Abstract
Recently, we serendipitously discovered that mice with the deficiency of the enzyme prolylcarboxypeptidase (PRCP) have elevated α-melanocyte-stimulating hormone (α-MSH) levels which lead to decreased food intake and weight loss. This suggests that PRCP is an endogenous inactivator of α-MSH and an appetite stimulant. Since a modest weight loss can have the most profound influence on reducing cardiovascular risk factors, the inhibitors of PRCP would be emerging as a possible alternative for pharmacotherapy in high-risk patients with obesity and obesity-related disorders. The discovery of a new biological activity of PRCP in the PRCP-deficient mice and studies of α-MSH function indicate the importance and complexity of the hypothalamic pro-opiomelanocortin (POMC) system in altering food intake. Identifying a role for PRCP in regulating α-MSH in the brain may be a critical step in enhancing our understanding of how the brain controls food intake and body weight. In light of recent findings, the potential role of PRCP in regulating fuel homeostasis is critically evaluated. Further studies of the role of PRCP in obesity are much needed.
Introduction
The hypothalamus is often times a target for newer potential obesity treatments due to its crucial role in food intake and metabolism. It is also established that food intake modulation is confounded by numerous players within the hypothalamus, allowing for the research and development of a diverse array of obesity management drug leads that have completely different underlying mechanisms. The melanocortin system is established amongst these systems; however, the cannabinoid system, among others, also has an established role. Speculation of a connection between the cannabinoid system and melanocortin system regarding food intake has existed due to their presence in nearby regions of the hypothalamus. Moreover, suboptimal doses of SR 141716 (rimonabant) together with suboptimal doses of α-melanocyte-stimulating hormone (α-MSH) are known to behave synergistically in order to reduce food intake. Attempts to develop a safe drug to treat obesity via blocking the CB1 receptor have proven to be elusive and controversial, as drugs such as rimonabant have had to struggle for approval due to numerous reported and suspected side effects, particularly depression. The role of the melanocortin system in food intake is well-established and prevention of the rapid inactivation of α-MSH may prove to be a better alternative pathway for potential obesity treatments. Recent studies suggest that prolylcarboxypeptidase (PRCP) involved in regulating blood pressure and inflammation is an appetite stimulant and, by consequence, PRCP inhibitors may prove to be a viable lead to treat obesity.
There have been numerous excellent reviews on melanocortin receptors.Citation1–Citation6 However, this article only reports the most current information about how the two tectonic physiological processes, namely the proteolytic enzymes of renin-angiotensin system (RAS) and proopiomelanocortin (POMC)-derived neuropeptide regulatory processes in the central nervous system might be shifting toward each other. Recent findings suggest that PRCP (a RAS enzyme) regulates α-MSH (a POMC-derived neuropeptide) levels, a theme addressed by the present review. While this article briefly introduces both PRCP- and α-MSH-mediated processes, it also outlines how brain PRCP may play a key role in controlling food intake and weight gain.
The current scope of prolylcarboxypeptidase
The PRCP-catalyzed reaction was initially found to be part of the pathway for angiotensin II (Ang II) metabolism in renal tissues, where PRCP appeared to control the total amount of Ang II. Odya and others demonstrated that PRCP metabolizes Ang II to angiotensin 1–7 (Ang 1–7) ().Citation7 The activation of Ang 1–7 receptor Mas (a G-protein-coupled protein) by Ang 1–7 results in the generation of nitric oxide (NO) and prostaglandins.Citation8 Thus, Ang 1–7 counteracts Ang II function, providing evidence that PRCP regulates the negative effects of Ang II such as high blood pressure and heart failure.Citation9 In addition, the activation of the Ang 1–7 receptor Mas may also lead to diminished cell proliferation through down-regulation of the phosphorylation and activation of Erk1 and Erk2 in the Erk1/Erk2 Map kinase signaling pathway.Citation10,Citation11 In theory, the PRCP inhibitors to target the production of pro-inflammatory prostaglandins and promote proliferation through the Ang 1–7 receptor Mas-dependent pathway represent a novel approach to suppress unwanted inflammation-causing prostaglandins.
Figure 1 The physiological action of prolylcarboxypeptidase (PRCP).
Abbreviations: α-MSH, α-melanocyte-stimulating hormone; HK high molecular weight kininogen; PK, prekallikrein.
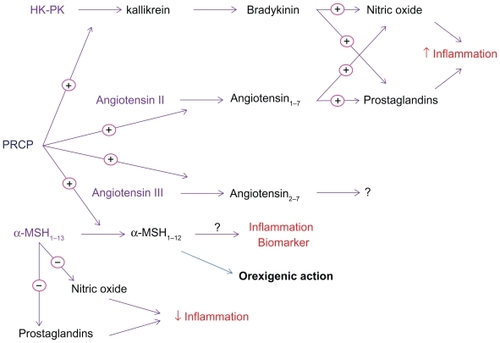
Later, it was shown that PRCP is one of several enzymes that convert Ang II to a unique bioactive molecule. In vitro studies showed that angiotensin-converting enzyme 2 (ACE2) is an exopeptidase that converts Ang II to Ang 1–7 at a much faster rate than PRCP.Citation12 These data suggest that Ang II is a poor substrate for PRCP. Clinical studies have provided reliable evidence that ACE2 is an essential regulator of angiotensin I (Ang I), Ang II, and angiotensin-induced cardiac hypertrophy.Citation13 Recent studies clearly show increased myocardial levels of Ang II and a significant decrease in Ang 1–7 in ACE2-deficient hearts, suggesting that the role of PRCP in metabolizing Ang II may be insignificant.Citation9 Taken together, these observations suggest that PRCP is a redundant catalyst contributing to alternate pathways for Ang II metabolism.
While the well-established cardiovascular and renal actions of Ang II are attributed to the angiotensin type 1 receptor (AT1R), much less is known about angiotensin III and its cardiovascular effects. For more than 30 years, it was known that PRCP metabolizes Ang III to Ang 2–7 ().Citation7 Soon after, studies demonstrated that Ang III is a pressor agent whose response, like that of Ang II, is mediated by AT1 receptors.Citation14,Citation15 Apparently, Ang III has multiple effects on renal function in the diseased kidney and can enhance renal disease through the overproduction of aldosterone, leading to arterial hypertension and/or atrial fibrillation.Citation16 Aldosterone maintains blood volume, pressure, and electrolyte balance. Its production is known to be regulated by renin, an enzyme produced in the kidneys. Renin increases in response to low blood pressure, decreased blood flow to the kidneys, or sodium deficiency. The elevation of renin results in an increase in synthesis and secretion of aldosterone. Studies indicate that Ang III also activates the secretion of aldosterone.Citation15 Recently, we have demonstrated that recombinant PRCP (rPCRP) metabolizes Ang III to Ang 2–7, removing phenylalanine (Phe).Citation8 It is tempting to speculate that PRCP might funnel the generation of angiotensin 3–4 (Ang 3–4) through Ang 2–7 (). If the over-secretion of aldosterone by Ang III is viewed as a trigger of arterial hypertension, then inactivation of Ang III by PRCP might lead to a decrease in blood pressure. Further studies are required to determine whether PRCP is critically important for regulating Ang III-induced hypertension and preserving renal structure and function. This is an important area of research to pursue given the increasing prevalence of cardiovascular disease and stroke in the older population.
The possible actions of another substrate of PRCP, plasma prekallikrein (PK, Fletcher factor), have recently begun to receive much attention. When the complex of high molecular weight kininogen (HK) and PK binds to endothelial membrane, PK is rapidly converted to kallikrein by PRCP.Citation17 The formed kallikrein then cleaves HK to liberate bradykinin (BK), which leads to NO and prostaglandin-I2 formation, as well as subsequent vasodilation, by activating constitutive bradykinin B2 and inducible bradykinin B1 receptors.Citation18,Citation19 The PRCP-dependent PK activation pathway might be considered an additional mechanism to preserve the availability of NO and prostacyclin as vasodilatory agents in vascular smooth muscle. We proposed that chronic PRCP inhibition might elevate blood pressure. In accordance, we have found that PRCPgt/gt mice have mild hypertension, suggesting a causative relationship between PRCP levels and signs of hypertension.Citation20
Local skeletal muscle ischemia and acidosis are shown to increase the generation of BK and prostaglandins, the two circulating products of the PRCP-induced cell activation ().Citation21 The increased acidotic response during exercise and inflammatory mediators such as BK and prostacyclin have been shown to cause abnormal exercise-related symptoms and autonomic responses in congestive heart failure syndrome.Citation22 Nonetheless, the long-term elevated concentrations of NO and prostacyclin through PRCP-dependent pathways may be detrimental and eventually responsible for cardiovascular diseases such as congestive heart disease. Since BK and Prostaglandins exacerbate the genesis of the symptoms of exercise intolerance in heart failure,Citation23 the inhibitors of PRCP might be effective in ameliorating the exercise-limiting symptoms.
Clinical studies demonstrate that PRCP is involved in the pathogenesis of inflammatory conditions such as rheumatoid arthritis and infection.Citation24 Melanocortin peptides have numerous effects on the host such as the modulation of fever, inflammation and appetite.Citation25 Recently, we showed that PRCP metabolizes alpha-melanocyte-stimulating hormone 1–13 (α-MSH1–13) to alpha-melanocyte-stimulating hormone 1–12 (α-MSH1–12),Citation26 (). α-MSH1–13 is a potent anti-inflammatory agent.Citation27 In addition to the specificity of cleavage, the cellular release of pro-inflammatory mediators seemed to be critical to PRCP actions. In theory, agents that increase production and effects of α-MSH1–13 could be used to counteract the effects of pro-inflammatory mediators such as bradykinin and cytokines ().
Obesity is known to cause inflammation and insulin resistance in the vasculature and non-vascular tissues involved in glucose metabolism.Citation28 Evidence suggests that hyperglycemia may contribute to defective NO-dependent vasodilation in diabetes.Citation29 The inducible NO synthase (iNOS) expression is elevated in adipose tissue of obese people compared to those of lean peopleCitation30 and is a mediator of inflammation and a key enzyme in insulin resistance.Citation31 The colocalization of α-MSH1–13 receptors (MC4R) with iNOS has been reported, suggesting a role for α-MSH1–13 in obese people.Citation32 The inactivation of α-MSH1–13 by PRCP provides a positive feedback loop for postprandial enhancement of food intake and inflammation by inhibiting α-MSH1–13 function, as shown in .Citation26 Since PRCP regulates the anorectic action of α-MSH1–13, this study highlights the presence of a newly recognized interaction between inflammation, obesity, and the expression and activity of PRCP ().Citation26 In view of the above studies, we consider that PRCP may be a key player in the obesity-associated metabolic complications, inflammatory response, and the host defense mechanism.
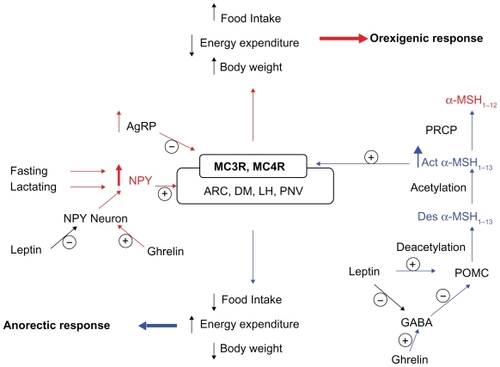
Figure 2 The role of the hypothalamus in food intake and metabolism.
Abbreviations: Act-α-MSH, acetylated α-MSH; AgRP, Agouti-related protein; ARC, arcuate nucleus of the hypothalamus; Des-α-MSH, desacetyl α-MSH; DM, dorsomedial hypothalamus; GABA, gamma-aminobutryric acid; LH, lateral hypothalamus; MC3R, melanocortin-3 receptor; MC4R, melanocortin-4 receptor; NPY, neuropetide y; POMC, pro-opiomelanocortin; PVN, paraventricular nucleus.
Prolylcarboxypeptidase’s physiological function and relationship with hypothalamic appetite-regulating pathways
The following sections emphasize pertinent findings, which best describe the theoretical perspective on the components of the central melanocortin system and stress the importance of PRCP influence in the melanogenic signaling pathway.
Synthesis of α–melanocyte – stimulating hormone
Pro-opiomelanocortin (POMC), a prohormone with molecular weight of 31 kDa, is ubiquitously expressed in various tissues of mammals.Citation33,Citation34 POMC expression in the central nervous system, however, is limited to the arcuate nucleus of the hypothalamus (ARC), nucleus tractus solitarius of the caudal medulla (NTS), and corticotrophs and melanotrophs of the anterior pituitary ().Citation35 The 1200 base pair POMC transcript encodes for the 267 amino acid prohormone with an N-terminal signal peptide of 26 residues.Citation36 As this precursor peptide passes through the Golgi stacks, it is targeted, via a specific signal peptide, into regulated secretory granules.Citation37 POMC undergoes extensive posttranslational modification within these secretory granules mediated by a family of serine proteases, the prohormone convertases (PCs), as illustrated in .
Figure 3 Schematic structure of pro-opiomelanocortin (POMC) and location of melanocyte-stimulating hormones (MSH).
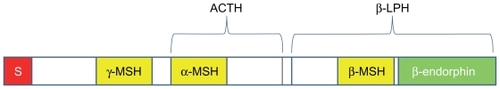
POMC is cleaved by prohormone convertase 1 (PC1) to produce 22 kDa pro-ACTH and β-lipoprotein hormone (β-LPH) ().Citation38 Pro-ACTH is further cleaved by PC1 to produce the N-terminus of POMC-joining peptide and 4.5 kDa ACTH. Prohormone convertase 2 (PC2) cleaves ACTH to ACTH 1–17 and corticotropin-like intermediate lobe peptide (CLIP) and β-LPH to γ-lipoprotein hormone (γ-LPH) and β-endorphin (). γ-LPH and the N-terminus of POMC are further modified to produce β-MSH and γ-MSH, respectively.
α-MSH is a tridecapeptide derived from POMC. The synthesis of α-MSH from POMC involves several specific enzymes in addition to PC1 and PC2. First, carboxypeptidase E cleaves the C-terminal basic amino acid residues of ACTH 1–17. The peptide is then amidated by peptidyl α-amidating monooxygenase (PAM) to produce desacetyl α-MSH (Des-α-MSH). Finally, Des-α-MSH is acetylated by N-acetyltransferase (NAT) to produce acetylated α-MSH (Act-α-MSH), as illustrated in . Act-α-MSH is more potent than Des-α-MSH in activating melanocortin receptor signaling and in reducing food intake, effects that are likely due to rapid degradation of Des-α-MSH. Guo et al have shown that total hypothalamic α-MSH levels are decreased in leptin-deficient ob/ob mice and increased in leptin-treated ob/ob and C57BL/6J mice. Leptin specifically enhances hypothalamic levels of Act-α-MSH without significantly affecting the amounts of Des-α-MSH, possibly by activating NAT in the POMC neurons.Citation39
Five distinct central melanocortin receptors with different physiological functions
The melanocortin receptors are G-protein-coupled receptors with characteristic seven transmembrane domains. So far, five melanocortin receptors have been identified in humans – MC1R–MC5R.Citation40–Citation43 The melanocortin receptors are Gs-coupled and signal via the adenylate cyclase-cAMP-protein kinase A second messenger pathway. However, depending on the cell type and the melanocortin receptor expression, signal transduction pathways other than cAMP may be activated which include inositol triphosphate-diacyl glycerol-protein kinase C (IP3-DAG-PKC) pathway, extracellular Ca2+ influx, MAP kinase pathway, and the JAK/STAT pathway.Citation44–Citation48 MC1R, MC3R, MC4R and MC5R show 40%–60% amino acid homology. The natural MSH peptides have a conserved sequence, His-Phe-Arg-Trp, which plays an important role in the binding of these peptides to specific melanocortin receptors.Citation49
MC1R was the first melanocortin receptor to be cloned and was isolated from human melanoma cell line.Citation40 α-, β- and γ-MSH and ACTH are the known agonists whereas agouti is the known antagonist of the MC1R. MC1R is expressed in human and mouse melanoma cells, human melanocytes, skin glands, and hair follicles.Citation40,Citation50–Citation53 α-MSH, agouti, and MC1R, therefore, play an important role in regulating skin pigmentation and hair color. The presence of MC1R in the testes and the pituitary has been demonstrated by Chhajlani et al.Citation54 MC1R expression in the central nervous system is limited to neurons of the periaqueductal grey in both rat and human brain.Citation55 However, MC1R is widely expressed in cells involved in inflammation such as endothelial cells, neutrophils, monocytes, macrophages, fibroblasts and astrocytes.Citation25,Citation56–Citation59 α-MSH has been shown to inhibit inflammation via MC1R-mediated decrease in the production of inflammatory cytokines such as IL-1, IL-6, and tumor necrosis factor-alpha (TNFα), as well as suppression of NF-κB.Citation60–Citation62
MC2R is encoded by a single gene localized to chromosome 18p11.2.Citation53 ACTH is the only known agonist of the MC2R.Citation63 Using in situ hybridization, Xia et al showed that MC2R is highly expressed in the adrenal cortex with the highest expression in the zona fasciculata and zona glomerulosa and relatively low expression in the zona reticularis.Citation64 These findings are consistent with the role of MC2R in mediating the effect of ACTH on the synthesis and release of corticosteroids. Besides the adrenal cortex, MC2R is expressed in murine adipocytes, which explains the lipolytic effect of ACTH.Citation59 However, human adipocytes do not express MC2R and there is no evidence to suggest that human adipose tissue is responsive to the lipolytic effect of ACTH.Citation54 These species differences in the expression of MC2R may be important when studying the role of melanocortins in obesity. MC2R expression has also been demonstrated in the skin along with three cytochrome enzymes involved in steroid hormone synthesis.Citation65 Kapas et al have shown that ACTH produced in the skin by keratinocytes stimulates DNA synthesis and induces cell proliferation via MC2R.Citation66 MC2R, therefore, appears to play an important role in cutaneous pathophysiology.
MC3R is encoded by a single gene localized to the q13.2–q13.3 region of chromosome 20.Citation67 Since this locus is associated with type 2 noninsulin-dependent diabetes mellitus (NIDDM), Mc3r could represent a candidate gene for NIDDM.Citation68 MC3R is unique in that it binds to α-, β- and γ-MSH with similar affinities. Using Northern blot hybridization and polymerase chain reaction, Gantz et al first showed that MC3R is expressed in brain, placental, and gut tissues but not in melanoma cells or in adrenal glands.Citation69 MC3R expression in the brain is highest in the hypothalamus especially in the arcuate nucleus, ventromedial nucleus, preoptic nucleus, lateral hypothalamic area, and posterior hypothalamic area.Citation43 Agouti-related protein (AgRP), which is normally expressed in the hypothalamus, is a potent antagonist of both MC3R and MC4R.Citation70 However, POMC and AgRP neurons in the arcuate nucleus of hypothalamus selectively express MC3R, and not MC4R, suggesting that MC3R might function as a presynaptic autoreceptor regulating the release of melanocortins.Citation71,Citation72 Physiological support for this hypothesis was provided by Cowley et al,Citation73 who demonstrated that the selective MC3R agonist, D-Trp-γ-MSH inhibited firing of GFP-labeled POMC neurons in the PVN. Also, Marks et al showed that peripheral administration of the MC3R agonist stimulates feeding via MC3R-mediated inhibition of the ARC POMC neurons.Citation74
An association between MC3R and human obesity has been identified by linkage studies. Several sequence variants have been found in the Mc3r coding region and in 5′ flanking sequences.Citation75 Mc3r variants are associated with subtle changes in weight, leptin levels, and insulin-glucose ratios, but none of these explain human morbid obesity.Citation76 A novel heterozygous mutation I183N in Mc3r was identified in two obese patients of the same family.Citation77 Functional characterization of I183N showed that this mutation completely abolished the activity of the mutated receptor to stimulate intracellular cAMP production, suggesting that I183N might play an important role in obesity.Citation78 Similarly, Tao et al showed that a novel mutation I335S in Mc3r results in complete loss of ligand binding and signaling suggesting that this mutation might contribute to obesity.Citation79 However, a recent study evaluating the functional consequences of all mutations found in Mc3r and Mc4r in severely obese North American adults concluded that Mc4r, but not Mc3r mutations are associated with severe obesity in this population.Citation80 Thus, the significance of Mc3r mutations in human obesity is still not conclusively established to date.
MC4R was first cloned by Gantz et al in 1993.Citation42 MC4R is a 332 amino acid protein encoded by a single gene, localized to chromosome 18q21.3. Using Northern blot analysis and in situ hybridization, MC4R was originally found to be expressed primarily in the brain. MC4R expression was notably absent in the adrenal cortex, melanocytes, and placenta.Citation42 MC4R is widely distributed in the central nervous system, especially in the cortex, hippocampus, amygdala, septum, corpus striatum, nucleus accumbens, hypothalamus, nucleus tractus solitarius, visual and motor nuclei of the brainstem, and the dorsal horn of the spinal cord.Citation81 α-MSH, β-MSH, and ACTH are the known agonists and AgRP is the known antagonist of the MC4R ().Citation70
Since MC4R is highly expressed in the hypothalamus and has a strong affinity for α-MSH it is believed to be a strong candidate for energy balance, appetite control, and body weight regulation. Mc4r knockout mice have been shown to develop a maturity onset obesity syndrome characterized by hyperphagia, hyperglycemia and hyperinsulinemia.Citation82 Since this syndrome is similar to the agouti obesity syndrome seen in Avy/– mice and AgRP-transgenic mice that overexpress agouti and AgRP respectively, it is speculated that the primary mechanism by which agouti and AgRP produce obesity is chronic antagonism of MC4R.Citation83 Cachexia, a chronic wasting syndrome characterized by loss of body weight and muscle mass, is commonly associated with diseases such as cancer and AIDS. MC4R −/ − mice and mice treated with AgRP are resistant to lipopolysaccharide- or tumor-induced cachexia, further supporting the role of MC4R in energy balance and body weight regulation.Citation84
MC5R is a 325 amino acid protein encoded by a single gene located on chromosome 18p11.2.Citation85 MC5R has been shown to bind to all melanocortins except γ-MSH.Citation86 MC5R is the most widely expressed melanocortin receptor. MC5R mRNA is expressed in the adrenal gland, adipose tissue, kidney, leukocytes, lung, lymph node, mammary gland, ovary, pituitary, testis, and uterus.Citation54 Mc5r is highly expressed in exocrine glands such as lacrimal, preputial, Harderian and sebaceous glands.Citation87 Mc5r-deficient mice have a severe defect in water repulsion and thermoregulation due to decreased production of sebaceous lipids. MC5R may therefore play an important role in peripheral thermoregulation. Studies in humans have shown that MC5R immunoreactivity is detectable in the epithelium and appendages, including the sebaceous, eccrine, and apocrine glands. However, analysis of Mc5r variations in patients with acne, hidradenitis suppurativa, and sebaceous gland dysfunction have failed to suggest a causative role of MC5R in these conditions.Citation88 Low levels of MC5R mRNA have also been reported in the central nervous system,Citation89 however, the physiological function of MC5R in the brain remains unclear. Linkage analysis in the Quebec Family StudyCitation90 revealed a significant association of Mc5r polymorphisms with body mass index, fat mass, and resting metabolic rate, thus providing some evidence for the possible role of MC5R in energy balance and body weight regulation.
Appetite is tightly controlled by the relative hypothalamic levels of α-MSH
ARC is the major site of POMC expression in the central nervous system. The POMC neurons, which produce α-MSH, also express another anorectic peptide cocaine-amphetamine-related transcript (CART). Cell bodies of the POMC/CART neurons are found throughout the rostrocaudal extent of the arcuate nucleus, as well as the periarcuate area of the hypothalamus. Within the hypothalamus, these neurons project to the periventricular nucleus, paraventricular nucleus (PVH), and the perifornical region.Citation91,Citation92 The POMC/CART neurons also project to the brainstem to innervate the rostral NTS, lateral reticular nucleus, ventrolateral medulla, nucleus ambiguous, and the spinal cord, as reviewed elsewhere.Citation93 Another critical component of the central melanocortin system within the ARC is the neurons expressing neuropeptide Y (NPY) and the potent MC3R/MC4R antagonist AgRP (). The NPY/AgRP neurons have the same distribution as the POMC/CART neurons within the hypothalamus, with the densest fibers innervating the PVH, dorsomedial hypothalamus (DMH), posterior hypothalamus, and septal regions around the anterior commissure.Citation94
The NPY/AgRP neurons form synapses with the POMC/CART neurons within the ARC, thus producing a neuronal network that is responsive to the modulatory effects of several appetite and body weight regulating hormones such as leptin, ghrelin, insulin, and peptide YY (PYY).Citation73,Citation95–Citation98 Leptin acts via hypothalamic receptors (Ob-R) to decrease feeding and increase thermogenesis, resulting in a decrease in body weight. POMC/CART and NPY/AgRP neurons in the ARC are the principal sites of leptin receptor expression and the source of potent neuropeptide hormones, α-MSH and NPY, which exert opposing effects on feeding and metabolism as shown in . Subpopulations of NPY/AgRP neurons that also express gamma-aminobutyric acid (GABA) send inhibitory collaterals to the POMC/CART neurons. GABA inhibits the POMC/CART neurons and blocks the anorexic effect of α-MSH ().Citation99 Using electrophysiological techniques, Cowley et al showed that leptin stimulates the POMC/CART neurons via two mechanisms: 1) depolarization through a nonspecific cation channel and 2) hyperpolarization of NPY/AgRP neurons, leading to a reduction in the release of GABA that, in turn, causes disinhibition of the POMC/CART neurons ().Citation73
Ghrelin, the endogenous ligand for growth hormone secretagogue receptor (GHS-R), is a potent stimulant of growth hormone release and plays an important role in appetite control and body weight regulation. Circulating ghrelin levels are markedly increased with fasting and before meals and decrease following meals.Citation100,Citation101 Plasma ghrelin levels are also influenced by long-term energy balance and are increased in anorexia and decreased in obesity.Citation102,Citation103 Within the ARC, GSH-R is expressed on the NPY/AgRP neurons which are thought to mediate the orexigenic effects of ghrelin. Central and peripheral administration of ghrelin induces c-Fos in these neurons and increases hypothalamic NPY and AgRP mRNA expression, thus antagonizing the anorexic effects of leptin.Citation95,Citation98,Citation104 Also, electrophysiological studies have shown that ghrelin directly activates the orexigenic NPY/AgRP neurons while coordinately inhibiting the anorexogenic POMC/CART neurons via increased GABA release on them ().Citation105 Stimulation of food intake by ghrelin is blocked by administration of NPY antagonist and is reduced in NPY −/ − mice.Citation95 Lastly, MC3R and MC4R knockout mice show reduced sensitivity to ghrelin as evidenced by decreased ghrelin-induced food intake and growth hormone secretion, thus suggesting an important role of MC3R and MC4R in mediating the orexigenic effects of ghrelin.Citation106
NPY is a potent hypothalamic orexigenic peptide, probably the most powerful stimulant of appetite known. NYP mRNA expression in the hypothalamus is significantly increased during lactation and fasting.Citation107 Central administration of NPY causes robust increase in food intake and body weight in rats.Citation108,Citation109 Chronic intracerebroventricular administration of NPY to normal rats produces hyperphagia, hyperinsulinemia, and liver and adipose tissue lipogenesis, thus mimicking the hormonal and metabolic changes of obesity.Citation110 Recent evidence suggests that ectopic overexpression of NPY in other areas of the hypothalamus such as PVH, lateral hypothalamus, and DMH also increases food intake and body weight and that NPY knockdown in the DMH ameliorates the hyperphagia, obesity, and diabetes of Otsuka Long-Evans Tokushima Fatty (OLETF) rats.Citation111,Citation112 Thus, NPY in the hypothalamus plays an important role in modulating food intake and body weight ().
NPY exerts its orexigenic effects probably by inhibiting the ARC POMC/CART neurons via its Y2 receptor.Citation113 NPY is metabolized by several peptidases in the plasma. Recent evidence suggests that NPY(1–36) is metabolized into three major fragments: NPY(3–36), NPY(3–35), and NPY(2–35), upon incubation with human serum.Citation114 Specific inhibitors of dipeptidyl peptidase 4, plasma kallikrein, and aminopeptidase P prevent the production of NPY(3–36), NPY(3–35), and NPY(2–36), respectively. Plasma kallikrein metabolizes NPY(3–36) to NPY(3–35). Since NPY(3–35) is unable to bind to NPY Y1, Y2, and Y5 receptors, NPY(3–35) may represent the major metabolic end product of the Y2/Y5 agonist, NPY(3–36).
Insight into the physiological functions of PRCP through genetic studies in mice
A recent study identified two splice variants of PRCP; the second isoform was named Prcp2 (NCBI: NM_199418). Unlike Prcp, Prcp2 has a longer transcript and a unique amino-terminal region. Although its full-length sequence is known, there is no evidence suggesting whether PRCP2 mRNA encodes a functional protein.Citation24
The Prcp gene is speculated to be a candidate gene for essential hypertension.Citation115 Mutational analysis of the human PRCP has led to a better understanding of PRCP-catalyzed reactions. Certain putative mutant forms of human PRCP apparently predispose the polymorphic carriers to cardiovascular diseases including hypertension and the risk of preeclampsia.Citation116 The E112D polymorphism in the Prcp gene leads to increased antihypertensive effect of benazepril treatment in hypertensive patients.Citation117 We have also demonstrated that PRCPgt/gt mice have mild hypertension.Citation20 Our recent studies demonstrated that the Prcp-null (PRCP−/ −) mice ate less and had even less fat than the mice with partial loss of the enzyme.Citation26 These observations suggest that PRCP is a genetic marker for weight regulation and putative PRCP single nucleotide polymorphism (SNP) variants are associated with mild hypertension. Continued identification of PRCP mutations, full-characterization of PRCP knockout mice, and studies with knock-in mice with PRCP/PRCP mutations will provide evidence that PRCP is a disease-causing gene for both obesity and hypertension. Meanwhile, we propose that the use of PRCP inhibitors should be strongly indicated by a diagnosis of obesity in patients with no systolic or diastolic deterioration.
Future perspectives
Obesity is an emerging worldwide public health hazard and is associated with significant morbidity and mortality. Although the physiological determinants of normal/abnormal eating behavior have been investigated, the underlying causes and mechanisms of dysregulation of food intake in obesity, type 2 diabetes, and metabolic syndrome are not well understood. The long-lasting challenge for clinicians and scientists in basic research to unfold the major cause of the dysregulation of the food intake is becoming close to the last battle. Clinical studies indicate that the molecular and cellular mechanisms by which leptin and alpha-melanocyte stimulation hormone (α-MSH) modulate each other’s activity result in the regulation of food intake and energy expenditure.Citation118 These studies suggest that α-MSH is intimately involved in the regulatory mechanism of obesity, energy expenditure, and body weight.
Recently, prolylcarboxypeptidase (PRCP) was found to be responsible for the control of food intake and energy expenditure at a central level. The molecular mechanisms underlying the suppression of food intake in PRCP-deficient mice or by the inhibitor of PRCP clearly provide physiological evidence that PRCP is an inactivator of α-MSH.Citation26 Thus, PRCP is emerging as a new identity involved in the control of food intake and energy metabolism.
Since α-MSH can activate both melanocortin 4 receptors (MC4R) and melanocortin 1 receptors (MC1R) in a decreasing order, the catalysis of α-MSH1–13 to α-MSH1–12 by PRCP would lead to the suppression of both MC4R and MC1R activation as shown in . Although there are various contributing factors for obesity, the recent research findings indicate that PRCP is involved in the development of weight gain and obesity. PRCP controls the balance between energy intake and energy expenditure via an α-MSH1–13-mediated mechanism. An increase in PRCP expression or activity may result in obesity due to an imbalance between energy intake and energy expenditure. Although regulation of α-MSH1–13-mediated MC4R activation described above is demonstrably important, the importance of the role of PRCP on the signaling mechanisms of MC1R in anti-inflammatory response remains an enigma ().Citation119 Additional studies are needed to determine whether or not PRCP regulates α-MSH1–13-mediated MC1R activation.
Figure 4 PRCP inhibitors provoke accumulation of α-MSH1–13 peptide leading to reduced body weight, inflammation, and pain.
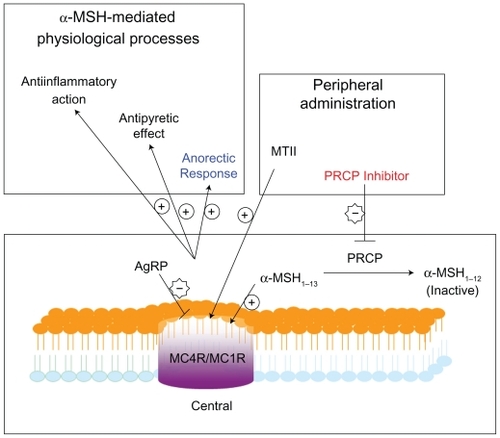
The cellular role of PRCP is beginning to be unraveled both at the molecular and physiological levels. The human PRCP mutation studies exhibit associations with diseases such as hypertension and preeclampsia. The upregulation of human PRCP expression during inflammation has been described.Citation24 On other hand, the PRCP knockout mice study demonstrates that PRCP is an appetite stimulant. Therefore, the pleiotropic effects of PRCP include increased NO and prostaglandin bioavailability, decreased vasoconstriction, and increased appetite.
In summary, knowledge of the role of PRCP in mouse appetite regulation has provided a new view of the ability of PRCP to influence obesity. Thus, research on PRCP-α-MSH interactions may be important to the further understanding of human obesity. In addition, the identification of PRCP as an inactivator of α-MSH should provide an attractive therapeutic target in the fight against obesity. However, due to its pleiotropic effects, the PRCP inhibitors must be scrutinized carefully to optimize their use in the treatment and prevention of obesity and obesity-related diseases.
Acknowledgment
This study was supported by NCRR/NIH P20RR021929 to SZ.
Disclosures
The authors declare that they have no competing interests, financial or otherwise with this work.
References
- Eves PC MacNeil S Haycock JW Alpha-Melanocyte stimulating hormone, inflammation and human melanoma Peptides 2006 27 444 452 16274844
- Lasaga M Debeljuk L Durand D Role of alpha-melanocyte stimulating hormone and melanocortin 4 receptor in brain inflammation Peptides 2008 29 1825 1835 18625277
- Oktar BK Alican I Modulation of the peripheral and central inflammatory responses by alpha-melanocyte stimulating hormone Curr Protein Pept Sci 2002 3 623 628 12470216
- Olszewski PK Bomberg EM Grace MK Alpha-melanocyte stimulating hormone and ghrelin: central interaction in feeding control Peptides 2007 28 2084 2089 17719137
- Konig S Luger TA Scholzen TE Monitoring neuropeptide-specific proteases: processing of the proopiomelanocortin peptides adrenocorticotropin and alpha-melanocyte-stimulating hormone in the skin Exp Dermatol 2006 15 751 761 16984256
- Gao Qian Horvath Tamas L Neurobiology of Feeding and Energy Expenditure Annual Review of Neuroscience 2007 30 367 398 Ref Type: Generic
- Odya CE Marinkovic DV Hammon KJ Purification and properties of prolylcarboxypeptidase (angiotensinase C) from human kidney J Biol Chem 1978 253 5927 5931 28321
- Dharmani M Mustafa MR Achike FI Effects of angiotensin 1–7 on the actions of angiotensin II in the renal and mesenteric vasculature of hypertensive and streptozotocin-induced diabetic rats Eur J Pharmacol 2007 561 144 150 17320855
- Kassiri Z Zhong J Guo D Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction Circ Heart Fail 2009 2 446 455 19808375
- Giani JF Gironacci MM Munoz MC Angiotensin-(1–7) has a dual role on growth-promoting signalling pathways in rat heart in vivo by stimulating STAT3 and STAT5a/b phosphorylation and inhibiting angiotensin II-stimulated ERK1/2 and Rho kinase activity Exp Physiol 2008 93 570 578 18448663
- Nie W Yan H Li S Angiotensin-(1–7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells Mol Immunol 2009 46 355 361 19041135
- Rice GI Thomas DA Grant PJ Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism Biochem J 2004 383 45 51 15283675
- Huentelman MJ Grobe JL Vazquez J Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats Exp Physiol 2005 90 783 790 16049057
- Felix D Khosla MC Barnes KL Neurophysiological responses to angiotensin-(1–7) Hypertension 1991 17 1111 1114 2045157
- Gammelgaard I Wamberg S Bie P Systemic effects of angiotensin III in conscious dogs during acute double blockade of the renin-angiotensin-aldosterone-system Acta Physiol (Oxf) 2006 188 129 138 16948800
- Al-Aloul B Li JM Benditt D Atrial fibrillation associated with hypokalemia due to primary hyperaldosteronism (Conn’s syndrome) Pacing Clin Electrophysiol 2006 29 1303 1305 17100688
- Shariat-Madar Z Mahdi F Schmaier AH Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator J Biol Chem 2002 277 17962 17969 11830581
- Zhao Y Qiu Q Mahdi F Assembly and activation of HK-PK complex on endothelial cells results in bradykinin liberation and NO formation Am J Physiol Heart Circ Physiol 2001 280 H1821 H1829 11247797
- Colman RW Schmaier AH Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes Blood 1997 90 3819 3843 9354649
- Adams GN Zhou Y Larusch G Prolylcarboxypeptidase murine hypomorphs are hypertensive and prothrombotic Journal of Thrombosis and Haemostasis 7 Supplement 2 XXII ISTH Congress, Abstract OC-MO-128 2009 Ref Type: Abstract
- Piepoli MF Scott AC Capucci A Skeletal muscle training in chronic heart failure Acta Physiol Scand 2001 171 295 303 11412141
- Scott AC Wensel R Davos CH Skeletal muscle reflex in heart failure patients: role of hydrogen Circulation 2003 107 300 306 12538432
- Scott AC Wensel R Davos CH Putative contribution of prostaglandin and bradykinin to muscle reflex hyperactivity in patients on Ace-inhibitor therapy for chronic heart failure Eur Heart J 2004 25 1806 1813 15474695
- Mallela J Yang J Shariat-Madar Z Prolylcarboxypeptidase: a cardioprotective enzyme Int J Biochem Cell Biol 2009 41 477 481 18396440
- Catania A Rajora N Capsoni F The neuropeptide alpha-MSH has specific receptors on neutrophils and reduces chemotaxis in vitro Peptides 1996 17 675 679 8804079
- Wallingford N Perroud B Gao Q Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents J Clin Invest 2009 119 2291 2303 19620781
- Chiao H Foster S Thomas R Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation J Clin Invest 1996 97 2038 2044 8621792
- Kim F Pham M Maloney E Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance Arterioscler Thromb Vasc Biol 2008 28 1982 1988 18772497
- Pieper GM Dondlinger L Glucose elevations alter bradykinin-stimulated intracellular calcium accumulation in cultured endothelial cells Cardiovasc Res 1997 34 169 178 9217887
- Bullo M Casas-Agustench P migo-Correig P Inflammation, obesity and comorbidities: the role of diet Public Health Nutr 2007 10 1164 1172 17903326
- Kaneki M Shimizu N Yamada D Nitrosative stress and pathogenesis of insulin resistance Antioxid Redox Signal 2007 9 319 329 17184170
- Tai MH Weng WT Lo WC Role of nitric oxide in alpha-melanocyte-stimulating hormone-induced hypotension in the nucleus tractus solitarii of the spontaneously hypertensive rats J Pharmacol Exp Ther 2007 321 455 461 17283224
- DeBold CR Menefee JK Nicholson WE Proopiomelanocortin gene is expressed in many normal human tissues and in tumors not associated with ectopic adrenocorticotropin syndrome Mol Endocrinol 1988 2 862 870 2845257
- Tatro JB Reichlin S Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents Endocrinology 1987 121 1900 1907 2822378
- Young JI Otero V Cerdan MG Authentic cell-specific and developmentally regulated expression of pro-opiomelanocortin genomic fragments in hypothalamic and hindbrain neurons of transgenic mice J Neurosci 1998 18 6631 6640 9712635
- Voisey J Carroll L van DA Melanocortins and their receptors and antagonists Curr Drug Targets 2003 4 586 597 14535656
- Cool DR Normant E Shen F Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice Cell 1997 88 73 83 9019408
- Rouille Y Duguay SJ Lund K Proteolytic processing mechanisms in the biosynthesis of neuroendocrine peptides: the subtilisin-like proprotein convertases Front Neuroendocrinol 1995 16 322 361 8557169
- Guo L Munzberg H Stuart RC N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin Proc Natl Acad Sci U S A 2004 101 11797 11802 15280541
- Chhajlani V Wikberg JE Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA FEBS Lett 1992 309 417 420 1516719
- Cone RD Mountjoy KG Robbins LS Cloning and functional characterization of a family of receptors for the melanotropic peptides Ann N Y Acad Sci 1993 680 342 363 8390157
- Gantz I Miwa H Konda Y Molecular cloning, expression, and gene localization of a fourth melanocortin receptor J Biol Chem 1993 268 15174 15179 8392067
- Roselli-Rehfuss L Mountjoy KG Robbins LS Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system Proc Natl Acad Sci U S A 1993 90 8856 8860 8415620
- Konda Y Gantz I DelValle J Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor J Biol Chem 1994 269 13162 13166 8175743
- Kojima I Kojima K Rasmussen H Role of calcium and cAMP in the action of adrenocorticotropin on aldosterone secretion J Biol Chem 1985 260 4248 4256 2579947
- Englaro W Rezzonico R Durand-Clement M Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells J Biol Chem 1995 270 24315 24320 7592642
- Buggy JJ Binding of alpha-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway Biochem J 1998 331 Pt 1 211 216 9512481
- Rodrigues AR Pignatelli D Almeida H Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism Mol Cell Endocrinol 2009 303 74 81 19428994
- Wikberg JE Melanocortin receptors: perspectives for novel drugs Eur J Pharmacol 1999 375 295 310 10443584
- Donatien PD Hunt G Pieron C The expression of functional MSH receptors on cultured human melanocytes Arch Dermatol Res 1992 284 424 426 1337693
- Suzuki I Cone RD Im S Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis Endocrinology 1996 137 1627 1633 8612494
- Xia Y Skoog V Muceniece R Polyclonal antibodies against human melanocortin MC1 receptor: preliminary immunohistochemical localisation of melanocortin MC1 receptor to malignant melanoma cells Eur J Pharmacol 1995 288 277 283 7774671
- Mountjoy KG Robbins LS Mortrud MT The cloning of a family of genes that encode the melanocortin receptors Science 1992 257 1248 1251 1325670
- Chhajlani V Distribution of cDNA for melanocortin receptor subtypes in human tissues Biochem Mol Biol Int 1996 38 73 80 8932521
- Xia Y Wikberg JE Chhajlani V Expression of melanocortin 1 receptor in periaqueductal gray matter Neuroreport 1995 6 2193 2196 8595200
- Hartmeyer M Scholzen T Becher E Human dermal microvascular endothelial cells express the melanocortin receptor type 1 and produce increased levels of IL-8 upon stimulation with alpha-melanocyte-stimulating hormone J Immunol 1997 159 1930 1937 9257858
- Wong KY Rajora N Boccoli G A potential mechanism of local anti-inflammatory action of alpha-melanocyte-stimulating hormone within the brain: modulation of tumor necrosis factor-alpha production by human astrocytic cells Neuroimmunomodulation 1997 4 37 41 9326743
- Star RA Rajora N Huang J Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone Proc Natl Acad Sci U S A 1995 92 8016 8020 7544012
- Boston BA Cone RD Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line Endocrinology 1996 137 2043 2050 8612546
- Luger TA Scholzen T Grabbe S The role of alpha-melanocyte-stimulating hormone in cutaneous biology J Investig Dermatol Symp Proc 1997 2 87 93
- Lipton JM Catania A Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH Immunol Today 1997 18 140 145 9078687
- Manna SK Aggarwal BB Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents J Immunol 1998 161 2873 2880 9743348
- Schioth HB Chhajlani V Muceniece R Major pharmacological distinction of the ACTH receptor from other melanocortin receptors Life Sci 1996 59 797 801 8761313
- Xia Y Wikberg JE Localization of ACTH receptor mRNA by in situ hybridization in mouse adrenal gland Cell Tissue Res 1996 286 63 68 8781213
- Slominski A Ermak G Mihm M ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin J Clin Endocrinol Metab 1996 81 2746 2749 8675607
- Kapas S Hagi-Pavli E Brown DW Direct effects of corticotrophin on oral keratinocyte cell proliferation Eur J Biochem 1998 256 75 79 9746348
- Gantz I Tashiro T Barcroft C Localization of the genes encoding the melanocortin-2 (adrenocorticotropic hormone) and melanocortin-3 receptors to chromosomes 18p11.2 and 20q13.2–q13.3 by fluorescence in situ hybridization Genomics 1993 18 166 167 8276410
- Mountjoy KG Wong J Obesity, diabetes and functions for proopiomelanocortin-derived peptides Mol Cell Endocrinol 1997 128 171 177 9140088
- Gantz I Konda Y Tashiro T Molecular cloning of a novel melanocortin receptor J Biol Chem 1993 268 8246 8250 8463333
- Ollmann MM Wilson BD Yang YK Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein Science 1997 278 135 138 9311920
- Bagnol D Lu XY Kaelin CB Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain J Neurosci 1999 19 RC26 10479719
- Jegou S Boutelet I Vaudry H Melanocortin-3 receptor mRNA expression in pro-opiomelanocortin neurones of the rat arcuate nucleus J Neuroendocrinol 2000 12 501 505 10844578
- Cowley MA Smart JL Rubinstein M Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus Nature 2001 411 480 484 11373681
- Marks DL Hruby V Brookhart G The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides 2006 27 259 264 16274853
- Li WD Joo EJ Furlong EB Melanocortin 3 receptor (MC3R) gene variants in extremely obese women Int J Obes Relat Metab Disord 2000 24 206 210 10702772
- Schalin-Jantti C Valli-Jaakola K Oksanen L Melanocortin-3-receptor gene variants in morbid obesity Int J Obes Relat Metab Disord 2003 27 70 74 12532156
- Lee YS Poh LK Loke KY A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity J Clin Endocrinol Metab 2002 87 1423 1426 11889220
- Rached M Buronfosse A Begeot M Inactivation and intracellular retention of the human I183N mutated melanocortin 3 receptor associated with obesity Biochim Biophys Acta 2004 1689 229 234 15276649
- Tao YX Functional characterization of novel melanocortin-3 receptor mutations identified from obese subjects Biochim Biophys Acta 2007 1772 1167 1174 17964765
- Calton MA Ersoy BA Zhang S Association of functionally significant Melanocortin-4 but not Melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study Hum Mol Genet 2009 18 1140 1147 19091795
- Mountjoy KG Mortrud MT Low MJ Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain Mol Endocrinol 1994 8 1298 1308 7854347
- Huszar D Lynch CA Fairchild-Huntress V Targeted disruption of the melanocortin-4 receptor results in obesity in mice Cell 1997 88 131 141 9019399
- Yen TT Gill AM Frigeri LG Obesity, diabetes, and neoplasia in yellow A(vy)/– mice: ectopic expression of the agouti gene FASEB J 1994 8 479 488 8181666
- Marks DL Ling N Cone RD Role of the central melanocortin system in cachexia Cancer Res 2001 61 1432 1438 11245447
- Fathi Z Iben LG Parker EM Cloning, expression, and tissue distribution of a fifth melanocortin receptor subtype Neurochem Res 1995 20 107 113 7739752
- bdel-Malek ZA Melanocortin receptors: their functions and regulation by physiological agonists and antagonists Cell Mol Life Sci 2001 58 434 441 11315190
- Chen W Kelly MA Opitz-Araya X Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides Cell 1997 91 789 798 9413988
- Hatta N Dixon C Ray AJ Expression, candidate gene, and population studies of the melanocortin 5 receptor J Invest Dermatol 2001 116 564 570 11286624
- Chhajlani V Muceniece R Wikberg JE Molecular cloning of a novel human melanocortin receptor Biochem Biophys Res Commun 1996 218 638 8561809
- Chagnon YC Chen WJ Perusse L Linkage and association studies between the melanocortin receptors 4 and 5 genes and obesity-related phenotypes in the Quebec Family Study Mol Med 1997 3 663 673 9392003
- Watson SJ Akil H Richard CWIII Evidence for two separate opiate peptide neuronal systems Nature 1978 275 226 228 211426
- Jacobowitz DM O’Donohue TL alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain Proc Natl Acad Sci U S A 1978 75 6300 6304 366617
- Cone RD Anatomy and regulation of the central melanocortin system Nat Neurosci 2005 8 571 578 15856065
- Broberger C Johansen J Johansson C The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice Proc Natl Acad Sci U S A 1998 95 15043 15048 9844012
- Shintani M Ogawa Y Ebihara K Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway Diabetes 2001 50 227 232 11272130
- Niswender KD Baskin DG Schwartz MW Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis Trends Endocrinol Metab 2004 15 362 369 15380807
- Challis BG Coll AP Yeo GS Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3–36) Proc Natl Acad Sci U S A 2004 101 4695 4700 15070780
- Kamegai J Tamura H Shimizu T Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression Endocrinology 2000 141 4797 4800 11108296
- Rao TL Kokare DM Sarkar S GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats Pharmacol Biochem Behav 2003 76 417 423 14643840
- Cummings DE Purnell JQ Frayo RS A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans Diabetes 2001 50 1714 1719 11473029
- Tschop M Wawarta R Riepl RL Post-prandial decrease of circulating human ghrelin levels J Endocrinol Invest 2001 24 RC19 21 11434675
- Ariyasu H Takaya K Tagami T Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans J Clin Endocrinol Metab 2001 86 4753 4758 11600536
- Tschop M Weyer C Tataranni PA Circulating ghrelin levels are decreased in human obesity Diabetes 2001 50 707 709 11289032
- Hewson AK Dickson SL Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats J Neuroendocrinol 2000 12 1047 1049 11069119
- Cowley MA Smith RG Diano S The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis Neuron 2003 37 649 661 12597862
- Shaw AM Irani BG Moore MC Ghrelin-induced food intake and growth hormone secretion are altered in melanocortin 3 and 4 receptor knockout mice Peptides 2005 26 1720 1727 16005545
- Wilding JP Ajala MO Lambert PD Additive effects of lactation and food restriction to increase hypothalamic neuropeptide Y mRNA in rats J Endocrinol 1997 152 365 369 9071956
- Clark JT Kalra PS Crowley WR Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats Endocrinology 1984 115 427 429 6547387
- Levine AS Morley JE Neuropeptide Y: a potent inducer of consummatory behavior in rats Peptides 1984 5 1025 1029 6549409
- Zarjevski N Cusin I Vettor R Chronic intracerebroventricular neuropeptide-Y administration to normal rats mimics hormonal and metabolic changes of obesity Endocrinology 1993 133 1753 1758 8404618
- Tiesjema B Adan RA Luijendijk MC Differential effects of recombinant adeno-associated virus-mediated neuropeptide Y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior J Neurosci 2007 27 14139 14146 18094253
- Yang L Scott KA Hyun J Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance J Neurosci 2009 29 179 190 19129396
- Garcia de YE Li S Fournier A Regulation of proopiomelanocortin gene expression by neuropeptide Y in the rat arcuate nucleus Brain Res 1995 674 112 116 7773678
- Abid K Rochat B Lassahn PG Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3–35: a new peptide generated by plasma kallikrein J Biol Chem 2009 284 24715 24724 19620246
- Watson BJr Nowak NJ Myracle AD The human angiotensinase C gene (HUMPCP) maps to 11q14 within 700 kb of D11S901: a candidate gene for essential hypertension Genomics 1997 44 365 367 9325062
- Wang L Feng Y Zhang Y Prolylcarboxypeptidase gene, chronic hypertension, and risk of preeclampsia Am J Obstet Gynecol 2006 195 162 171 16681991
- Yan ZHANG Xiu-mei Hong Hou-xun E112D polymorphism in the prolylcarboxypeptidase gene is associated with blood pressure response to benazepril in Chinese hypertensive patients Chinese Med J 2009 122 2461 2465
- Arai K Shibasaki T [Genetic abnormalities of regulatory mechanism of appetite] Nippon Rinsho 2001 59 449 455 11268592
- Ichiyama T Sakai T Catania A Systemically administered alpha-melanocyte-stimulating peptides inhibit NF-kappaB activation in experimental brain inflammation Brain Res 1999 836 31 37 10415402