Abstract
The incidence of diabetes is directly related to the incidence of obesity, which is at epidemic proportions in the US. Cardiovascular disease is a common complication of diabetes, which results in high morbidity and mortality. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear hormone receptors that regulate lipid and glucose metabolism. PPAR-α agonists such as fenofibrate and PPAR-γ agonists such as the thiozolidinediones have been used to treat dyslipidemia and insulin resistance in diabetes. Over the past few years research has discovered the role of PPARs in the regulation of inflammation, proliferation, and angiogenesis. Clinical trials looking at the effect of PPAR agonists on cardiovascular outcomes have produced controversial results. Studies looking at angiogenesis and proliferation in various animal models and cell lines have shown a wide variation in results. This may be due to the differential effects of PPARs on proliferation and angiogenesis in various tissues and pathologic states. This review discusses the role of PPARs in stimulating angiogenesis. It also reviews the settings in which stimulation of angiogenesis may be either beneficial or harmful.
Keywords:
Introduction
Obesity, diabetes and cardiovascular disease are at epidemic proportions in the US.Citation1,Citation2 A large amount of clinical and basic research has been done to elucidate the pathophysiology of these disease processes. Peroxisome proliferator-activated receptors (PPARs) are a group of nuclear hormone receptors that control the expression of networks of genes regulating lipid metabolism and insulin resistance.Citation3 They have been shown to affect inflammation, proliferation, immune function and angiogenesis.Citation3 There are three PPAR isotypes, PPAR-α, PPAR-β/δ, and PPAR-γ. They form heterodimers with the retinoid X receptors and bind to specific DNA sequences, called peroxisome proliferator response elements (PPRE), in the promoter regions of their target genes. PPARs exhibit isotype-specific tissue expression patterns. PPAR-α is primarily expressed in organs with significant fatty acid catabolism. PPAR-β/δ is expressed in nearly all cell types and the level of expression seems to depend on the amount of angiogenesis, cell proliferation, and differentiation occurring in that specific tissue.Citation4 PPAR-γ is found in adipose tissue and at lower levels in immune cells vascular tissue and some organs. PPAR-γ exists in two protein isoforms, PPAR-γ1 and PPAR-γ2, with different lengths of the N-terminal. The PPAR-γ2 isoform is predominantly expressed in adipose tissue, whereas PPAR-γ1 is relatively widely expressed.Citation5 Expression of each isoform is driven by a specific promoter that confers the distinct tissue expression patterns. There are also two other mRNA variants of PPAR-γ, proteins identical to PPAR-γ1: PPAR-γ3, which is restricted to macrophages, adipose tissue, and colon, and PPAR-γ4, the tissue distribution of which is unclear at this time.Citation5 Human PPAR-γ plays a critical physiological role as a central transcriptional regulator of both adipogenic and lipogenic programs. Its transcriptional activity is induced by the binding of endogenous and synthetic lipophilic ligands, which has led to the determination of many roles for PPAR-γ in pathological states such as type 2 diabetes, atherosclerosis, inflammation, and cancer. The role of PPARs has traditionally been recognized as antiproliferative and antiangiogenic in a large number of disease states including cancer and cardiovascular disease.Citation4 These studies have led to clinical trials with PPAR agonists to evaluate their benefits in cancer and cardiovascular disease. The results of some of these trials especially in cardiovascular disease have been mixed and hence controversial. The results obtained with a PPAR-γ agonist pioglitazone do suggest a better impact on the lipid profile compared to rosiglitazone (the former lowers triglyceride significantly and has less adverse effects on low-density lipoprotein [LDL] cholesterol), and at least a mixed result (the primary composite endpoint was not reduced significantly but myocardial infarction, stroke, and death were reduced by 16%), in an outcome trial – PROspective pioglitAzone Clinical Trial In macroVascular Events (PROACTIVE).Citation6 Rosiglitazone on the other hand was found to increase cardiovascular events in a large restrospective analysis study.Citation7 This has led to a lot of recent research into PPARs that is contrary to the traditional literature in their role as inhibitors of angiogenesis. This review will examine the role and evidence of PPARs as promoters of angiogenesis, the mechanisms involved, and the implications thereof.
Angiogenesis, type 2 diabetes, and cardiovascular disease
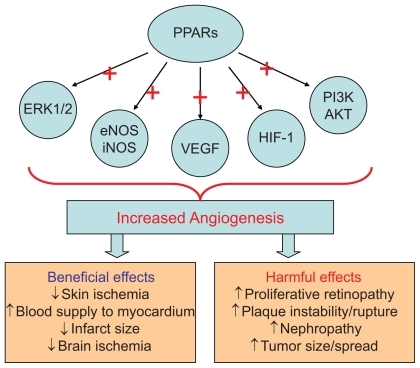
Angiogenesis is described as the formation of new capillaries from the existing vasculature. This process involves the breakdown of the extracellular matrix and formation of an endothelial tube. Angiogenesis is an important physiologic process in the female reproductive cycle, wound healing, and bone formation. Angiogenesis is also a crucial step in several disease states including cancer, diabetic retinopathy, rheumatoid arthritis, stroke, and ischemic coronary artery disease.Citation8–Citation10 Neoangiogenesis has harmful as well as beneficial effects in the setting of type 2 diabetes and cardiovascular disease.Citation10 In the setting of diabetes, there is abnormal regulation and signaling of vascular endothelial growth factor (VEGF) and its receptor Flk-1.Citation11 This may lead to increased levels of circulating VEGF, resulting in increased permeability of vascular structures throughout the body. In the retina, this results in the formation of protein-rich exudates containing VEGF that induces a local inflammatory response resulting in capillary sprouting. A similar process might take place in the arterial wall, thereby promoting capillary sprouting and plaque destabilization.Citation12 At the same time, the lack of Flk-1 activation in endothelial cells and abnormal VEGF-dependent activation of monocytes impair the arteriogenic response that requires monocyte recruitment, and monocyte and endothelial cell migration and proliferation.Citation11 This could lead to a deficient angiogenic response in ischemic tissue. VEGF/Flk-1 signaling may also be required for bone marrow release of circulating endothelial progenitor cells that play a role in endothelial function and arteriogenesis.Citation13 The abnormal release of endothelial progenitors could further reduce arteriogenic response. This has therapeutic implications in terms of vascularization and survival of skin grafts in patients with diabetes as well as vascularization of the ischemic myocardium. An important mechanism by which PPARs seem to regulate angiogenesis is via VEGF.Citation11,Citation12 It would therefore appear that PPARs have a role in regulating both beneficial and harmful effects of angiogenesis thereby leading to controversial results ().
The other factor influencing the results of angiogenesis studies is the use of PPAR agonists that have pleotropic effects. PPAR-α agonists such as fibrates stimulate pathways that do not depend on PPAR-α.Citation14 PPAR-γ agonists such as thiozolidinediones (TZDs) have PPARγ independent actions on proliferative and inflammatory pathways.Citation14 Therefore to conclude that the effects of commonly used PPAR agonists on angiogenesis are specifically due to PPAR activation is at best controversial.Citation15
PPAR-α and angiogenesis
Studies supporting antiproliferative properties of PPAR-α
PPAR-α agonists such as fibrates have long been used clinically to treat dyslipidemia. Several studies using fibrates have shown that they inhibit proliferation, angiogenesis and tumor growth.Citation16 In the Fenofibrate Intervention in Event Lowering in Diabetes (FIELD) study fenofibrate treatment demonstrated a significant 30% reduction in the need for laser therapy in patients with and without known diabetic retinopathy, and more particularly in the first course of laser treatment for both macular edema and proliferative retinopathy.Citation17 In addition, fenofibrate treatment was associated with less albuminuria progression and reduced risk of non traumatic distal amputations.Citation17 Pozzi et al treated mice injected with tumor cells with Wy-14643, a selective PPAR-α ligand. Wy-14643 treated mice showed marked reductions in tumor growth and vascularization.Citation15 All these responses were absent in PPAR-α−/− mice thus suggesting that these antiproliferative effects might be PPARα mediated. Several other animal studies show the antiproliferative properties of fenofibrate ().Citation18,Citation19
Table 1 Effect of PPARs on angiogenesis
Studies supporting proangiogenic role of PPAR-α
However recent studies using specific non fibrate PPAR-α agonists or PPAR-α knockout mice have shown that PPAR-α may be involved in stimulating angiogenesis (). Biscetti et al used a selective synthetic PPAR-α agonist to demonstrate that activation of PPAR-α leads to endothelial tube formation in an endothelial/interstitial cell co-culture assay.Citation20 This study also showed that neovascularization occurs with PPARα activation in a murine corneal angiogenic model. In contrast no angiogenesis was found in PPAR-α knockout mice treated with this synthetic agonist.Citation20 In another study iloprost, an angiogenesis agent, was not able to induce angiogenesis in PPAR-α knockout mice.Citation21 In both these studies angiogenesis was stimulated through a PPAR-α induced VEGF upregulation. Fauconnet et al showed that activation of PPAR-α increased VEGF expression via a transcriptional activation of the VEGF promoter.Citation22
Although the majority of studies point towards the antiproliferative, antiangiogenic properties of PPAR-α, this may be due to the use of fibrates as agonists in these experiments. A lot more research needs to be done using methods such as spontaneous PPAR-α activation, overexpression, silencing and knockout mice, rather than using chemical agonists and antagonists which might have pleotropic effects unrelated to PPAR-α.
PPAR-β/δ and angiogenesis
Recently developed synthetic ligands and genetically modified mice models for PPAR-β/δ have increased our knowledge of its role in metabolism, inflammation, and angiogenesis. A large range of naturally occurring ligands bind to PPAR-β/δ, including 14 to 18 carbon saturated fatty acids, 16 to 20 carbon polyunsaturated fatty acids, prostaglandin A1 and carbaprostacyclin.Citation23 However, whether any of these are physiological ligands is controversial. Retinoic acid and several synthetic ligands can also specifically activate PPAR-β/δ.Citation24
Studies supporting proangiogenic role of PPAR-β/δ
Studies generally show that PPAR-β/δ may be involved in stimulating angiogenesis (). The PPAR-β/δ agonist GW501516 stimulated human umbilical vein endothelial cells proliferation and increased VEGF expression.Citation25 In another experiment GW501516 increased angiogenesis and promoted endothelial tube formation in a PPAR-β/δ and VEGF dependent manner.Citation26 Gaudel et al showed that PPAR-β/δ activation resulted in a 1.5-fold increase in capillary number in mouse skeletal muscle.Citation27 In a study of tissue samples obtained from human colorectal carcinomas and matched adjacent tissues, VEGF expression, microvascular density, and venous vessel invasion increased as the expression of PPAR-β/δ and cyclooxygenase (COX)-2 increased.Citation28 He et al performed a set of experiments in human endothelial progenitor cells (EPCs) which showed that impaired tube formation and cell proliferation induced by inactivation of COX-1 were rescued by treatment with a PPAR-β/δ agonist. Furthermore, transfection of PPAR-β/δ siRNA into EPCs decreased the capillary formation in vivo after transplantation of these human EPCs into nude mice.Citation29 Growth of syngeneic PPAR-β/δ wild-type tumors was suppressed in PPAR-β/δ−/− mice, along with a decreased blood flow and hyperplastic vasculature.Citation30 Muller et al reviewed a number of recent studies that suggest that stromal PPAR-β/δ regulates tumor endothelial cell proliferation and promotes differentiation leading to the properly orchestrated events required for tumor blood vessel formation.Citation31 These data do suggest that PPAR-β/δ may indeed be proangiogenic under certain conditions.
Mechanisms by which PPAR-β/δ activation either suppresses or stimulates angiogenesis are not well studied. A large number of studies implicate increased VEGF and Flt-1 expression.Citation32,Citation33
PPAR-γ and angiogenesis
PPAR-γ is probably the most studied PPAR, likely due to the use and development of several PPAR-γ agonists such as thiozolidinediones in the treatment of type 2 diabetes. Endogenous ligands for PPAR-γ include long chain polyunsaturated fatty acids and their derivatives, 15-deoxy-Δ12, 14-prostaglandin J2 (15d-PGJ2).Citation4 Other natural ligands include nitrolinoleic acids. 15d-PGJ2 has been found to upregulate the expression of PPAR-γ and also the DNA binding and transcriptional activity.Citation34 Synthetic ligands include TZDs and various nonsteroidal anti-inflammatory drugs.Citation35
Studies supporting antiproliferative properties of PPAR-γ
PPAR-γ has widespread effects involving, inflammation, atherosclerosis, obesity, diabetes, and cancer.Citation36 PPAR-γ agonists directly inhibit tumor cell growth, induce cell differentiation, and apoptosis in various cancer types ().Citation37 TZDs have been shown to decrease post angioplasty neointimal hyperplasia in both animals and humans ().Citation38,Citation39 PPAR-γ ligands have been shown to inhibit and stimulate angiogenesis (). Inhibition by PPAR-γ ligands can occur through direct effects on the endothelium or through indirect effects on the net balance of proangiogenic and antiangiogenic mediators.Citation37 PPAR-γ expressed in choroidal endothelial cells inhibits the differentiation and proliferation of those cells.Citation38,Citation39 Rosiglitazone inhibited endothelial cell proliferation and migration and decreased VEGF-induced tubule formation in human umbilical vein endothelial cells.Citation40,Citation41 In another study PPAR-γ ligands stimulated endothelial cell caspase-mediated apoptosis.Citation42 15d-PGJ2, an endogenous ligand of PPAR-γ, induces growth inhibition, differentiation, and apoptosis of tumor cells.Citation43 PPAR-γ activation interrupts NF-kβ signaling with subsequent blockade of proinflammatory gene expression.Citation43 Pioglitazone and rosiglitazone inhibit the effects of growth factors such as bFGF and VEGF. Endothelial cell migration is also inhibited by both compounds.Citation44 Thus natural and synthetic ligands of PPAR-γ exhibit antiangiogenic properties under certain conditions.
Studies supporting proangiogenic role of PPAR-γ
However, PPAR- ligands have also been shown to stimulate the angiogenic pathway (). In bovine aortic endothelial cells, prolonged treatment with troglitazone increased VEGF and endothelial nitric oxide (NO) production with no change in endothelial nitric oxide synthase (eNOS) expression.Citation45 In cultured rat myofibroblasts, activation of PPAR-γ by troglitazone and 15-dPGJ2 induced VEGF expression and augmented tubule formation.Citation46 In mice treated with rosiglitazone, angiogenesis was stimulated in adipose tissue with increased expression of VEGF and angiopoeitin-4 (Ang-4). Ang-4 stimulated endothelial cell growth and tubule formation. Citation47 In rats with focal cerebral ischemia, rosiglitazone treatment enhanced neurologic improvement and reduced the infarct size by reducing caspase-3 activity, increasing the number of endothelial cells, and increasing eNOS expression.Citation48 In the setting of diabetes, PPAR-γ agonists may promote revascularization of ischemic tissue. Diabetic mice with induced unilateral hind limb ischemia, when treated with pioglitazone showed normalization of VEGF, upregulation of eNOS activity, and partial restoration of blood flow recovery.Citation49 In mice treated with pioglitazone, VEGR-receptor-2 positive EPCs were upregulated and migratory capacity was increased. In vivo angiogenesis was increased 2-fold.Citation50 In an endothelial/interstitial cell co-culture assay, treatment with PPAR-γ agonists stimulated production of VEGF. In the same study, corneas treated with the same PPAR-γ agonists increased phosphorylation of eNOS.Citation20
Few studies have evaluated angiogenesis in humans. Pioglitazone treatment has been shown to increase serum VEGF, IL-8, and angiogenin levels in patients with type 2 diabetes.Citation51 In another study thiozolidinedione use in patients with type 2 diabetes was associated with diabetic macular edema.Citation52
PGC-1α and angiogenesis
Peroxisome proliferator-activated receptor (PPAR)-gamma coactivator 1alpha (PGC-1α) is a nuclear transcriptional coactivator that regulates several important metabolic processes, including mitochondrial biogenesis, adaptive thermogenesis, respiration, insulin secretion and gluconeogenesis. Citation53 PGC-1α also co-activates PPAR-α, PPAR-β/δ, and PPAR-γ which are important transcription factors of genes regulating lipid and glucose metabolism.Citation53 Recently Arany and colleagues have shown that PGC-1α stimulates angiogenesis in ischemic tissues. Using a combination of muscle cell assays and genetically modified mice that over or underexpess PGC-1α, they showed that PGC-1α is a powerful inducer of VEGF expression. PGC-1α did not involve HIF-1 but activated the nuclear receptor, estrogen-related receptor-α (ERR-α).Citation33 PGC-1α−/− mice are viable, suggesting that PGC-1α is not essential in embryonic vascularization but they show a striking failure to reconstitute blood flow in a normal manner to the limb after an ischaemic insult.Citation54 Transgenic expression of PGC-1α in skeletal muscle is protective against ischemic insults. This suggests that PGC-1α plays a more important role in a disease state rather than a physiologically healthy state.
Mechanisms by which PPARs may stimulate angiogenesis
PPARs seem to have a protective role in ischemic tissues, including brain, cardiac and skin. A part of this may be by stimulating angiogenesis and improving blood supply. Hypoxia is a trigger for the development of angiogenesis. One of the key mediators in hypoxia-induced angiogenesis is hypoxia inducible factor (HIF-1), which is induced in hypoxic cells and binds to hypoxia response element (HRE). HIF-1 mediates the transcriptional activation of several genes that promote angiogenesis, including VEGF, angiopoeitin (Ang-1, Ang-2), and matrix metalloproteinases (MMP-2, MMP-9).Citation55 15-deoxy-delta(12, 14)-prostaglandin J(2) (15d-PGJ(2)), a PPAR-γ agonist, has been shown to induce HIF-1 expression and thereby angiogenesis ().Citation34 However pioglitazone has been shown to suppress the induction of HIF-1.Citation56 Conditions that influence the stimulation or suppression of HIF activation by PPAR-γ are largely unknown.
Several studies suggest that eNOS synthase activation is required for angiogenesis that may be protective under certain conditions.Citation57–Citation59 In one study pioglitazone reduced the myocardial infarct size in part via activation of eNOS.Citation60 PPAR-α activation has also been shown to protect the type 2 diabetic rat myocardium against ischemia-reperfusion injury via the activation of the NO pathway (, ).Citation61 However, stimulation of the inducible nitric oxide (iNOS) pathway can lead to undesirable angiogenesis that may be contribute to pathological states such as proliferative retinopathy. PPARs in fact have been shown to suppress iNOS expression, thereby suppressing undesirable angiogenesis.Citation62,Citation63 Here again the factors that allow for activation of eNOS and suppression of iNOS is largely unknown.
The most studied pathway by which PPARs may stimulate angiogenesis is the VEGF pathway. VEGF can stimulate angiogenesis via stimulation of the ERK1/2 pathway. PPAR-β/δ activation has been shown to increase VEGF expression and thereby stimulate angiogenesis ().Citation26 In some studies PPAR-α and PPAR-γ have also been shown to increase VEGF expression.Citation47,Citation48 However the majority of studies still show that PPAR activation suppresses VEGF expression. The end result of whether PPAR activation suppresses or stimulates VEGF expression seems to lie in the pathological condition in which its actions are observed (). It is likely that PPAR activation results in increased VEGF expression in conditions where new blood vessel formation is required, such as ischemic skin flaps, brain, or cardiac tissue ischemia. On the other hand, pathological angiogenesis such as in the eye or within an atherosclerotic plaque is suppressed by PPAR activation via a suppression of VEGF ().
Recently some studies indicate that PPARs may increase the expression and activation of the phosphatidylinositol-3-kinase (PI3K/AKT) pathway.Citation61,Citation64 The PI3K/AKT pathway stimulates angiogenesis.Citation59,Citation65 Again the majority of studies show that PPAR activation inhibits PI3K/AKT activation.
It is very likely that a large amount of variation found in different studies is due to the use of agonists and antagonists of the PPAR receptors that exhibit direct PPAR-independent effects. Most study designs do not distinguish between direct effects and indirect effects of various pharmacological agonists/antagonist used. Fibrates and TZDs have both been shown to have direct independent effects on inflammation, proliferation and angiogenesis. Hence it is difficult to conclude that all the pro and antiangiogenic effects seen in various studies are a result of PPAR activation exclusively.
Clinical significance and conclusions
Some compounds such as TZDs and fibrates are routinely used in patients with diabetes, dyslipidemia, and cardiovascular disease. Other compounds such as partial agonists or dual agonists of PPAR-α and PPAR-γ are in development. The effects of these newer compounds, on angiogenesis and cardiovascular disease are yet to be determined. Current evidence from clinical trials suggest a mixed picture. TZD treatment in patients with type 2 diabetes has been shown to be associated with macular edema. On the other hand, the FIELD study using fenofibrate showed a decrease in the need for laser treatments in patients with diabetic retinopathy. The PROACTIVE study showed that pioglitazone trended to decrease certain cardiovascular endpoints. In some studies, rosiglitazone increased the risk of cardiovascular events. In other studies such as ACCORD and VADT, TZD treatment was not associated with increased cardiovascular event risk. Several factors, including the study design, PPAR receptor affinity, and the PPAR-independent actions of these compounds, possibly play a role in the differences in results seen. The duration of the pathological state and the vasculature of the effected organ likely play a role in whether PPARs prove beneficial or harmful. In conclusion it may be prudent to summarize that at this point the evidence suggests that PPARs can either stimulate or inhibit angiogenesis, depending on the biological context and pathological process.
Disclosures
The authors declare no conflicts of interest.
References
- WeeCCHamelMBHuangADavisRBMittlemanMAMcCarthyEPObesity and undiagnosed diabetes in the USDiabetes Care2008311813181518509212
- WestphalSAObesity, abdominal obesity, and insulin resistanceClin Cornerstone20089233119046737
- CalkinACThomasMCPPAR agonists and cardiovascular disease in diabetesPPAR Res2008245410
- DuanSZIvashchenkoCYUsherMGMortensenRMPPAR-gamma in the cardiovascular systemPPAR Res2008745804
- KnouffCAuwerxJPeroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacologyEndocr Rev20042589991815583022
- ErdmannEDormandyJWilcoxRMassi-BenedettiMCharbonnelBPROactive 07: pioglitazone in the treatment of type 2 diabetes: results of the PROactive studyVasc Health Risk Manag2007335537017969365
- NissenSEWolskiKEffect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causesN Engl J Med20073562457247117517853
- FolkmanJAngiogenesis in cancer, vascular, rheumatoid and other diseaseNat Med1995127317584949
- CarmelietPBaesMMetabolism and therapeutic angiogenesisN Engl J Med20083582511251218525050
- MartinAKomadaMRSaneDCAbnormal angiogenesis in diabetes mellitusMed Res Rev20032311714512500286
- SimonsMAngiogenesis, arteriogenesis, and diabetes: paradigm reassessed?J Am Coll Cardiol20054683583716139133
- QaumTXuQJoussenAMVEGF-initiated blood-retinal barrier breakdown in early diabetesInvest Ophthalmol Vis Sci2001422408241311527957
- PitchfordSCFurzeRCJonesCPWengnerAMRankinSMDifferential mobilization of subsets of progenitor cells from the bone marrowCell Stem Cell20094627219128793
- Jandeleit-DahmKACalkinATikellisCThomasMDirect antiatherosclerotic effects of PPAR agonistsCurr Opin Lipidol200920242919133407
- PozziAIbanezMRGaticaAEPeroxisomal proliferator-activated receptor-alpha-dependent inhibition of endothelial cell proliferation and tumorigenesisJ Biol Chem2007282176851769517405874
- GrabackaMReissKAnticancer properties of PPARalpha – effects on cellular metabolism and inflammationPPAR Res2008930705
- ScottRO’BrienRFulcherGEffects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) studyDiabetes Care20093249349818984774
- KasaiTMiyauchiKYokoyamaTAiharaKDaidaHEfficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine modelsAtherosclerosis200618827428016325819
- GizardFAmantCBarbierOPPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4aJ Clin Invest20051153228323816239970
- BiscettiFGaetaniEFlexASelective activation of peroxisome proliferator-activated receptor (PPAR)alpha and PPAR gamma induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanismDiabetes2008571394140418268046
- BiscettiFGaetaniEFlexAPeroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulationJ Vasc Res20094610310818617751
- FauconnetSLascombeIChabannesEDifferential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cellsJ Biol Chem2002277235342354311980898
- WangNPPAR-delta in Vascular PathophysiologyPPAR Res2008164163
- BerryDCNoyNAll-trans-retinoic acid represses obesity and insulin resistance by activating both PPAR{beta}/{delta} and RARMol Cell Biol2009293286329619364826
- StephenRLGustafssonMCJarvisMActivation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell linesCancer Res2004643162317015126355
- PiquerasLReynoldsARHodivala-DilkeKMActivation of PPARbeta/delta induces endothelial cell proliferation and angiogenesisArterioscler Thromb Vasc Biol200727636917068288
- GaudelCSchwartzCGiordanoCAbumradNAGrimaldiPAPharmacological activation of PPARbeta promotes rapid and calcineurin-dependent fiber remodeling and angiogenesis in mouse skeletal muscleAm J Physiol Endocrinol Metab2008295E297E30418492772
- YoshinagaMKitamuraYChaenTThe simultaneous expression of peroxisome proliferator-activated receptor delta and cyclooxygenase-2 may enhance angiogenesis and tumor venous invasion in tissues of colorectal cancersDig Dis Sci2009541108111418720000
- HeTLuTd’UscioLVLamCFLeeHCKatusicZSAngiogenic function of prostacyclin biosynthesis in human endothelial progenitor cellsCirc Res2008103808818511850
- Muller-BrusselbachSKomhoffMRieckMDeregulation of tumor angiogenesis and blockade of tumor growth in PPARbeta-deficient miceEmbo J2007263686369817641685
- MullerRKomhoffMPetersJMMuller-BrusselbachSA Role for PPARbeta/delta in Tumor Stroma and TumorigenesisPPAR Res2008534294
- WangDWangHGuoYCrosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progressionProc Natl Acad Sci U S A2006103190691907417148604
- HollingsheadHEKillinsRLBorlandMGPeroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta) ligands do not potentiate growth of human cancer cell linesCarcinogenesis2007282641264917693664
- KimEHSurhYJ15-deoxy-Delta12, 14-prostaglandin J2 as a potential endogenous regulator of redox-sensitive transcription factorsBiochem Pharmacol2006721516152816987499
- LehmannJMLenhardJMOliverBBRingoldGMKliewerSAPeroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugsJ Biol Chem1997272340634109013583
- PershadsinghHAPeroxisome proliferator-activated receptor-gamma: therapeutic target for diseases beyond diabetes: quo vadis?Expert Opin Investig Drugs200413215228
- GiaginisCTsantili-KakoulidouATheocharisSPeroxisome proliferator-activated receptor-gamma ligands: potential pharmacological agents for targeting the angiogenesis signaling cascade in cancerPPAR Res2008431763
- RosmarakisESFalagasMEEffect of thiazolidinedione therapy on restenosis after coronary stent implantation: a meta-analysis of randomized controlled trialsAm Heart J200715414415017584567
- DesouzaCVGeretyMHamelFGLong-term effects of a PPAR-gamma agonist, pioglitazone, on neointimal hyperplasia and endothelial regrowth in insulin resistant ratsVascul Pharmacol20074618819417141574
- PanigrahyDSingerSShenLQPPARgamma ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesisJ Clin Invest200211092393212370270
- SheuWHOuHCChouFPLinTMYangCHRosiglitazone inhibits endothelial proliferation and angiogenesisLife Sci2006781520152816297938
- Bishop-BaileyDHlaTEndothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Delta12, 14-prostaglandin J2J Biol Chem1999274170421704810358055
- GiriSRattanRSinghAKSinghIThe 15-deoxy-delta12,14-prostaglandin J2 inhibits the inflammatory response in primary rat astrocytes via down-regulating multiple steps in phosphatidylinositol 3-kinase-Akt-NF-kappaB- p300 pathway independent of peroxisome proliferator-activated receptor gammaJ Immunol20041735196520815470065
- AljadaAO’ConnorLFuYYMousaSAPPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesisAngiogenesis20081136136718810647
- ChoDHChoiYJJoSAJoINitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathwaysJ Biol Chem20042792499250614593122
- ChintalgattuVHarrisGSAkulaSMKatwaLCPPAR-gamma agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblastsCardiovasc Res20077414015017320065
- GealekmanOBurkartAChouinardMNicoloroSMStraubhaarJCorveraSEnhanced angiogenesis in obesity and in response to PPAR-gamma activators through adipocyte VEGF and ANGPTL4 productionAm J Physiol Endocrinol Metab2008295E1056E106418728224
- ChuKLeeSTKooJSPeroxisome proliferator-activated receptor-gamma-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemiaBrain Res2006109320821816696956
- HuangPHSataMNishimatsuHSumiMHirataYNagaiRPioglitazone ameliorates endothelial dysfunction and restores ischemia-induced angiogenesis in diabetic miceBiomed Pharmacother200862465217692499
- GenschCCleverYPWernerCHanhounMBohmMLaufsUThe PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cellsAtherosclerosis2007192677416876172
- VijaySKMishraMKumarHTripathiKEffect of pioglitazone and rosiglitazone on mediators of endothelial dysfunction, markers of angiogenesis and inflammatory cytokines in type-2 diabetesActa Diabetol200946273318758684
- FongDSContrerasRGlitazone use associated with diabetic macular edemaAm J Ophthalmol2009147583586e119181303
- FinckBNKellyDPPeroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and diseaseCirculation20071152540254817502589
- AranyZFooSYMaYHIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alphaNature20084511008101218288196
- HickeyMMSimonMCRegulation of angiogenesis by hypoxia and hypoxia-inducible factorsCurr Top Dev Biol20067621725717118268
- LeeKSKimSRParkSJPeroxisome proliferator activated receptor-gamma modulates reactive oxygen species generation and activation of nuclear factor-kappaB and hypoxia-inducible factor 1alpha in allergic airway disease of miceJ Allergy Clin Immunol200611812012716815147
- ChenJCuiXZacharekARobertsCChoppMeNOS mediates TO90317 treatment-induced angiogenesis and functional outcome after stroke in miceStroke2009402532253819443804
- HowellKCostelloCMSandsMDooleyIMcLoughlinPL-arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertensionAm J Physiol Lung Cell Mol Physiol2009296L1042L105019346433
- NamkoongSKimCKChoYLForskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signalingCell Signal20092190691519385062
- YasudaSKobayashiHIwasaMAntidiabetic drug pioglitazone protects the heart via activation of PPAR-{gamma} receptors, PI3-kinase, Akt, and eNOS pathway in a rabbit model of myocardial infarctionAm J Physiol Heart Circ Physiol2009296H1558H156519286954
- BulhakAAJungCOstensonCGLundbergJOSjoquistPOPernowJPPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: involvement of the PI3-Kinase/Akt and NO pathwayAm J Physiol Heart Circ Physiol2009296H719H72719151258
- CuzzocreaSPisanoBDugoLRosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammationEur J Pharmacol2004483799314709329
- TaoLLiuHRGaoEAntioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemiaCirculation20031082805281114610009
- PedchenkoTVGonzalezALWangDDuBoisRNMassionPPPeroxisome proliferator-activated receptor beta/delta expression and activation in lung cancerAm J Respir Cell Mol Biol20083968969618566335
- MaJSawaiHOchiNPTEN regulate angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cellsMol Cell Biochem2009 May 13 Epub ahead of print
- PanigrahyDKaipainenAHuangSPPARalpha agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibitionProc Natl Acad Sci U S A200810598599018199835
- MinutoliLAntonuccioPPolitoFPeroxisome proliferator activated receptor beta/delta activation prevents extracellular regulated kinase 1/2 phosphorylation and protects the testis from ischemia and reperfusion injuryJ Urol20091811913192119237170
- LimHJLeeSParkJHPPAR delta agonist L-165041 inhibits rat vascular smooth muscle cell proliferation and migration via inhibition of cell cycleAtherosclerosis200920244645418585719
- BorlandMGForemanJEGirroirEELigand activation of peroxisome proliferator-activated receptor-beta/delta inhibits cell proliferation in human HaCaT keratinocytesMol Pharmacol2008741429144218687807
- PiquerasLSanzMJPerrettiMActivation of PPAR{beta}/{delta} inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine releaseJ Leukoc Biol20098611512219389799