Abstract
Preclinical data on extracts of and preparations derived from beans of Phaseolus vulgaris are reviewed as potential remedies for use in controlling food consumption, body weight, lipid accumulation, and glycemia. A growing body of evidence suggests that acute and chronic administration of P. vulgaris derivatives reduces food intake (including highly palatable foods), body weight, lipid deposit, and glycemia in rats exposed to multiple experimental procedures. Two possible lectin-mediated mechanisms of action have been proposed: (a) inhibition of α-amylase, resulting in a reduced carbohydrate metabolism and absorption; (b) phytohemoagglutinin-induced modulation of the activity of cholecystokinin and glucagon-like peptides, resulting in a reduced appetite. Preliminary clinical data, as well as reports focusing on the use of several traditional medicines, apparently extend these findings to humans. Should these initial clinical data be confirmed by future surveys, P. vulgaris derivatives might constitute novel remedies for the treatment of obesity and metabolic syndrome. Future studies are also expected to identify active structures leading to the development of new pharmaceutical agents.
This paper reviews the accumulating lines of experimental evidence suggesting that extracts of beans from Phaseolus vulgaris (Fabaceae) may be capable of reducing food intake (including highly palatable foods and fluids), body weight, lipid deposit, and glycemia in different, validated animal models of overeating, obesity, diabetes, and metabolic syndrome. A brief mention of the most relevant surveys testing P. vulgaris preparations on food intake and glycemia in humans is also given.
The genus P. vulgaris includes all species of legume seeds normally known as common beans. Archeological investigations showed that common beans originated on the American Continent, specifically in southern United States, Mexico, Central America, and the northern part of South America. In particular, the species P. vulgaris was introduced into Europe in the sixteenth century and since then it has become a very important crop in many regions of the world. Legume seeds are among the richest food sources of proteins, amino acids, complex carbohydrates, dietary fibers, and oligosaccharides for human and animal nutrition.Citation1
P. vulgaris extracts and food intake in laboratory animals
Preclinical investigations have unanimously reported how the acute, repeated administration of extracts of P. vulgaris, as well as some of their isolated ingredients, reduced food intake, body weight, and lipid accumulation in lean and obese laboratory animals.Citation2–Citation13
Specifically, a study was performed to investigate the effect of a P. vulgaris extract mixed with a starch-enriched chow on food intake and body weight in young, lean Hooded Lister rats.Citation6 Restricted amounts of food were made available to rats to ensure the entire supply of P. vulgaris extract was consumed by each rat. The results of this study indicated a significant reduction in body weight gain in rat groups consuming chow mixtures containing 20 and 40 mg/die P. vulgaris extract. The extract used in this study had a high content of α-amylase inhibitors, suggesting that the possible mechanism of action underlying the reducing effect produced by this P. vulgaris extract on body weight gain was constituted by inhibition of the pancreatic enzyme α-amylase, hampering starch metabolism and reducing feed efficiency (ie, food was less efficaciously converted into energy and, in turn, into body mass). Notably, the reduction in body weight gain secondary to exposure to the P. vulgaris extract was associated to a decrease in body content of lipids. Similar data were generated by a previous study, in which rats were fed with chow containing α-amylase inhibitors from P. vulgaris; rats displayed a decrease in body weight gain and lipid accumulation.Citation4
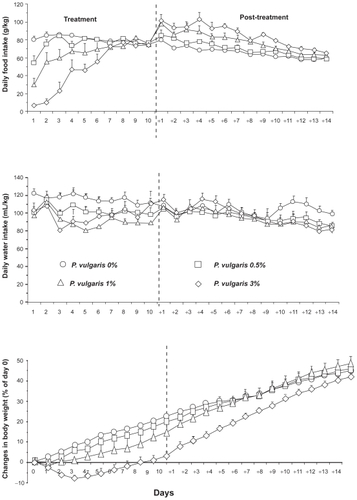
Two studies evaluated the effect of prolonged (700 to 800 consecutive days) exposure to a starch-enriched diet containing a P. vulgaris preparation.Citation6,Citation7 One of these two studies was designed to ensure that rats exposed to the 90 g/kg kidney bean-based diet and pair-fed control rats (a) weighed approximately 100 g at the start of the experiment and (b) entirely consumed a fixed daily supply of food (resulting, in the treated rat group, in the consumption of the full daily dose of P. vulgaris extract).Citation7 As shown in , feed efficiency (defined as the body weight gain over the amount of food intake) was largely lower, especially over the first 3-month period, in P. vulgaris extract-treated rats than in control rats. Additionally, a significant reduction in body content of lipids was observed throughout the study in the rat group exposed to the P. vulgaris extract-containing diet when compared to the rat group exposed to the P. vulgaris extract-free diet.Citation7 In the second study, control rats (exposed to a P. vulgaris extract-free diet) had a mean body weight gain of approximately 660 g; conversely, rats consuming the diet including the P. vulgaris extract displayed a mean body weight gain of approximately 470 g.Citation6
Figure 1 Reducing effect of the prolonged (700 consecutive days) ingestion of a Phaseolus vulgaris preparation, mixed in a starch-enriched diet, on feed efficiency [defined as the body weight gain (g) over the amount (g) of food intake] in Hooded Lister rats. Adapted from Grant G, Dorward PM, Buchan WC, Armour JC, Pusztai A. Consumption of diets containing raw soya beans (Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: effects on body composition and organ weights. Br J Nutr. 1995;73:17–29.Citation7 Copyright © 1995 with permission of Cambridge University Press.
![Figure 1 Reducing effect of the prolonged (700 consecutive days) ingestion of a Phaseolus vulgaris preparation, mixed in a starch-enriched diet, on feed efficiency [defined as the body weight gain (g) over the amount (g) of food intake] in Hooded Lister rats. Adapted from Grant G, Dorward PM, Buchan WC, Armour JC, Pusztai A. Consumption of diets containing raw soya beans (Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: effects on body composition and organ weights. Br J Nutr. 1995;73:17–29.Citation7 Copyright © 1995 with permission of Cambridge University Press.](/cms/asset/c723dc0e-556f-4509-8e1c-8e91f8e3961a/dmso_a_4236_f0001_b.jpg)
An additional study investigated the effect of repeated (21 consecutive days) daily administration, by intragastric gavage, of a single dose (50 mg/kg) of an extract of P. vulgaris prepared to contain high amounts of α-amylase inhibitors on daily food intake and body weight in Wistar rats given access to a starch-enriched diet.Citation2 Administration of the P. vulgaris extract resulted in a 15% reduction, in comparison to vehicle-treated control rats, in food intake over the 21-day treatment period; this effect was associated with a reduction in body weight gain (+52.0 g per rat and −1.3 g per rat in the vehicle- and P. vulgaris extract-treated rat groups, respectively, at the end of the treatment period).
Notably, these results were subsequently replicated in a study using rats with streptozotocin-induced diabetes.Citation13 The repeated (22 consecutive days) daily administration of a single dose (100 mg/kg) of the P. vulgaris extract used in the previous study with Wistar rats (see also above) resulted in an approximately 25% reduction in daily food intake.Citation12 This effect was paralleled by a reduction in body weight gain.
Beside the action of α-amylase inhibitors, another mechanism for the reducing effect of P. vulgaris extracts on food intake and body weight has been proposed. As described in detail below, this mechanism involves phytohemoagglutinin, a lectin present at high levels in P. vulgaris. A series of experiments has been conducted to test this hypothesis. Specifically, an extract of P. vulgaris characterized by a high phytohemoagglutinin content was mixed to the diet; genetically obese Zucker rats were exposed to restricted daily amounts of food to ensure ingestion of the entire daily dose of phytohemoagglutinin by all rats.Citation9 Repeated exposure to this diet resulted in reductions of 25% and 20%, in comparison to control rats (given an identical daily amount of phytohemoagglutinin-free chow), in body weight gain and body fat content, respectively. In an additional experimentCitation11 purified phytohemoagglutinin from P. vulgaris beans was acutely administered (by intragastric gavage), at a dose of 100 mg/kg, to fasted Wistar rats given access to regular rat chow. Treatment with phytohemoagglutinin resulted in a marked reduction in food intake throughout the entire dark phase of the light/dark cycle (ie, the period of maximal activity in rats). The reducing effect of phytohemoagglutinin had a relatively slow onset, being manifest after approximately 5 hours; at the end of the dark phase, food intake was reduced by approximately 40% in phytohemoagglutinin-treated rats in comparison to vehicle-treated rats.
This laboratory has recently conducted a study aimed at assessing the effect of a P. vulgaris extract prepared in order to produce a potential dual action: inhibition of α-amylase and a phytohemoagglutinin-induced anorectic effect. To this end BeanBlock® (prepared by Indena SpA, Milan, Italy), the extract tested, exerted an inhibitory activity on α-amylase equal to 1400 U/mg and an hemoagglutinating activity equal to 16 HAU/mg. The extract was added to a starch-enriched diet (Altromin RP 1000®; Rieper, Vandoies, Italy); pellets containing 0%, 0.5%, 1%, and 3% P. vulgaris extract were prepared. Singly housed, male adult Wistar rats (Charles River Laboratories, Calco, Italy) were divided into 4 groups of n = 6 to 7, matched for body weight, and fed with the above-mentioned food pellets with unlimited access for 24 hours/day over 10 consecutive days (treatment phase). Rats from all groups were then given unlimited access to the plain diet (with 0% P. vulgaris extract) for an additional 14 consecutive days (post-treatment phase). Food and water intake, as well as rat body weight, were recorded once a day.
Exposure to the four different diets resulted in a concentration-dependent suppression in daily food intake over the first 3 days of treatment (, top panel). On days 1 and 2, daily food intake in the rat group exposed to the diet containing 3% P. vulgaris extract was approximately 90% lower than that recorded in control rats (fed with the 0% P. vulgaris extract). However, on continuing treatment, tolerance to the anorectic effect of the P. vulgaris extract developed, as indicated by the virtually complete lack of any difference in daily food intake among the four rat groups from day 8 of treatment (, top panel). This tolerance developed even though rats exposed to the most concentrated diets ingested daily amounts of P. vulgaris extract 2 to 3 times higher than those ingested during the first days of treatment. In the post-treatment phase, a relatively long period of overeating developed; this phenomenon was dependent on concentration of the P. vulgaris extract included in the previous rat diet and peaked up to 50%, in comparison to control rats, on day +4 in the 3% P. vulgaris extract-treated rat group (, top panel). Overeating tended to decrease progressively and vanished when rat body weight virtually achieved control values (see below).
Figure 2 Reducing effect of the repeated (10 consecutive days) ingestion of a Phaseolus vulgaris extract, mixed – at the concentrations of 0%, 0.5%, 1% and 3% – to a starch-enriched diet, on daily food (top panel) and water (center panel) intake, as well as changes in body weight (expressed as percent of baseline) (bottom panel) in Wistar rats. Each point is the mean ± SEM of n = 6 to 7 rats. Hatched vertical lines indicate the end of the 10-day treatment phase and the start of the 14-day post-treatment phase. ANOVA results – Food intake, treatment phase: Fdiet (3,21) = 10.42, P < 0.0005; Ftime (9,189) = 22.58, P < 0.0001; Finteraction (27,189) = 8.79, P < 0.0001; Food intake, post-treatment phase: Fdiet (3,21) = 12.93, P < 0.0001; Ftime (13,273) = 43.78, P < 0.0001; Finteraction (39,273) = 1.69, P < 0.01; Water intake, treatment phase: Fdiet (3,21) = 3.47, P < 0.05; Ftime (9,189) = 2.38, P < 0.05; Finteraction (27,189) = 0.97, P > 0.05; Water intake, post-treatment phase: Fdiet (3,21) = 0.40, P > 0.05; Ftime (13,273) = 14.76, P < 0.0001; Finteraction (39,273) = 2.60, P < 0.0001; Body weight changes, treatment phase: Fdiet (3,21) = 7,44, P < 0.005; Ftime (9,189) = 79.94, P < 0.0001; Finteraction (27,189) = 3.23, P < 0.0001; Body weight changes, post-treatment phase: Fdiet (3,21) = 2.31, P > 0.05; Ftime (13,273) = 458.37, P < 0.0001; Finteraction (39,273) = 6.55, P < 0.0001.
Reduced daily water intake was observed only in the rat group consuming the 3% P. vulgaris extract; however, this effect vanished on continuing treatment (, center panel). Erratic differences in daily water intake were observed among the four rat groups during the 14-day post-treatment period (, center panel).
In agreement with the reducing effect on food intake, rat body weight was greatly reduced in a concentration-dependent fashion by the ingestion of P. vulgaris extract (, bottom panel). Specifically, in the rat group exposed to the diet containing 3% P. vulgaris extract, the reduction in body weight peaked up to 8% on day 3, respect to values recorded immediately prior to start of the experiment. In the post-treatment phase, overeating (see above) led to a regaining of rat body weight (, bottom panel); indeed, rate of rat body weight increase varied between control rats and animals previously exposed to the P. vulgaris extract.
P. vulgaris extracts and intake of palatable food in laboratory animals
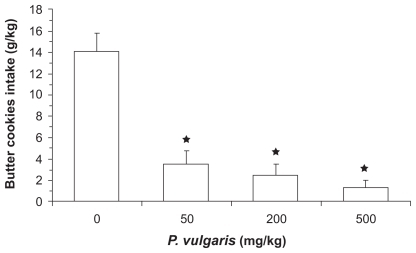
Recent lines of evidence indicate that P. vulgaris extracts may also be effective in reducing intake of highly palatable foods. As an example, a recent study by this laboratory found that the acute administration of a P. vulgaris extract (BeanBlock®) suppressed the intake of butter cookies in pre-satiated rats. Specifically, singly housed adult male Wistar rats (Charles River Laboratories) were habituated to feed [standard rat chow (Mucedola, Settimo Milanese, Italy)] for 3 hours per day (the first 3 hours of the dark phase); no food was available during the remaining 21 hours. Water was available 24 hours per day. This procedure led to fully satiated rats at the end of the 3-hour feeding period. On the test day, the 3-hour period of access to regular food was followed by 1-hour availability of the highly palatable butter cookies. Rats were divided into 4 groups of n = 7, matched for body weight and food intake during the last 3 days preceding the experiment. The P. vulgaris extract was administered intragastrically at doses of 0, 50, 200, and 500 mg/kg at the time of removal of the regular food (immediately prior to presentation of the butter cookies). Intake of butter cookies was recorded at the end of the 1-hour exposure period.
Even though rats were fully satiated, vehicle-treated rats consumed large amounts of butter cookies over the 1-hour exposure period. Acute administration of the P. vulgaris extract resulted in a dose-dependent suppression of this extra-intake of food. Specifically, intake of butter cookies in rats treated with 50, 200, and 500 mg/kg P. vulgaris extract was approximately 75%, 85%, and 90% lower, respectively, than that recorded in vehicle-treated rats ().
Figure 3 Reducing effect of the acute administration of a Phaseolus vulgaris extract on intake of butter cookies in pre-fed satiated Wistar rats having a 1-hour access to butter cookies. Each bar is the mean ± SEM of n = 7 rats. ANOVA results: F (3; 27) = 245.55, P < 0.0001; *P < 0.05 with respect to vehicle-treated rats (Newman-Keuls test).
Another experiment (this laboratory, unpublished results) found that the same P. vulgaris extract previously tested in the experiment with the butter cookies (see above) dose-dependently suppressed the polydipsic-like consumption of a highly palatable, chocolate-flavored beverage in rats. Notably, the P. vulgaris extract was shown to be more potent and effective in reducing intake of the chocolate-flavored beverage than regular food pellets. In that particular study, the chocolate-flavored beverage provided a modest caloric supply (<1/15 than that provided by regular food pellets), resulting in a beverage mostly consumed by rats because of its palatability rather than its caloric properties. This suggests that P. vulgaris extracts may exert their suppressing effects on palatable food with mechanisms other than those related to the nutritive properties of food.
P. vulgaris extracts and glycemia in laboratory animals
P. vulgaris extracts have also been found to reduce glycemiaCitation12–Citation14 and glucose absorptionCitation5 in laboratory animals. As an example, the study by Tormo et al in which rats were repeatedly treated with 50 mg/kg of P. vulgaris extract (with a high content of α-amylase inhibitors) and fed with a starch-enriched diet, demonstrated that the reducing effect of P. vulgaris extract on food intake and body weight was associated with a steady reduction in glycemia, measured once every other day and 1 hour after lights-off (this time schedule was likely chosen to measure the glycemia derived from the last daily meal, that usually occurs in the last period of the dark phase of the light/dark cycle under which rats were housed).Citation12 An additional experiment found that acute administration of the same P. vulgaris extract reduced glycemia in rats forcedly administered (by intragastric gavage) with a fixed amount (2 g/kg) of potato starch.Citation12
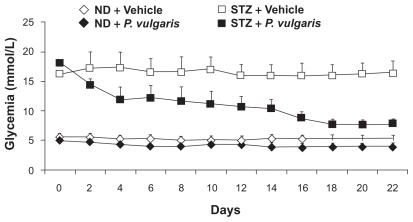
Similar results were collected in rats made diabetic by treatment with streptozotocin.Citation13 Specifically, newborn rats were treated with streptozotocin to induce pancreatic injury, resulting in reduction of insulin content and hyperglycemia; in adulthood, rats were treated daily and for 22 consecutive days with 100 mg/kg P. vulgaris extract; glycemia was assessed every other day, 1 hour after lights-off. Treatment with P. vulgaris extract resulted in (a) a marked and progressively increasing reduction in glycemia in streptozotocin-treated rats, reaching – at the end of the treatment – the values of vehicle-treated nonstreptozotocin-treated rats (), and (b) a less marked – although significant – reduction in glycemia in non-streptozotocin-treated rats ().
Figure 4 Reducing effect of the repeated (22 consecutive days) administration of a Phaseolus vulgaris extract on glycemia in control (ND) and streptozotocin-treated (STZ) rats. Glycemia was monitored every other day. Adapted from Tormo MA, Gil-Exojo I, Romero de Tejada A, Campillo JE. White bean amylase inhibitor administered orally reduces glycaemia in type 2 diabetic rats. Br J Nutr. 2006;96:539–544. Copyright © 2006 with permission of Cambridge University Press.
When P. vulgaris extracts with high contents of phytohemoagglutinin and low contents of α-amylase inhibitors were tested, a lack of effect on glycemia was the most common outcome. As an example, repeated exposure to fixed amounts of phytohemoagglutinin-enriched diets resulted in slight, if any, reductions in glycemia in normal and obese rats.Citation9–Citation15
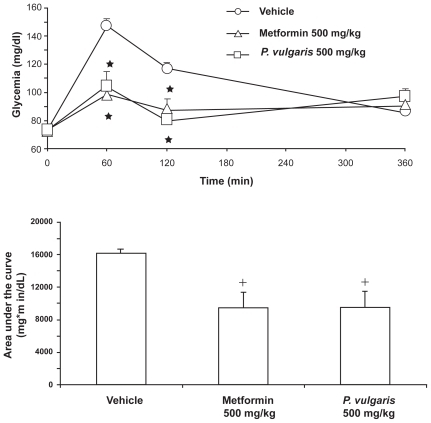
A recent experiment by this laboratory compared the effect of identical doses of a P. vulgaris extract (BeanBlock®), prepared to contain both α-amylase inhibitors and phytohemoagglutinin, and the standard anti-hyperglycemic drug, metformin, in rats. Specifically, singly housed adult male Wistar rats were fasted 24 hours before the start of the experiment. On the test day, rats were divided into 4 groups of n = 7 to 8, matched for body weight and glycemia, and treated intragastrically with vehicle, 500 mg/kg metformin (Sigma, Milan, Italy), and 500 mg/kg P. vulgaris extract. Thirty minutes later, rats were given 9 g/kg food [starch-enriched chow (Altromin RP 1000®; Rieper, Vandoies, Italy); an amount totally consumed – in less than 60 minutes – by fasted rats]. Glycemia was determined 0, 60, 120, and 360 minutes after food presentation. A small (0.05 mL) blood sample was collected from the tip of the tail of each rat and analyzed enzymatically [GL5 Analox® (Analox Ltd, London, UK)].
Metformin and P. vulgaris extract exerted a similar effect, inducing a reduction in glycemia of comparable magnitude at both 60- and 120-minute recording times (, top panel). Accodingly, metformin and P. vulgaris extract reduced to a similar extent the area under the curve of the time-course of glycemia (, bottom panel). These data indicate that P. vulgaris extracts may produce a reduction in post-prandial glycemia closely resembling that produced by metformin in rats exposed to a semi-naturalistic setting of a large meal after several hours fasting.
Figure 5 Similar reducing effect of a Phaseolus vulgaris extract and metformin on time-course of glycemia (top panel) and area under the curve of the time-course of glycemia (bottom panel) in Wistar rats given a 1-hour (corresponding to the 0- to 60-minute time interval) access to a starch-enriched diet and water. Each point or bar is the mean ± SEM of n = 7 to 8 rats. ANOVA results – Time-course: Ftreatment (2,20) = 9.13, P < 0.005; Ftime (2,40) = 21.95, P < 0.0001; Finteraction (4,40) = 10.95, P < 0.0001; Area under the curve: F (2,20) = 7.23, P < 0.005. *P < 0.005 with respect to values of vehicle-treated rats at the same time interval (Newman-Keuls test); +P < 0.05 with respect to values of vehicle-treated rats (Newman-Keuls test).
Possible mechanisms of action
Review of the existing literature suggests the involvement of two possible mechanisms of action in the reducing effect of P. vulgaris extracts on food intake, body weight, and glycemia. Both these mechanisms focus on the role of phytohemoagglutinin and α-amylase inhibitors. The latter constituents belong to the class of lectins, highly represented in different cereals and pulses, including P. vulgaris. These lectins – together with arcelins, another type of lectins abundant in P. vulgaris – are homologous proteins displaying high degrees (40% to 95%) of aminoacid sequence similarity.Citation16–Citation19
Pancreatic α-amylase is an enzyme that catalyzes hydrolysis of α-(1,4)-glycosidic bonds of starch polymers.Citation20 Thus, inhibition of α-amylase results in the suppression of starch metabolism and, in turn, a decrease in glycemia.Citation12–Citation20 It has also been reported that α-amylase inhibitors delay gastric emptying, producing feelings of satiety,Citation21–Citation22 thus resulting in reduced food intake.Citation12–Citation13
Phytohemoagglutinin is known to bind to the stomach epithelial cells and to the brush border membrane of small intestine, cecum, and colon.Citation5,Citation11,Citation23–Citation25 This binding results in the stimulation of the release of cholecystokinin and glucagon-like peptides,Citation11,Citation23–Citation26 two hormones playing a relevant role in digestive processes and also in the central control of appetite. In close agreement with the latter hypothesis, recent data from this laboratory indicate that treatment with the cholecystokinin receptor typeA (CCKA) antagonist, lorglumide, blocked the reducing effect of a P. vulgaris extract (BeanBlock®) on food intake in rats (this laboratory, unpublished results), suggesting that phytohemoagglutinin-stimulated release of cholecystokinin and CCKA receptors are major players in the appetite-reducing effect of P. vulgaris extracts.
Another CCKA receptor-mediated action of phytohemoagglutinin is the stimulation of pancreatic secretion of α-amylase in rats;Citation27–Citation29 this should result in an accelerated metabolism of ingested starch and, in turn, in a stimulation of food intake and increase in glycemia. However, this effect seems to be of limited relevance in the overall control of appetite and food intake, as it is likely overwelmed by the opposite, anorectic and hypoglycemic effects of phytohemoagglutinin itself and α-amylase inhibitors.
Human data and conclusions
As reported above and recently discussed,Citation10,Citation30 accumulating lines of experimental evidence consistently indicate that extracts of P. vulgaris are effective in reducing appetite, body weight, lipid accumulation, hedonic properties of food, starch absorption and metabolism, and glycemia in different species of laboratory animals.
These data are consonant with reports on a traditional use, mainly in Central American and European Countries, of P. vulgaris preparations as “antidiabetic” remedies. Accordingly, considerable benefit – especially during the developmental stages of the disease – has been reported in individuals taking P. vulgaris or preparations containing P. vulgaris as a main constituent.Citation30,Citation31–Citation35
An interesting clinical trial, conducted in healthy subjects, found that intraluminal administration of a P. vulgaris-derived α-amylase inhibitor suppressed (a) amylase activity in duodenum, jejunum, and ileum, (b) early post-prandial glycemia rise, and (c) late post-prandial glycemia fall (secondary to a marked decrease in post-prandial insuline release).Citation36–Citation37 Confirmation of the hypoglycemic effect of a P. vulgaris-derived α-amylase inhibitor is provided by a further studyCitation38 in which the α-amylase inhibitor was administered to healthy subjects, resulting in a significant reduction in post-prandial glycemia. Further, Brugge and RosenfeldCitation39 investigated the effect of a P. vulgaris-derived α-amylase inhibitor on hydrogen content in breath air after a starch-rich meal (spaghetti) in healthy subjects; breath hydrogen is inversely correlated with carbohydrate absorption. The P. vulgaris-derived α-amylase inhibitor produced a 2-fold increase in hydrogen excretion rate compared to control subjects, suggesting a decreased carbohydrate absorption and/or metabolism. Similar results were collected by Boivin et al.Citation38 In a recent additional series of studies, tendencies toward a reduction in body weight and waist circumference were observed in healthy subjects taking a preparation of P. vulgaris. Citation40–Citation42 Finally, a more recent randomized, double-blind, placebo-controlled trial found that a 2-month treatment with a dietary supplement made up of P. vulgaris and Cynara scolymus extracts increased the feeling of satiation (measured by the Haber’s scale for hunger/satiety scoring) in healthy overweight and obese subjects.Citation43
Together, these data suggest that extracts of P. vulgaris may constitute potentially interesting, novel remedies for the treatment of overweight and metabolic syndrome. Future studies, designed to confirm and extend those currently available in literature, are needed. Confirmation of the above-mentioned, promising data would also allow a re-evaluation of P. vulgaris preparations, the reputation of which has frequently been hampered by the development of commercial preparations supported by large advertising campaign but devoid of any clinically demonstrated efficacy.
Acknowledgments and disclosures
The authors are grateful to S. Anne Farmer for language editing of the manuscript. The authors declare no conflicts of interest.
References
- GeilPBAndersonJWNutrition and health implications of dry beans: a reviewJ Am Coll Nutr1994135495587706585
- KakadeMLEvansRJGrowth inhibition of rats fed raw navy beans (Phaseolus vulgaris)J Nutr1966901911985922107
- MaranesiMCareniniGGentiliPNutritional studies on anti alpha-amylase: I) Influence on the growt rate, blood picture and biochemistry and histological parameters in ratActa Vitaminol Enzymol198462592696335947
- MaranesiMBarzantiVBiagiPLCareniniGGentiliPNutritional studies on anti alpha-amylase: II) Lipid metabolism investigation: fatty acid composition of organs and tissuesActa Vitaminol Enzymol198463473536335948
- DonatucciDALienerIEGrossCJBinding of navy bean (Phaseolus vulgaris) lectin to the intestinal cells of the rat and its effect on the absorption of glucoseJ Nutr1987117215421603694292
- GrantGDorwardPMPusztaiAPancreatic enlargement is evident in rats fed diets containing raw soybeans (Glycine max) or cowpeas (Vigna unguiculata) for 800 days but not in those fed diets based on kidney beans (Phaseolus vulgaris) or lupinseed (Lupinus angustifolius)J Nutr1993123220722157505319
- GrantGDorwardPMBuchanWCArmourJCPusztaiAConsumption of diets containing raw soya beans (Glycine max), kidney beans (Phaseolus vulgaris), cowpeas (Vigna unguiculata) or lupin seeds (Lupinus angustifolius) by rats for up to 700 days: effects on body composition and organ weightsBr J Nutr19957317297857911
- PusztaiAGrantGDuguidTInhibition of starch digestion by α-amylase inhibitor reduces the efficiency of utilization of dietary proteins and lipids and retards the growth of ratsJ Nutr1995125155415627782910
- PusztaiAGrantGBuchanWCBardoczSde CarvalhoAFEwenSWLipid accumulation in obese Zucker rats is reduced by inclusion of raw kidney bean (Phaseolus vulgaris) in the dietBr J Nutr1998792132219536866
- PusztaiABardoczSEwenSWBUses of plant lectins in bioscience and biomedicineFrontiers in Bioscience2008131130114017981618
- BaintnerKKissPPfullerUBardoczSPusztaiAEffect of orally and intraperitoneally administered plant lectins on food consumption of ratsActa Physiologica Hungarica2003909710712903908
- TormoMAGil-ExojoIRomero de TejadaACampilloJEHypoglycaemic and anorexigenic activities of an α-amylase inhibitor from white kidney beans (Phaseolus vulgaris) in Wistar ratsBr J Nutr20049278579015533267
- TormoMAGil-ExojoIRomero de TejadaACampilloJEWhite bean amylase inhibitor administered orally reduces glycaemia in type 2 diabetic ratsBr J Nutr20069653954416925860
- KotaruMIwamiKYehHYIbukiFIn vivo action of alpha-amylase inhibitor from cranberry bean (Phaseolus vulgaris) in rat small intestineJ Nutr Sci Vitaminol (Tokyo)1989355795882699495
- BardoczSGrantGPusztaiAThe effect of phytoaemagglutinin at different dietary concentrations on the growth, body composition and plasma insulin of the ratBr J Nutr1996766136268942367
- MorenoJChrispeelsMJA lectin gene encodes the α-amylase inhibitor of the common beanProc Natl Acad Sci U S A198986788578892682631
- IshimotoMSuzukiKIwanagaMKikuchiFKitamuraKVariation of seed – amylase inhibitors in the common beanTheor Appl Genet199590425429
- SharmaVSuroliaAAnalyses of carbohydrate recognition by legume lectins: size of the combining site loops and their primary specificityJ Molec Biol19972674334459096236
- LeeSCGeptsPLWhitakerJRProtein structures of common bean (Phaseolus vulgaris) α-amylase inhibitorsJ Agric Food Chem2002506618662712381161
- SantimoneMKoukiekoloRMoreauYPorcine pancreatic alpha-amylase inhibition by the kidney bean (Phaseolus vulgaris) inhibitor (α-AI1) and structural changes in the α-amylase inhibitor complexBiochim Biophys Acta2004169618119014871659
- JainNKBoivinMZinsmeisterARBrownMLMalageladaJRDi MagnoEPEffect of ileal perfusion of carbohydrates and amylase inhibitor on gastrointestinal hormones and emptyingGastroenterology1989963773872463204
- JainNKBoivinMZinsmeisterARDi MagnoPThe ileum and carbohydrate-mediated feedback regulation of postprandial pancreatico-biliary secretion in normal humansPancreas199164955051719522
- KingTPPusztaiAGrantGSlaterDImmunogold localization of ingested kidney bean (Phaseolus vulgaris) lectins in epithelial cells of the rat small intestineHistochem J1986184134203536802
- BardoczSGrantGEwenSWReversible effect of phytohaemagglutinin on the growth and metabolism of rat gastrointestinal tractGut1995373533607590430
- HerzigKHBardoczSGrantGNustedeRFölschURPusztaiARed kidney bean lectin is a potent cholecystokinin releasing stimulus in the rat inducing pancreatic growthGut1997413333389378388
- RådbergKBiernattMLinderothAZabielskiRPierzynowskiSGWeströmBREnteral exposure to crude red kidney bean lectin induces maturation of the gut in suckling pigsJ Anim Sci2001792669267811721847
- KordásKBurghardtBKisfalviKBardoczSPusztaiAVargaGDiverse effects of phytohaemagglutinin on gastrointestinal secretions in ratsJ Physiol (Paris)200094313610761686
- BaintnerKKissPBardoczSPusztaiAEffect of orally administered plant lectins on intestinal liquor accumulation and amylase activity in ratsActa Physiol Hung2004909710712903908
- BaintnerKKissPPikliAPeumansWBardoczSPusztaiAOrigin and mediation of secretion induced by oral phytoemagglutinin (PHA) in ratsActa Physiol Hung20049122123316438116
- ObiroWCZhangTJiangBThe nutraceutical role of the Phaseolus vulgaris alpha-amylase inhibitorBr J Nutr200810011218331662
- Román-RamosRFlores-SáenzJLPartida-HernándezGLara-LemusAAlarcón-AguilarFExperimental study of the hypoglycemic effect of some antidiabetic plantsArch Invest Med (Mex)19912287931819981
- Roman-RamosRFlores-SaenzJLAlarcon-AguilarFJAnti-hyperglycemic effect of some edible plantsJ Ethnopharmacol19951125328569244
- PetlevskiRHadzijaMSlijepcevicMJureticDEffect of ‘antidiabetis’ herbal preparation on serum glucose and fructosamine in NOD miceJ Ethnopharmacol20017518118411297848
- Andrade-CettoAHeinrichMMexican plants with hypoglycaemic effect used in the treatment of diabetesJ Ethnopharmacol20059932534815964161
- HelmstädterAAntidiabetic drugs used in Europe prior to the discovery of insulinPharmazie20076271772017944329
- LayerPCarlsonGLDi MagnoEPPartially purified white bean amylase inhibitor reduces starch digestion in vitro and inactivates intraduodenal amylase in humansGastroenterology198588189519022581844
- LayerPZinsmeisterARDi MagnoEPEffects of decreasing intraluminal amylase activity on starch digestion and postprandial gastrointestinal function in humansGastroenterology19869141482423408
- BoivinMZinsmeisterARGoVLDi MagnoEPEffect of a purified amylase inhibitor on carbohydrate metabolism after a mixed meal in healthy humansMayo Clin Proc1987622492552436011
- BruggeWRRosenfeldMSImpairment of starch absorption by a potent amylase inhibitorAm J Gastroenterol1987827187222440298
- UdaniJHardyMMadsenDCBlocking carbohydrate absorption and weight loss: a clinical trial using Phase 2 brand proprietary fractionated white bean extractAltern Med Rev20049636915005645
- UdaniJSinghBBBlocking carbohydrate absorption and weight loss: a clinical trial using a proprietary fractionated white bean extractAltern Ther Health Med200713323717658120
- CellenoLTolainiMVD’AmoreAPerriconeNVPreussHGA Dietary supplement containing standardized Phaseolus vulgaris extract influences body composition of overweight men and womenInt J Med Sci20074455217299581
- RondanelliMOrsiniFOpizziAGiacosaABombardelliEVillaniSThe effect of 2-mo administration of a Phaseolus vulgaris and Cynara scolymus complex on feeling of satiation in healthy, overweight peopleEuropean Journal of Obesity20092Suppl 2234