Eli Lilly) at a daily dose of 20 μg plus an oral placebo or oral alendronate (Fosamax®; Merck) at a daily dose of 10 mg plus an injectable placebo. They also received supplementation with calcium carbonate (at a dose of 1000 mg of elemental calcium) and vitamin D (at a dose of 800 IU). According to the results of the first 18 months, the anabolic agent teriparatide appeared to show significant skeletal benefits in patients with GIOP, compared with the bisphosphonate alendronate.Citation67 In a recent trial comparing a bisphosphonate with teriparatide in postmenopausal women with osteoporosis, teriparatide therapy was associated with increased areal and volumetric BMD and estimates of bone strength at the lumbar spine, compared with alendronate.Citation67,Citation68 Although the time course of changes in markers of bone turnover in this trial resembled that observed in postmenopausal women, the magnitude of gains in BMD in the teriparatide group was less than that seen previously.Citation68 This differential response may reflect the characteristic ability of glucocorticoids to inhibit osteoblast and osteocyte function profoundly by several mechanisms, including the stimulation of apoptosis.Citation69 In this study, patients in the teriparatide group had fewer new vertebral fractures than did patients in the alendronate group, although the overall number of fractures was small. Bisphosphonates have been associated with a reduced incidence of vertebral fractures in this patient population in randomized trials of alendronate,Citation31,Citation68 in pooled studies of risedronate,Citation70 and in a non-randomized, open-label study of ibandronate.Citation38 Although there were more non-vertebral fractures in the teriparatide group than in the alendronate group in this study, the difference was not significant. In previous studies of teriparatide, there was a reduction in non-vertebral fractures in postmenopausal women with osteoporosis.Citation29,Citation30 The standard of care for patients at risk for glucocorticoid-associated bone loss and osteoporosis includes a choice of antiresorptive agents. However, for patients with established osteoporosis who are at high risk for fracture, more aggressive and expensive therapy may be warranted. Patients in this trial had lower BMD and more prevalent fractures than those in previous trials involving patients with GIOP, which suggests an even greater need for an efficacious intervention.Citation31,Citation69,Citation70–Citation74 The occurrence of sporadic hypercalcemia was more frequent in the teriparatide group than in the alendronate group.Citation75
In a 36-month study BMD at the lumbar spine and femoral neck increased significantly more in the teriparatide compared with alendronate group. Fewer patients had new radiographic vertebral fractures in the teriparatide than the alendronate group. The number of patients with new non-vertebral fractures was not significantly different between groups versus alendronate. There was no significant difference between groups in the number of patients with ≥1 adverse event (91% teriparatide versus 86% alendronate).Citation65,Citation76 It should be mentioned that the therapy with teriparatide at various doses causes osteosarcoma and abnormalities in bone tissue in the rat model.Citation73,Citation74,Citation77–Citation79 On the other hand, in the study of Neer et al and other major clinical trials with teriparatide no osteosarcomas were found.Citation73
On the basis of the known pathophysiology of GIOP, teriparatide might be considered as a therapeutic strategy for patients at high risk for fracture.Citation65,Citation66
According to the literature, there are no recommendations for prevention and treatment of GIOP in children or premenopausal women. This treatment is always individual.Citation63 General principles of management include preferring other than oral glucocorticoids, minimizing the oral dose when possible and attention to nutrition, exercise and calcium and vitamin D status.Citation80
Many factors influence a patient’s decision to begin GIOP therapies and to adhere them over time, such as the results and quality of evidence-based medicine studies, the clinician’s know-how, and the patient’s values, preferences and compliance. According the most national and international studies effective therapies of GIOP such as the use of sufficient calcium, vitamin D and early prescription for bisphoshonates can lower the risk of fragility fractures. Despite accumulating evidence, GIOP therapies are underutilized.Citation81
There are no multicenter, prospective, randomized, double-blinded clinical studies with enough patients, unlike postmenopausal osteoporosis, which could define effective treatment modalities to reduce vertebral and non-vertebral fractures. In clinical practice we have only trials with surrogate markers (like BMD, bone turnover markers) that can be correlated with fracture-risk reduction.
Monitoring of therapy
The role of monitoring the effects of bone-sparing agents in GIOP, using either BMD or biochemical markers of bone turnover, has not been established. Depending on the rate of bone loss prior to treatment, significant treatment responses in individuals may be detectable within 1 to 2 years using dual energy X-ray absorptiometric measurements of bone density. However, in individuals taking high doses of glucocorticoids, large changes in BMD may be detectable earlier and measurement at 6 months may be appropriate. The spine is the preferred site for monitoring because of the low precision error of bone density measurements at this site. Bone loss from the spine during the first year of glucocorticoid therapy may vary between 3% and 10%; since the precision error of measurements is approximately 1%, a loss of more than 3% (the least significant change) is likely to be significant. Rates of bone loss are less during established glucocorticoid therapy and in this situation the target is to increase BMD above the least significant change, ie, an increase of more than 3%. Bone resorption markers, such as N-telopeptide or C-telopeptide of type I collagen, show similar changes with treatment in individuals taking glucocorticoids to those in women with postmenopausal osteoporosis. However, bone resorption markers may also be affected by changes in inflammatory activity and hence a decrease following initiation of glucocorticoid therapy may reflect suppression of disease activity rather than reduced bone resorption.Citation22
There are many recommendations for the prevention and treatment of GIOP. Here we describe the most important.
Recommendations for the prevention and treatment of GIOP (American College of Rheumatology 2001)Citation82
The Committee recommends obtaining a baseline measurement of BMD at the lumbar spine and/or hip when initiating long-term (ie, >6 months) glucocorticoid therapy. Longitudinal measurements may be repeated as often as every 6 months for monitoring glucocorticoid-treated patients to detect bone loss. In patients who are receiving therapy to prevent bone loss, annual follow-up measurements are probably sufficient. These recommendations divide patients into two groups in terms of glucocorticoid use.
A – patient beginning therapy with glucocorticoid (prednisone equivalent of 5 mg/day) with plans for treatment duration of 3 months:
– Modify lifestyle risk factors for osteoporosis (smoking cessation or avoidance, reduction of alcohol consumption if excessive, instruct in weight-bearing physical exercise)
– Initiate calcium supplementation
– Initiate supplementation with vitamin D (plain or activated form)
– Prescribe bisphosphonate (use with caution in premenopausal women)
B – patient receiving long-term glucocorticoid therapy (prednisone equivalent of 5 mg/day)
– Modify lifestyle risk factors for osteoporosis (smoking cessation or avoidance, reduction of alcohol consumption if excessive, instruct in weight-bearing physical exercise)
– Initiate calcium supplementation
– Initiate supplementation with vitamin D (plain or activated form)
– Prescribe treatment to replace gonadal sex hormones if deficient or otherwise clinically indicated
– Measure BMD at lumbar spine and/or hip
if BMD is not normal (T-score below −1.0 SD)
– Prescribe bisphosphonate (use with caution in premenopausal women)
– Consider calcitonin as second-line agent if patient has contraindication to or does not tolerate bisphosphonate therapy
if BMD is normal
– Follow up and repeat BMD measurement either annually or biannually.
The American Society for Bone and Mineral Research 2004Citation83
In patients receiving prednisone more than 3 months 5 mg/day and with risk factors of osteoporosis (premenopausal women, men over 50 years, low BMI, prior osteoporotic fracture, high doses of glucocorticoids, diseases that leads to secondary osteoporosis, low calcium intake, immobilization, family history of osteoporosis) – measure BMD:
A – T-score below −2.5 SD
– start treatment with oral or intravenous bishosphonates
– calcium, vitamin D
B–T-score −1.5 to −2.5 SD
– consider treatment with oral or intravenous bisphosphonates
– calcium, vitamin D
C–T-score more than −1.5 SD
– calcium, vitamin D
– measure BMD after 12 months of glucocorticoid therapy.
The Dutch Society for Rheumatology 2004Citation84
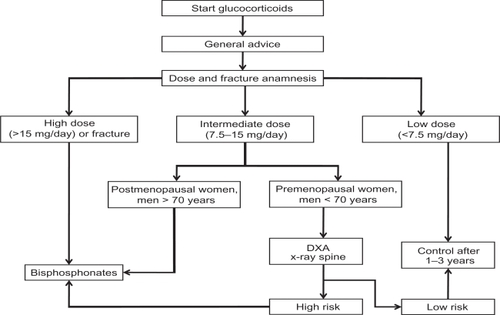
The stream diagram () shows the diagnostic and therapeutic steps in making decisions for the prevention of GIOP. Factors that influence this decision include the dose of glucocorticoids and the presence of other risk factors such as age, sex, previous fracture, and BMD. The main message is that treatment with bisphosphonates should be started immediately in patients at high risk (high dose of glucocorticoids, prevalent fracture, postmenopausal women, and elderly men).
Figure 2 Diagnostic and therapeutic steps in making decisions for the prevention of glucocorticoid-induced osteoporosis. Reproduced from Geusens PP, de Nijs RNJ, Lems WF, et al. Prevention of glucocorticoid osteoporosis: a consensus document of the Dutch Society for Rheumatology. Ann Rheum Dis. 2004;63:324–325. Copyright © 2004, with permission from BMJ Publishing Group Ltd.
In the Slovak Republic we have currently accepted recommendations for patients with long-term glucocorticoid use. These recommendations reflect information about the efficiency of teriparatide.
Recommendations for prevention of GIOP in the Slovak republicCitation63
Patients receiving long-term glucocorticoid therapy (>3 months >7.5 mg prednisone/day or cumulative dose 27 g/year) – without risk factors:
Peventive actions – reducing the dose of glucocorticoids to a minimum, exclusion of all risk factors, instruct in weight-bearing physical exercise, BMI > 19, calcium supplementation 1000 to 1500 mg per day, vitamin D supplementation 800 IU per day.
Measure BMD at lumbar spine and hip:
– T-score > −2.0 SD measure BMD after 12 months of glucocorticoid therapy
– T-score ≤ −2.0 SD treatment with bisphosphonates
– T-score ≤ −2.9 SD treatment with teriparatide – osteoporotic fracture–treatment with teriparatide
Patients receiving long-term glucocorticoid therapy (>3 months >7.5 mg prednisone/day or cumulative dose 27 g/year) – with risk factors
Peventive actions – reducing the dose of glucocorticoids to a minimum, exclusion all risk factors, instruct in weight-bearing physical exercise, BMI > 19, calcium supplementation 1000 to 1500 mg per day, vitamin D supplementation 800 IU per day.
Measure BMD at lumbar spine and hip:
– T-score > −1.5 SD measure BMD after 12 months of glucocorticoid therapy
– T-score ≤ −1.5 SD treatment with bisphosphonates
– T-score ≤ −2.9 SD treatment with teriparatide – osteoporotic fracture – treatment with teriparatide.
Conclusion
Glucocorticoids are widely used to treat a number of medical disorders. The administration of oral glucocorticoids is associated with a significant increase in fracture risk at the hip and spine. Measurement of BMD using dual energy x-ray absorptiometry is currently recommended for assessment of fracture risk in individuals treated with glucocorticoids. In general, the pharmacological agents that have undergone assessment for the prevention and treatment of GIOP are similar to those used for postmenopausal osteoporosis. But, according to character of GIOP, the indications for treatment have to be earlier in comparison with postmenopausal osteoporosis.
Disclosures
The authors declare no conflicts of interest.
References
- PayerJKillingerZBone changes in hypercorticismRheumatol199913181183
- Van StaaTPLeufkensHGCooperCThe epidemiology of corticosteroid-induced osteoporosis: a meta-analysisOsteoporos Int20021377778712378366
- Dale CarbonareLArlotMEChavassieuxPMComparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosisJ Bone Miner Res2001169710311149495
- Van StaaTPLeufkensHGMAbenhaimLOral corticosteroids and fracture risk: relationship to daily and cumulative dosesRheumatol20003913831389
- Van StaaTLeufkensHGMAbenhaimLUse of oral corticosteroids and risk of fracturesJ Bone Miner Res200015993100010841167
- De GregórioLHLacativaPGMelazziACGlucocorticoid-induced osteoporosisArq Bras Endocrinol Metabol20065079380117117304
- Van StaaTPThe pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosisCalcif Tissue Int20067912913716969593
- EastellRReidDMCompstonJECooperCA UK consensus gruop on management of glucocorticoid-induced osteoporosis: an updateJ Intern Med19982442712929797491
- HooymanJRMeltonLJNelsonAMO’FallonWMRiggsBLFractures after rheumatoid arthritis – a population based studyArthritis Rheum198427135313616508860
- CooperCCouplandCMitchellMRheumatoid arthritis, corticosteroid therapy and the risk of hip fractureAnn Rheum Dis19955449527880122
- PayerJRovenskýJKillingerZLexicon of Osteoporosis SAP200730
- KanisJAJohnellOOdenAFRAX and the assessment of fracture probability in men and women from the UKOsteoporos Int20081938539718292978
- WeinsteinRSJilkaRLParfittAMManolagasSCInhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids – potential mechanisms of their deleterious effects on boneJ Clin Invest19981022742829664068
- WeinsteinRSNicholasRWManolagasSCApoptosis of ostecytes on glucocorticoid-induced osteonecrosis of the hipJ Clin Endocrinol Metab2000852907291210946902
- WeinsteinRSChenJRPowersCCStewartSAPromotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoidsJ Clin Invest20021091041104811956241
- PlotkinLIWeinsteinRSParfittAMRobersonPKPrevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitoninJ Clin Invest19991041363137410562298
- MorrisHANeedAGO’LoughlinPDHorowitzMBridgesANordinBECMalabsorption of calcium in corticosteroid-induced osteoporosisCalcif Tissue Int1990463053082110853
- SambrookPBirminghamJKellyPKemplerSPrevention of corticosteroid osteoporosis – a comparison of calcium, calcitriol and calcitoninN Engl J Med1993328174717527684512
- PearceGTabenskyDADelmasPDBakerHWSeemanECorticosteroid-induced bone loss in menJ Clin Endocrinol Metab1998838018069506731
- AlesciSDe MartinoMUIliasIGlucocorticoid-Induced Osteoporosis: From Basic Mechanisms to Clinical AspectsNeuroimmunomodulation20051211915756049
- HeffernanMPSaagKGRobinsonJKPrevention of osteoporosis associated with chronic glucocorticoid therapyJAMA2006295130016541489
- Guidelines Writing Group for the Royal College for Physicians of LondonOsteoporosis: Clinical Guidelines for Prevention and TeatmentLondonRoyal College for Physicians2002
- AagaardEMLinPModinGWPrevention of glucocorticoid-induced osteoporosis: provider practice at an urban county hospitalAm J Med199910745646010569300
- LeeYHLimYWLingPSInadequate dietary calcium intake in elderly patients with hip fracturesSingapore Med J2007481117112118043839
- LemsWFVan VeenGJGerritsMIEffect of low-dose prednisone (with calcium and calcitriol supplementation) on calcium and bone metabolism in healthy volunteersBr J Rheumatol19983727339487247
- RovenskyJPayerJBisphosphonates. Dictionary of RheumatologyNewYorkSpringerWien200924
- MokCCTongKHToCHRisedronate for prevention of bone mineral density loss in patients receiving high-dose glucocorticoids: a randomized double-blind placebo-controlled trialOst Int200819357364
- RingeJDDorstAFaberHIntermittent intravenous ibandronate injections reduce vertebral fracture risk in corticosteroid-induced osteoporosis: results from a long-term comparative studyOst Int200314801807
- ReidDMHughesRALaanRFEfficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trialJ Bone Miner Res2000151006101310841169
- ReidDMAdamiSDevogelaerJPChinesAARisedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapyCalcif Tissue Int20016924224711730260
- BodyJJGaichGAScheeleWHA randomized double-blind trial to compare the efficacy of teriparatide (recombinant human parathyroid hormone 1–34) with alendronate in postmenopausal women with osteoporosisJ Clin Endocrinol Metab2002874528453512364430
- PayerJČiernyDKillingerZŠulkováIBehuliakMCelecPPreferences of patients with postmenopausal osteoporosis treated with bisphosphonates – the VIVA II studyJIMR2009371225122919761708
- PayerJKillingerZŠulkováICelecPPreferences of patients receiving bisphosphonates – How to influence the therapeutic adherenceBiomed Pharmacother20086212212417888616
- RovenskyJPayerJOsteonecrosis of the Jaw. Dictionary of RheumatologyNewYorkSpringerWien2009158
- MillerPDBisphosphonates: Pharmacology and Use in the Treatment of Osteoporosis: Bisphosphonate Safety OsteoporosisElsevier200817331736
- AbrahamsenBEikenPEastellRSubtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a registered-based national cohort studyJ Bone Miner Res2009241095110219113931
- SinghAPOral bisphosphonates may cause esophageal cancerN Engl J Med200913608990
- AdachiJDBensenWGBellMJSalmon calcitonin nasal spray in the prevention of corticosteroid-induced osteoporosisBr J Rheumatol1997362552599133941
- CruseLMValerianoJVaseyFBPrevalence of evaluation and treatment of glucocorticoid-induced osteoporosis in menJ Clin Rheumatol20061222122517023807
- YamadaHLong-term effect of 1 alpha-hydroxyvitamin D, calcium and thiazide administration on glucocorticoid-induced osteoporosisNippon Naibunpi Gakkai Zasshi1989656036142792461
- GrotzWHRumpLCNiessenASchmidt-GaykHTreatment of osteopenia and osteoporosis after kidney transplantationTransplantation199866100410089808483
- Garcia-DelgadoIPrietoSGil-FraguasLRoblesECalcitonin, etidronate and calcidiol treatment in bone loss after cardiac transplantationCalcif Tissue Int1997601551599056163
- BiandaTLinkaAJungaGBrunnerHPrevention in osteoporosis in heart transplantation recipients: a comparison of calcitriol with calcitonin and pamidronateCalcif Tissue Int20006711612110920215
- VälimäkiMJKinnunenKTähteläRLoyttyniemiEA prospective study of bone loss and turnover after cardiac transplantation: effect of calcium supplementation with or without calcitoninOsteoporos Int19991012813610501793
- CremerJStruberMWagenbrethIMnischelskyJProgression of steroid-associated osteoporosis after heart transplantationAnn Thoracic Surg199967130133
- ValeroMALoinazCLarrodenaLLeonMCalcitonin and bisphosphonate treatment in bone loss after liver transplantationCalcif Tissue Int19955715197671159
- RizzatoGTosiGSchiraldiGMontemurroLSistiDSBone protection with salmon calcitonin (sCT) in the long-term steroid therapy of chronic sarcoidosisSarcoidosis19885991033227195
- LuengoMPonsFMartinez de OsabaMJPicadoCPrevention of further bone mass loss by nasal calcitonin in patients on longterm glucocorticoid therapy for asthma: a two year follow up studyThorax199449109911027831624
- CharlwoodCManningEMCRobinsonJFraserWDComparison of pamidronate, calcitonin and cyclical etidronate in the treatment of osteoporosis associated with steroid therapyJ Bone Miner Res199712Suppl 1S510
- HealeyJHPagetSAWilliams-RussoPSzatrowskiTPA randomized controlled trial of salmon calcitonin to prevent bone loss in corticosteroid-treated temporal arteritis and polymyalgia rheumaticaCalcif Tissue Int19965873808998681
- KotaniemiAPiiraainenHPaimelaLLeirisalo-RepoMIs continuous intranasal salmon calcitonin effective in treating axial bone loss in patients with active rheumatoid arthritis receiving low dose glucocorticoid therapy?J Rheumatol199623187518798923359
- HallGMDanielsMDoyleDVSpectorTDEffect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroidsArthritis Rheum199437149915057945476
- LaneNERoeBGenantHKArnaudCParathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trialJ Clin Invest1998102162716339788977
- DrinkwaterBLNilsonKOttSChesnutCHBone mineral density after resumption of menses in amenorrheic womenJAMA19862563803823723725
- DrinkwaterBLNilsonKChesnutCHBrennerWJShainholtzSSouthworthWBBone mineral content of amenorrheic and eumenorrheic athletesN Engl J Med19843112772816738640
- DelmasPDBjarnasonNHMitlakBHRavouxACHusterWJDraperMEffects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal womenN Engl J Med1997337164116779385122
- EttingerBBlackDMMitlakBHKnickerbockerRKNickelsenTGenantHKfor the Multiple Outcomes of Raloxifene Evaluation (MORE) InvestigatorsReduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trialJAMA199928263764510517716
- ReidIRWattieDJEvansMCStapletonJPTestosterone therapy in glucocorticoid-treated menArch Intern Med1996156117311778639011
- PetakSMBaskinHJBergmanDADickeyRANankinHA AACE clinical practice guidelines for the evaluation and treatment of hypogonadism in adult male patients. http://www.aace.com/clin/guides/hypogonadism.html. Accessed December 20, 2000.
- JinLBriggsSLChandrasekharSChirgadzeNYClawsonDCrystal structure of human parathyroid hormone 1–34 at 0,9-A resolutionJ Biol Chem2000275272382724410837469
- ČiernyDKillingerZPayerJPlace of teriparatide and intact parathormone in treatment of osteoporosisSlov Lek200717226230
- ŠtěpánJGlucocorticoid-induced osteoporosis: New options of prevention and treatmentInterní Med200810323326
- PayerJKillingerZBrázdilováKTreatment algorithm of glucocorticoid induced osteoporosisVnitř Lék200955506511
- NeerRMClaudeDAJoseRZEffect of Parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med200134414311441
- SaagKGZanchettaJRDevogelaerJPAdlerRASeeKDalskyGPTeriparatide versus Alendronate for Treatment of Glucocorticoid-Induced Osteoporosis: 36-month ResultsN Engl J Med2008Suppl 1S49
- OrwollESScheeleWHPaulSThe effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosisJ Bone Miner Res20031891712510800
- McClungMRSan MartinJMillerPDOpposite bone remodeling effects of teriparatide and alendronate in increasing bone massArch Intern Med20051651762176816087825
- KeavenyTMDonleyDWHoffmannPFMitlakBHGlassEVSan MartinJAEffects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosisJ Bone Miner Res20072214915717042738
- O’BrienCAJiaDPlotkinLIGlucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strenghtEndocrinology20041451835184114691012
- AdachiJDSaagKGDelmasPDTwo-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized double-blind, placebo-controlled extension trialArthritis Rheum20014420221111212161
- SambrookPNKotowiczMNashPPrevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium, and alendronate plus calciumJ Bone Miner Res20031891992412733733
- WallachSCohenSReidDMEffects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapyCalcif Tissue Int20006727728511000340
- NeerRMArnaudCDZanchettaJREffect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosisN Engl J Med20013441434144111346808
- SaagKGEmkeyRSchnitzerTJAlendronate for the prevention and treatment of glucocorticoid-induced osteoporosisN Engl J Med19983392922999682041
- CohenSLevyRMKellerMRisedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, paralel-group studyArthritis Rheum1999422309231810555025
- de NijsRNJacobsJWLemsWFAlendronate or alfacalcidol in glucocorticoid-induced osteoporosisN Engl J Med200635567568416914703
- SaagKGShaneEBoonenSTeriparatide or alendronate in glucocorticoid-induced osteoporosisN Engl J Med20073572028203918003959
- VahleJLSatoMLongGGSkeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for two years and relevance to human safetyToxicol Pathol20023031232112051548
- BetancourtMWirfelKLRaymondAKOsteosarcoma of bone in a patient with primary hyperparathyroidism: a case reportJ Bone Miner Res20031816316612510819
- AdlerRACurtisJWeinsteinRSSaagKGManagement of Glucocorticoid-Induced Osteoporosis in Children OsteoporosisElsevier20081156
- KennelKASwigloBAMontoriVMEvidence-based osteoporosi careMarcusRFeldmanDNelsonDARosenCJOsteoporosisThird Ed2Elsevier Academic Press200816291650
- Recommendations for the Prevention and Treatment of Glucocorticoid-Induced OsteoporosisAmerican College Of Rheumatology Ad Hoc Committee On Glucocorticoid-Induced OsteoporosisArthritis Rheum2001441496150311465699
- SaagKGPrevention of glucocorticoid-induced osteoporosisSouth Med J20049755555815255421
- GeusensPPde NijsRNJLemsWFPrevention of glucocorticoid osteoporosis: a consensus document of the Dutch Society for RheumatologyAnn Rheum Dis20046332432514962971
- CanalisEMazziottiGGuitinaAGlucocorticoid-induced osteoporosis: pathophysiology and therapyOsteoporosis Int20071813191328