Abstract
Objective
The purpose of the trial was to examine the effects of amantadine, a N-methyl-D-aspartate (NMDA) antagonist, on the oral glucose tolerance test (OGTT) plus insulin, glucagon and neurotransmitters circulating levels. Previous findings showed that hyperinsulinism and type 2 diabetes are positively associated with neural sympathetic and adrenal sympathetic activities, respectively. These peripheral sympathetic branches depend on the pontine (A5-noradrenergic) and the rostral ventrolateral (C1-adrenergic) medullary nuclei. They are excited by glutamate axons which act at NMDA postsynaptic receptors.
Research design and methods
One OGTT plus placebo and one OGTT plus oral amantadine test were carried out two weeks apart in 15 caucasic normal voluntary humans. Noradrenaline, adrenaline, dopamine, plasma-free serotonin, platelet serotonin, glucose, glucagon, and insulin were measured throughout the 180-minute testing period.
Results
Maximal reductions of plasma glucose and glucagon plus exacerbated insulin rises were significantly greater throughout the oral glucose plus amantadine test than those registered throughout the oral glucose plus placebo challenge. The above findings were paralleled by greater than normal noradrenaline/adrenaline plasma ratio increases. In addition, maximal reductions of the platelet serotonin and plasma serotonin circulating values contrasted with the normal rises of these parameters, always registered during the glucose load plus placebo challenge.
Conclusion
This study supports the theory that amantadine might be a powerful antidiabetic tool and could be added to the therapeutic arsenal against type 2 diabetes.
Introduction
The interaction between alpha- and beta-islet cells is responsible for plasma glucose homeostasis.Citation1 Predominance of the former and the latter are responsible for hyperglycemiaCitation2–Citation4 and hypoglycemia,Citation4,Citation5 respectively. In addition, this binomial peripheral crosstalk is modulated by several physiological factors which include both peripheral neuroendocrine and central nervous system (CNS) mechanisms.Citation6,Citation7 However, this physiological stability can be disrupted by many stressor factors such as exercise and restraint,Citation8,Citation9 neuropharmacological agents such as tianeptine (enhancer of serotonin uptake),Citation10 doxepin (inhibitor of serotonin uptake),Citation11 or dopaminergic-blocking agents (such as sulpiride, haloperidol).Citation12,Citation13 Quantitative and/or qualitative changes of the above factors might lead to hyper- or hypoglycemic disorders. Most research work dealing with this issue has investiged the peripheral mechanisms involved in the alpha-cell versus beta-cell crosstalk (endocrine, gastrointestinal, and neuroautonomic); however, little research work has been devoted to the understanding of the CNS circuitry responsible for the alpha- versus beta-cells interaction and/or CNS versus peripheral autonomic nervous system (ANS) parameters. The investigation of such crosstalk might redound in findings able to lead to the outlining of adequate neuropharmacological therapeutical strategies addressed to normalize the peripheral disorders responsible for the alpha-cells ↔ beta-cells imbalance.
Evidence have demonstrated that insulin excites neural sympathetic activityCitation14,Citation15 while glucagon excites adrenal sympathetic activity.Citation16–Citation18 In addition, both sympathetic branches of the peripheral ANS depend on the A5 pontomedullary nucleus that releases noradrenaline (NA)Citation19 and the C1 medullary nuclei which secrete adrenaline (Ad).Citation20–Citation24 Furthermore, both CNS nuclei interchange direct inhibitory axons whose neurotransmitters act at post-synaptic alpha-2 receptors, which crowd both nuclei.Citation18–Citation25 Finally, experimental evidence has demonstrated that secretion of both peripheral hormones (insulin and glucagon) would depend on the interaction between two opposite neuroendocrine circuits. According to the above, we decided to investigate the effect of amantadine, a glutamic acid (N-methyl-D-aspartate [NMDA]) antagonist, which inhibits the C1 (Ad) but not the A5 (NA) nuclei, and provokes absolute reduction of plasma adrenaline concentration when administered to normal subjects during fasting conditions.Citation26–Citation31
Methods
One oral glucose tolerance test (OGTT) plus placebo and one OGTT plus oral amantadine (100 mg) test were carried out two weeks apart in 15 caucasic normal voluntary humans (seven males and eight females), whose ages ranged from 25 to 58 years (mean ± standard error of mean [SEM] = 38.4 ± 7.2) (–). The study was conducted in accordance with the guidelines in the Declaration of Helsinki. Written informed consent was obtained after the purpose, nature and potential risks had been explained to the subjects. The experimental protocol was approved by the Ethical Committee of FUNDAIME. All subjects were within 10% of ideal body weight, and none had undergone abdominal surgery or were taking any medications. None of the subjects had physical or psychiatric illness. Subjects were rated on a modified Hamilton Depression Rating Scale for depression and all of them completed the self-rating Beck Depression InventoryCitation11 in order to discard subjects with depression. Pregnancy, lactation, smoking and/or alcoholism factors also excluded subjects.
Table 1 Correlations found during the oral glucose tolerance + placebo test performed in 15 normal voluntary human (seven males and eight females)
Table 2 Correlations found during the oral glucose tolerance + amantadine test performed in 15 normal voluntary human (seven males and eight females)
Table 3 Changes in SBP, DBP, and HR induced in 15 normal voluntary healthy humans (eight males and seven females) when one OGTT plus placebo and one OGTT plus amantadine tests were carried out two weeks apart
Analytical methods
The assessment of circulating neurotransmitters throughout the OGTT + placebo test carried out according to the present protocol ratified other previously published research studies.Citation10,Citation11 Noradrenaline, adrenaline, dopamine, plasma free serotonin (f5-HT), platelet serotonin (p5-HT), glucose, glucagon, and insulin were measured throughout the 180-minute testing period. For all parameters, the samples were assayed in duplicate and all determinations were made simultaneously. We used reverse-phase, ion-pair high performance liquid chromatography with electrochemical detection for the detection of monoamines. Optimization of chromatographic conditions and attainment of adequate quantification parameters allowed us to maximize sensitivity and reproducibility.
All tests were performed on recumbent subjects after 14 hours of fasting. A heparinized venous catheter was inserted into a forearm vein at least 30 minutes before beginning the tests. Blood samples were collected at 0, 30, 60, 90, 120, and 180 minutes. Each subject drank a 30% glucose solution (1 g/kg of ideal body weight) plus placebo or an oral dose of amantadine (100 mg = one capsule). Blood for catecholamines and serotonin assays was transferred to plastic tubes, each containing 20 mg of EDTA plus 10 mg of sodium bisulphite/ml of solution. The tubes were carefully inverted and placed on ice. The blood was promptly centrifuged at 600 rpm for 15 minutes at 4°C in order to obtain platelet-rich plasma. Two milliliters of platelet-rich plasma, obtained for determination of platelet serotonin (p5-HT), were taken and stored at −70°C until assayed. The remaining blood was again centrifuged at 7,000 rpm. The supernatant, platelet-poor plasma, was divided into four portions for determination of insulin, glucagon, catecholamines and free serotonin (f5-HT), after which all portions were stored at −70°C until assayed. Plasma glucose samples were immediately processed.
Reagents and standards
Noradrenaline, adrenaline, dopamine, serotonin creatinine sulphate, dihydroxybenzylamine, sodium octyl sulphate, dibutylamine, acid-washed aluminium oxide, Na2HPO4, citric acid and EDTA were purchased from Sigma-Aldrich (St. Louis, MO, USA). Microfilters were purchased from Whatman Inc. (Florham Park, NY, USA) through Merck S.A. (Caracas, Venezuela). Acetonitrile and 2-propanol were obtained from Merck, S.A. Glass-distilled water was de-ionized and filtered through a Milli-Q reagent grade water system (Millipore, Bedford, MA, USA). Solvents were filtered through a 0.2 μm Millipore filter and were vacuum de-aerated. Standard solutions (1 mmo1/L) were prepared in 0.1 mo1/L perchloric acid and diluted to the desired concentration.
Equipment
Liquid chromatography was performed using Waters 515 HPLC pump (Waters Corporation, Milford, MA. USA) equipped with a Rheodyne valve injector 7125i, which was fitted with a 50 μL sample loop (Rheodyne; Berodine, Berkeley, CA, USA). A 15 cm × 4.6 mm inner diameter Discovery C18 column packed with octadecylsilane 5 μm particles was preceded by a column prefilter of 2 μm porosity, both from Supelco/Sigma-Aldrich. The detection system was a Waters 460 Electrochemical Detector (Waters Corporation). The potential of the working electrode (glass carbon) was set at + 0.61 V versus the Ag–AgCl reference electrode for the detection of catecholamines and 0.70 V versus the Ag–AgCl for the detection of indolamines. The chromatograms were registered and quantified with Empower software (Waters Corporation). The results were corrected for the volume of EDTA added.
Analytical assays
Plasma catecholamines
The assay was performed by extraction of the catecholamines onto 20 mg of alumina followed by their elution with 200 μL of 1.0 mo1/L HClO4 using regenerated cellulose microfilters 0.2 μm pore size (Whatman Inc.). We calibrated the instrument with standard plasma. After incubation with acid-washed aluminum oxide, a plasma pool of free catecholamines was processed similarly to plasma samples, but 20 μL of a standard solution of noradrenaline, adrenaline, and dopamine (50, 25, and 25 ng/mL, respectively) were added to the plasma pool. Both the standard plasma and the sample plasma were supplemented with 20 μL of internal standard (100 ng/mL of dihydroxybenzylamine). The mobile phase was KH2PO4 6.8045 g/L, EDTA 0.100 g/L and di-N-butylamine 100 μl/L. Sodium octyl sulphate was added as ion-pair agent in a concentration of 0.6125 g/L with the pH adjusted to 5.6. The flow rate was 0.400 mL/min. The sensitivity of this method for noradrenaline, adrenaline and dopamine was 6.4, 5.8, and 2.0 pg/mL, respectively. The intraassay coefficients of variation were 2.8%, 4.0%, and 4.0 %, respectively. The inter-assay coefficients of variation were 6.7%, 4.5%, and 4.3%, respectively.
Plasma indolamines
After sonication of platelet-rich plasma to disrupt the platelets (Ultrasonic Liquid Processor, model 385; Heat Systems Ultrasonics Inc., Farmingdale, NY, USA), both platelet-rich and platelet-poor plasma were processed in the same way: 200 μL of 3.4 mol/L perchloric acid and 50 μl of 5-hydroxy-tryptophan solution (114.5 μg/mL) as internal standard, were added to 1 mL of plasma vortexed and centrifuged at 10,000 rpm for 15 minutes at 4°C. The supernatant was filtered through a 0.22 μm membrane (Millipore) and 10 μL was injected into the column. Calibration runs were generated by spiking blank platelet-poor plasma with 50 μL of a solution containing 5-HT (10 μg/mL) and 50 μL of 5-hydroxy-tryptophan (114.5 μg/mL). This standard plasma was processed in the same manner as the samples. The mobile phase was citric acid 3.8424 g/L, sodium acetate 4.1015 g/L, EDTA 0.100 g/L, di-N-butylamine 100 μl/L, and 30 ml/L of 2-propanol. Sodium octyl sulphate was added as ion-pair agent in a concentration of 4.25 mg/L with a pH of 5.0. The flow rate was 0.610 mL/min. The sensitivity of the method for serotonin was 0.1 ng/mL. The intra-assay coefficients of variation for p5-HT and f5-HT were 6.2% and 8.7%, respectively.
Plasma insulin
This was determined in duplicate by insulin ELISA kit (Cosmo Bio, Carlsbad, CA, USA).
Plasma glucagon
Plasma glucagon was determined by radioimmunoassay (RIA) kit GL-32K (Linco Research; Millipore). In our hands, the intraassay variation was 4.5 ± 1.2 and 11.1 ± 2.8 for the interassay variation.
Plasma glucose
Plasma glucose concentration was measured in duplicate with a glucose oxidase methodCitation32 using Glucose Analyzer II (Beckman Instruments, Fullerton, CA, USA).
Statistical methods
Results are presented as the mean ± SEM. Statistical significance was set at *P < 0.05; **P < 0.02; ***P < 0.01 (versus 0 min value). Multivariate ANOVA with repeated measurements, paired t test, and correlation coefficients (exploratory factor analysis) were used. dBase Stats™ (Ashton Tate, Chicago, IL, USA) and StatView SE + Graphics (SAS Institute, Cary, NC, USA) were used for statistical analyses.
Results
Oral glucose + placebo test
Suppression of plasma glucagon concentration was observed throughout the test with a nadir of 40.0 ± 5.0 pg/mL at the 90-minute period. Maximal rise of glucose was registered at the 30-minute period, whereas minimal mean values were reached at the 180-minute period (). Noradrenaline and platelet serotonin circulating parameters showed significant and sustained rises which paralleled insulin increases and opposed plasma glucose reductions. Significant reduction of adrenaline values were registered throughout the test. Dopamine did not show significant changes (). In addition to the above, although both noradrenaline and platelet serotonin concentrations were raised after the oral glucose load, the noradrenaline/p5-HT ratio showed significant falls throughout the OGTT (). No significant f5-HT changes were registered throughout the test. All of our subjects fell asleep and displayed rapid eye movements (REM) during OGTT, especially accentuated at the 90-minute and 180-minute periods.
Figure 1 The addition of an oral dose of amantadine (100 mg) to an oral glucose load potentiated both the glucose and glucagon reductions and increased postprandial insulin levels in 15 normal healthy subjects (seven males and eight females), whose ages ranged from 25 to 58 years (mean ± SEM = 38.4 ± 7.2).
Abbreviation: SEM, standard error of mean.
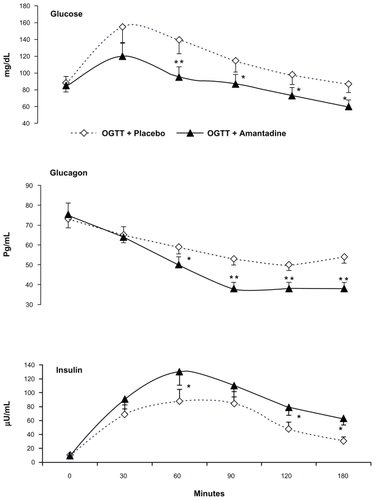
Figure 2 The addition of an oral dose of amantadine (100 mg) to an oral glucose load enhanced the normal noradrenaline and dopamine rises, always observed throughout the OGTT + placebo test. Conversely, maximal reduction of adrenaline opposed the former rises.
Abbreviations: OGTT, oral glucose tolerance test; SEM, standard error of mean.
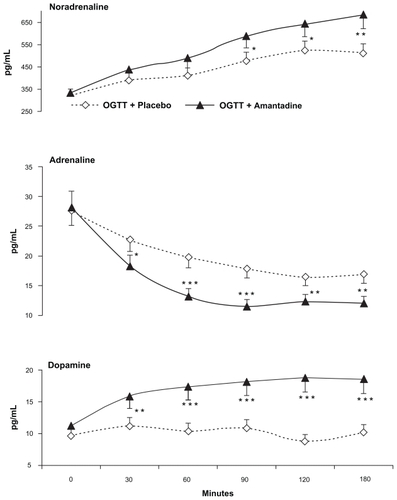
Figure 3 The insulin/glucagon ratio as well as the noradrenaline/adrenaline ratio showed paralleled increases throughout the oral glucose tolerance test when 100 mg of oral amantadine was given to 15 normal healthy subjects (seven males and eight females), whose ages ranged from 25 to 58 years (mean ± SEM = 38.4 ± 7.2).
Abbreviation: SEM, standard error of mean.
Oral glucose + amantadine test
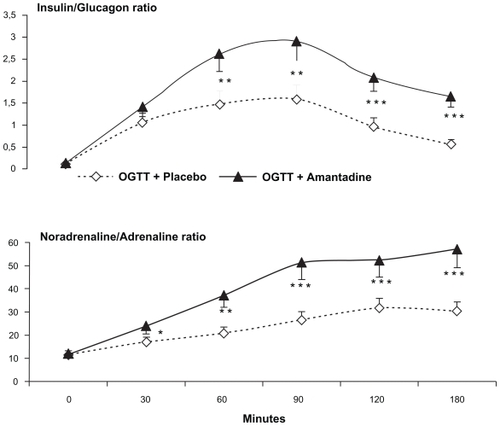
Subjects showed drowsiness but not REM sleep throughout the test. Adrenaline showed maximal and sustained decreases throughout the post-amantadine periods. Conversely, significant and sustained noradrenaline plus dopamine rises opposed the adrenaline falls. In addition, abrupt fall of f5-HT and p5-HT parameters were also registered throughout the OGTT + amantadine challenge ().
Figure 4 Both platelet serotonin (p5-HT) and plasma serotonin (f5-HT) showed maximal decreases throughout the test when an oral dose of amantadine (100 mg) was added to an oral glucose load. These findings contrast with the significant rises of both parameters observed throughout the oral glucose load + placebo challenge.
Abbreviation: SEM, standard error of mean.
Maximal and sustained reductions of both plasma glucose and glucagon were observed. Small and sustained but significant insulin rises were registered throughout post-glucose + amantadine periods (). Maximal fall of systolic blood pressure and heart rate, were also observed throughout the post-drug + glucose periods.
Discussion
The results obtained from the present study demonstrated that the addition of a small dose of amantadine (100 mg) to the OGTT was able to enhance the insulinogenic plus hypoglycemic effects normally triggered by the ingested sugar. These effects were paralleled by the enhancement of the normal neural sympathetic overactivity, always registered throughout the OGTT as well as by the accentuation of the fall of the adrenaline plus glucagon plasma levels that occurs after an oral glucose load. Both insulin and glucagon cross the blood–brain barrier and excite the A5 (NA)Citation14,Citation17,Citation33,Citation34 and the C1 (Ad)Citation16,Citation33–Citation36 pontomedullary nuclei, respectively. The former is responsible for the peripheral neural (noradrenergic) sympathetic activity whereas the latter excites adrenal sympathetic glands, which secrete 80% of adrenaline. The fact that glutamatergic axons innervate and excite the C1 (Ad) but not the A5 (NA) neurons (by acting at NMDA receptors) explains why amantadine (a NMDA antagonist) interferes with the adrenal but not with the neural sympathetic activity. With respect to this, experimental evidences obtained from our laboratory, demonstrated that a small dose of amantadine (administered without glucose) provokes absolute minimization of the adrenaline plasma concentration.Citation24,Citation29
In addition to all the above, some other factors should be considered in order to understand the ability by amantadine to annul the glucagon-induced excitatory effect at the CNS C1 (Ad) + peripheral adrenal sympathetic axis, which would explain its insulinogenic + hypoglycemic effects. For instance, the drug was able to provoke maximal reduction of both plasma serotonin and platelet serotonin concentration. This circulating indolamine exerts an inhibitory effect at the beta-cell level and in addition is able to excite alpha-cells, which secrete glucagon.Citation37–Citation41 Furthermore, plasma serotonin is able to excite the adrenal glands,Citation39,Citation42 directly. Thus, the reduction of plasma serotonin plus platelet serotonin circulating values registered in the present study should be taken into account in order to understand the hypoglycemic effect triggered by amantadine, when this drug was added to an oral glucose load.
The minimization of the circulating serotonin concentration (plasma serotonin + platelet serotonin) registered in the present study might find an explanation in the overwhelming neural sympathetic over adrenal sympathetic activity triggered by amantadine. It should be remembered that the release of serotonin from the enterochromaffin cells depends on the excitatory parasympathetic drive which should be interfered by the enhancement of the neural sympathetic activity (noradrenaline/adrenaline ratio) registered in this study.Citation40,Citation43–Citation48 The minimization of the plasma acetylcholine concentration, which competes with serotonin for the platelet uptake, fits well with the maximal reduction of the plasma serotonin values registered during the post amantadine periods. This postulation is in accordance with the maximal fall of systolic blood pressure and heart rate, observed throughout the post-drug periods. Both cardiovascular parameters are positively correlated with the peripheral adrenal sympathetic activity greatly minimized in the present study because of the inhibition by amantadine of the C1 (Ad) medullary nuclei.
In addition to the above, it has been shown that other drugs like tianeptine, which also minimizes plasma serotonin concentration is able to enhance plasma insulin secretion.Citation10 This fact should be attributed to the direct inhibitory effect exerted by this indolamine at the beta-cell level.
The enhancement of the noradrenaline/adrenaline plasma ratio registered in the present study indicates that the overwhelming neural sympathetic activity annulled the peripheral adrenal sympathetic branch. This peripheral neuroautonomic profile depends on the absolute predominance of the A5 (NA) pontomedullary nucleus over the C1 (Ad) medullary nuclei.Citation24,Citation49 The above CNS A5 (NA) versus C1 (Ad) predominance which redounded in the peripheral neural sympathetic plus beta-cells hyperactivity merits some additional comments. With respect to this, it should be known that sympathetic nerves innervate and excite alpha-cells by acting at alpha-2 receptors but not beta-cells.Citation20–Citation22,Citation50 However, glucagon released from the former excites beta-cells which send GABA-inhibitory drive to alpha-cells.Citation41 In addition to the above, it should be remembered that both adrenaline and glucagon are positively correlated with glucogenolysis and hyperglycemia both of which are absolutely minimized in the present research study.
According to all the above, we postulate that the postprandial hypoglycemic + insulinogenic effects provoked by the addition of a small dose of amantadine to the OGTT depends on peripheral + central nervous system mechanisms. The former should be attributed to the minimization of the inhibitory effect of circulating serotonin (plasma serotonin + platelet serotonin) plus the suppression of the glucogenolytic effect by adrenaline. At the central nervous system the above phenomena would depend on the inhibition of the C1 (Ad) medullary nuclei plus the absolute predominance of the A5 (NA) nucleus. Both insulin and glucagon cross the blood brain barrier and excite the A5 (NA) and the C1 (Ad) pontomedullary nuclei, respectively thus, the inhibition of the latter triggered by amantadine provoked the abrupt suppression of the adrenal glands secretion which favored the absolute predominance of the A5 (NA) neural sympathetic branch that resulted in the enhancement of insulin/glucagon ratio, registered in this study. This postulation receives definite support from overwhelming evidence quoted in several review articles and books.Citation24,Citation29,Citation41,Citation51,Citation52
Summarizing all the above, it is possible to postulate that the hypoglycemic + hypoglucagonemic + insulinogenic effects triggered by the addition of amantadine to an oral glucose load would depend of both peripheral plus central nervous system mechanisms. The former should be associated to the minimization of the alpha-cells + adrenal glands secretion whereas the latter should be associated with the inhibition of the C1 (Ad) plus the disinhibition of the A5 (NA), responsible for the peripheral adrenal sympathetic and neural sympathetic branches axis, respectively.
Disclosures
The authors report no conflicts of interest in this work.
References
- GökeBIslet cell function: alpha and beta cells – partners towards normoglycaemiaInt J Clin Pract Suppl20081592718269435
- FanelliCGPorcellatiFRossettiPBolliGBGlucagon: the effects of its excess and deficiency on insulin actionNutr Metab Cardiovasc Dis200616Suppl 1528534
- CryerPEGlucagon and hyperglycaemia in diabetesClin Sci (Lond)2008114958959018197838
- BanerjiMAImpaired beta-cell and alpha-cell function in African-American children with type 2 diabetes mellitus –“Flatbush diabetes”Pediatr Endocrinol Metab200215Suppl 1493501
- GellingRWVuguinPMDuXQPancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and massAm J Physiol Endocrinol Metab20092973E695E70719602585
- RizzaRACryerPEGerichJERole of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemiaJ Clin Invest1979641627136413
- LamCKChariMLamTKCNS regulation of glucose homeostasisPhysiology (Bethesda)20092415917019509126
- LechinFvan der DijsBBenaimMStress versus depressionProg Neuropsychopharmacol Biol Psychiat1996206899950
- LechinFColl-GarciaEvan der DijsBBentolilaAPeñaFRivasCEffects of captivity on glucose tolerance in dogsExperientia1979357876877573210
- LechinFvan der DijsBPardey-MaldonadoBBaezSLechinMEEffect of tianeptine on glucose tolerance with insulin secretion in human: Potential anti-diabetic effect of the drugOpen Neuroendocrinol J200921019
- LechinFvan der DijsBLechinADoxepin therapy for postprandial symptomatic hypoglycemic patients neurochemical, hormonal and metabolic disturbancesClin Sci19918043733841673882
- LechinFvan der DijsBHaloperidol and insulin releaseDiabetologia1981201787009289
- LechinFColl-GarciaEvan der DijsBBentolilaAPeñaFRivasCThe effects of dopaminergic blocking agents on the glucose tolerance test in six humans and six dogsExperientia1979357886887477842
- ChristensenNJHilstedJEggerMTeuscherAFrierBMHepburnDAPlasma noradrenaline, human insulin, and hypoglycaemiaLancet1989334867412681269
- LechinFvan der DijsBLechinMEffects of an oral glucose load on plasma neurotransmitters in humans: involvement of REM sleep?Neuropsychobiology1992261–24111361970
- AbdelmelekHFechtaliTFilali-ZegzoutiYResponsiveness of plasma catecholamines to intracerebroventricular injection of glucagon in Muscovy ducklingsJ Neural Transm2001108779380111515745
- FisherSJBrüningJCLannonSKahnCRInsulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemiaDiabetes20055451447145115855332
- DampneyRAGoodchildAKRobertsonLGMontgomeryWRole of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological studyBrain Res198224922232356128058
- ByrumCEGuyenetPGAfferent and efferent connections of the A5 noradrenergic cell group in the ratJ Comp Neurol198726145295422440916
- LoewyADFranklinMFHaxhiuMACNS monoamine cell groups projecting to pancreatic vagal motor neurons: a transneuronal labeling study using pseudorabies virusBrain Res19946381–22482607515322
- LoewyADMarsonLParkinsonDPerryMASawyerWBDescending noradrenergic pathways involved in the A5 depressor responseBrain Res19863861–23133243096495
- LoewyADHaxhiuMACNS cell groups projecting to pancreatic parasympathetic preganglionic neuronsBrain Res199362023233307690304
- ElenkovIJWilderRLChrousosGPViziESThe sympathetic nerve – an integrative interface between two supersystems: the brain and the immune systemPharmacol Rev200052459563811121511
- LechinFvan der DijsBCentral nervous system circuitries underlying two types of peripheral autonomic nervous system disordersOpen Neurosci J200824150
- MaiorovDNWiltonERBadoerEPetrieDHeadGAMalpasSCSympathetic response to stimulation of the pontine A5 region in conscious rabbitsBrain Res199981522272369878751
- RossCARuggieroDAParkDHTonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressinJ Neurosci1984424744946699683
- MorrisonSFCallawayJMilnerTAReisDJRostral ventrolateral medulla: a source of the glutamatergic innervation of the sympathetic intermediolateral nucleusBrain Res199156211261351724740
- ZhouWFuLWTjen-A-LooiSCGuoZLLonghurstJCRole of glutamate in a visceral sympathoexcitatory reflex in rostral ventrolateral medulla of catsAm J Physiol Heart Circ Physiol20062913H1309H131816632546
- LechinFvan der DijsBCrosstalk between the autonomic nervous system and the central nervous system: Mechanistic and therapeutic considerations for neuronal, immune, vascular, and somatic based diseasesMaieseKNeurovascular Medicine: Pursuing Cellular Longevity for Healthy AgingNew York, NYOxford University Press2009101152
- LiuRHFungSJReddyVKBarnesCDLocalization of glutamatergic neurons in the dorsolateral pontine tegmentum projecting to the spinal cord of the cat with a proposed role of glutamate on lumbar motoneuron activityNeuroscience19956411932087708205
- GerendaiIHalászBCentral nervous system structures connected with the endocrine glands. findings obtained with the viral transneuronal tracing techniqueExp Clin Endocrinol Diabetes2000108638939511026751
- KadishAHLittleRLSternbergJCA new and rapid method for the determination of glucose by measurement of rate of oxygen consumptionClin Chem196814116131
- RoweJWYoungJBMinakerKLStevensALPallottaJLandsbergLEffect of insulin and glucose infusions on sympathetic nervous system activity in normal manDiabetes19813032192257009270
- HegedüsLChristensenNJSestoftLAbnormal regulation of sympathetic nervous activity and heart rate after oral glucose in type 1 (insulin-dependent) diabetic patientsDiabetologia19832532422466642090
- SunMKCentral neural organization and control of sympathetic nervous system in mammalsProg Neurobiol19954731572338719915
- TanakaMMatsumotoYMurakamiTHisaYIbataYThe origins of catecholaminergic innervation in the rostral ventromedial medulla oblongata of the ratNeurosci Lett1996207153568710209
- JacobyJHBryceGFThe acute pharmacologic effects of serotonin on the release of insulin and glucagon in the intact ratArch Int Pharmacodyn Ther19782352254270310665
- AdeghateEPoneryASPallotDParvezSHSinghJDistribution of serotonin and its effect on insulin and glucagon secretion in normal and diabetic pancreatic tissues in ratNeuro Endocrinol Lett199920531532211460094
- de LeivaATanenbergRJAndersonGGreenbergBSenskeBGoetzFCSerotoninergic activation and inhibition: effects on carbohydrate tolerance and plasma insulin and glucagonMetabolism1978275511520147982
- LechinFvan der DijsBLechinMPlasma neurotransmitters throughout an oral glucose tolerance test in essential hypertensionClin Exp Hypertens19931512092408096777
- LechinFvan der DijsBCentral nervous system circuitry involved in the hyperinsulinism syndrome [review]Neuroendocrinology200684422223417167239
- CarvalhoFBarrosDSilvaJHyperglycemia induced by pharmacological activation of central serotonergic pathways depends on the functional integrity of brain CRH system and 5-HT3 receptorsHorm Metab Res200537848248816138260
- TobeTEnterochromaffin cells and carcinoid syndromeNippon Rinsho19743247457504858960
- ReynoldsDJLeslieRAGrahame-SmithDGHarveyJMLocalization of 5-HT3 receptor binding sites in human dorsal vagal complexEur J Pharmacol198917411271302612576
- LechinFvan der DijsBNeuropharmacological therapy of carcinoid syndromeNeuroendocrinology200581313713815976511
- LechinFvan der DijsBIntestinal pharmacomanometry and glucose tolerance: evidence for two antagonistic dopaminergic mechanisms in the humanBiol Psychiatry198116109699717306619
- LechinFvan der DijsBGlucose tolerance, non-nutrient drink and gastrointestinal hormonesGastroenterology19818012167004993
- LechinFvan der DijsBOrozcoBCholecystokinin (CCK) and secretin and pancreatic secretion of insulin and glucagonDig Dis Sci200247112422242312452373
- LechinFvan der DijsBCentral nervous system circuitry and peripheral neural sympathetic activity responsible for essential hypertensionCurr Neurovasc Res20063430732517109626
- SaitoMSaitohTInoueSAlpha 2-adrenergic modulation of pancreatic glucagon secretion in ratsPhysiol Behav1992516116511711353630
- LechinFvan der DijsBHernandez-AdrianGDorsal Raphe (DR) vs Median Raphe (MR) serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: Relevance for neuropharmacological therapyProg Neuropsychopharmacol Biol Psychiatry200630456558516436311
- LechinFvan der DijsBLechinMENeurocircuitry and Neuroautonomic Disorders: Reviews and Therapeutic StrategiesBasel, SwitzerlandKarger2002