Abstract
Telmisartan, a selective angiotensin II type 1 receptor blocker (ARB), has been investigated in many trials, in particular, in order to assess its antihypertensive effect in various situations and its ability to protect organs susceptible to hypertension. In addition to its antihypertensive properties, it has positive metabolic and vascular effects (partly because of its sustained action). Several large-scale trials have focused on the effect of telmisartan on cardiovascular morbidity and mortality, including comparisons of that with an angiotensin-converting enzyme inhibitor in subjects at high vascular risk. Telmisartan was used in the largest ARB research programme (the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial [ONTARGET] and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease [TRANSCEND] trial).
Blood pressure and vascular risk management
Hypertension is an extremely common pathology worldwide, and its effects on certain organs, such as the heart, the arteries, the kidneys, and the brain or retina, are well characterized. Many trials have shown that hypertension is a continuous, independent risk factor for cardiovascular (CV) disease. Once a patient has been diagnosed with hypertension, the practitioner should assess the cause and effects of the hypertension before initiating the pharmaceutical treatment.Citation1 It is also important to assess the overall CV risk of the patient with hypertension, which is evaluated on the basis of not only blood pressure (BP) readings but also other risk factors such as age, gender, dyslipidemia, diabetes mellitus, smoking, and excess abdominal weight or obesity. Risk should also be estimated using specific markers, such as albuminuria or increased plasma creatinine, left ventricular hypertrophy (LVH), and subclinical vascular damage, and preexisting CV, cerebrovascular, and renal pathologies are also taken into account.Citation2 Once overall CV risk has been assessed, it should be taken into account for planning the treatment strategy, and all of the following variables can be adjusted: initiation time; therapeutic class and even the specific molecule used; dosage; monotherapy or combination therapy from the outset; and prescription of a lipid-lowering drug, an antidiabetic drug, or an antiplatelet drug. In all cases, it is essential to advise the patient about diet and lifestyle factors.
Patient adherence: monotherapy vs combination therapy
Whether first-line treatment for hypertension should be based on a single drug or a combination will not be addressed here. However, once antihypertensive treatment is indicated, everything must be done to ensure that it is as effective as possible, meaning that individual BP targets must be met and the regimen must be tolerated. The patient’s compliance is dependent on both these parameters. If the BP targets are reached, especially if they are reached quickly, and if the treatment is well tolerated, compliance is more likely. Another significant factor is the number of tablets to be taken by the patient each day. In 18,806 newly diagnosed patients with hypertension older than 35 years, significant positive correlation was shown between combination antihypertensive therapy and compliance.Citation3 In the same trial, good compliance was associated with a 38% decrease in the risk of CV events when compared with poor compliance. These findings indicate that well-tolerated treatments that are effective at lowering the BP and preventing CV events are the way forward, as are fixed combinations in cases of multiple antihypertensive therapy. However, the optimal drug treatment management and education with the aim of reducing CV risk comes at a price. A recent trial showed that the most effective approaches to improving compliance with antihypertensive (and also lipid-lowering) therapy are intensive and multifaceted and are therefore likely to be expensive.Citation4
Pharmacology and the effect of telmisartan on biological parameters and the endothelium
Pharmacology
The renin–angiotensin–aldosterone system (RAAS) is involved in most of CV, cerebral, and renal problems. Its activation is an adaptive mechanism, but it can become overactive, leading to hypertension, heart failure, and impaired glomerular function. Angiotensin II-mediated activation of the angiotensin II type 1 (AT1) receptor has numerous effects, such as vasoconstriction, sodium and water retention, vascular and myocardial fibrosis and hypertrophy, and sympathetic nervous system activationCitation5 (). RAAS blockade is used for therapeutic reasons in various branches of medicine, hence the development of aldosterone antagonists, angiotensin-converting enzyme (ACE) inhibitors, AT1 receptor blockers (ARBs), and more recently a direct renin inhibitor (aliskiren).
Table 1 Biological effects of telmisartan
On physiological and pathophysiological levels, ARBs antagonize angiotensin II more effectively than ACE inhibitors, in particular, by suppressing the effect of angiotensin II produced by alternative enzyme pathways such as those based on trypsin, cathepsin, and heart chymase. Furthermore, contrary to ACE inhibitors, ARBs do not suppress, and in fact even increase, angiotensin II subtype 2 (AT2) receptor stimulation, thereby inhibiting vascular growth and apoptosis and promoting cell differentiation and vasodilatation.
Seven active oral ARBs – losartan, irbesartan, valsartan, candesartan, telmisartan, eprosartan, and olmesartan – are currently used in clinical practice. Among these ARBs, telmisartan has an advantageous kinetic profile with a longer half-life, higher lipophilicity, larger distribution volume, and negligible renal clearance.Citation6,Citation7 These properties mean that it can readily enter the tissue compartments by effectively blocking the RAAS (insurmountable angiotensin II inhibition) both systemically and locally. Moreover, telmisartan blocks the AT1 receptor for longer and has a greater binding affinity than other ARBs.Citation6–Citation8 Its affinity for the AT1 receptor is 3,000 times higher than its affinity for the AT2 receptor.Citation9 It also dissociates from AT1 receptors very slowly, which explains its sustained action.Citation8,Citation10 Results of pharmacological trials have led to a recommended dose of 20–80 mg for hypertension.
Biological effects
Many trials have shown that agents that target the RAAS have positive effects on thrombosis, platelet aggregation, and inflammation, although these effects vary from one compound to another. In a 1-month trial conducted on 36 patients with hypertension (16 treated with telmisartan and 20 with the ACE inhibitor perindopril), telmisartan had a better anticoagulant and rheological effects than perindopril, with decreases in the levels of soluble endothelial protein C receptor and fibrinogen.Citation11
Telmisartan has a particularly attractive metabolic profile when compared with other ARBs. It is the strongest stimulator of peroxisome proliferator-activated receptor-γ (PPAR-γ), an intracellular regulator of lipid and glucose metabolism, exerting anti-inflammatory, antioxidative, and antiproliferative effects on vascular cells.Citation12,Citation13 This property explains its beneficial metabolic effects – which are unique among the ARBs – on glucose and triglyceride levels and insulin sensitivity.Citation14 In patients with hypertension with impaired glucose tolerance, telmisartan improves insulin resistance more effectively than losartan.Citation15 It has been clearly demonstrated that ACE inhibitors and ARBs lead to the development of fewer new cases of type 2 diabetes.Citation16 In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), telmisartan 80 mg was as effective as ramipril 10 mg in preventing new-onset diabetes in patients at high vascular risk.Citation17 This is significant when we consider that, in the Heart Outcomes Prevention Evaluation (HOPE) trial, there was a relative risk reduction of 34% for diabetes in the ramipril group when compared with placebo group.Citation18
Recently, a substudy of the Telmisartan versus Ramipril in Renal Endothelium Dysfunction (TRENDY) trial, including 87 patients with type 2 diabetes, showed that telmisartan leads to a significant increase in adiponectin (a peptide hormone produced in adipose tissue) compared with ramipril.Citation19 This is particularly interesting because adiponectin has beneficial effects on atherogenesis, endothelial function, vascular remodeling, inflammation, and insulin resistance.Citation20
Telmisartan and endothelial dysfunction
Endothelial dysfunction is now a recognized marker for CV risk.Citation1 Many conditions, including hypertension and diabetes, are associated with endothelial dysfunction. RAAS blockers appear to be a particularly interesting solution to this vascular anomaly. A recent trial on patients with hypertension with impaired glucose tolerance showed that telmisartan improved endothelial function (brachial flow-mediated dilatation) to a greater extent than did losartan.Citation15 In patients with type 2 diabetes, both telmisartan 40–80 mg and ramipril 5–10 mg significantly increased nitric oxide (NO) activity in the renal endothelium.Citation21 This positive effect on endothelial function goes a long way in explaining why telmisartan improves vascular prognosis.
Antihypertensive efficacy and end-organ protection
Telmisartan: proven efficacy against hypertension
Several trials and a meta-analysis have assessed BP-lowering effect of telmisartan.Citation22 In the meta-analysis, the mean clinical BP reduction observed in 408 patients with grade 1–2 hypertension after 8–12 weeks administration of telmisartan 40–80 mg was −15.5 mmHg for systolic BP and −11.3 mmHg for diastolic BP. Naturally, telmisartan was not only compared to placebo but, more interestingly, also compared to other antihypertensive agents, including ACE inhibitors and ARBs. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 1 and 2 trials compared the efficacy of telmisartan 40–80 mg with that of ramipril 5–10 mg on 24-hour BP readings.Citation23–Citation25 BP decreased more sharply with telmisartan 80 mg than with ramipril 10 mg (–12.7/–8.8 mmHg vs −7.9/–5.4 mmHg, P < 0.0001), especially in subjects with a marked morning BP surge.
Telmisartan 80 mg+ hydrochlorothiazide(HCTZ) was compared with valsartan 160 mg + HCTZ in an 8-week trial of 1,181 patients with hypertension.Citation26 BP decreased more sharply in the telmisartan group than in the valsartan group (–24.6/–18.2 mmHg vs −22.5/–17.0 mmHg, P < 0.05). The antihypertensive effect of telmisartan was greater than not only that of valsartan but also that of losartan.Citation27,Citation28 Thus, in a trial comparing the antihypertensive efficacy of telmisartan 40 or 80 mg + HCTZ and losartan 50 mg + HCTZ in 805 patients with grade 1–2 hypertension, both telmisartan doses were more effective than losartan at normalizing BP in the 6 hours preceding the morning dose.Citation29
Telmisartan has also been shown to be effective against hypertension in overweight and obese patients with diabetes. In the SMOOTH trial conducted on 840 patients who presented these comorbidities, telmisartan 80 mg + HCTZ was more effective than valsartan 160 mg + HCTZ at lowering the 24-hour BP over 10 weeks, and over the last 6 hours of the therapeutic window.Citation30 Elderly patients with difficult-to-control isolated systolic hypertension have also benefited from telmisartan. Thus, the ATHOS trial of 872 subjects older than 60 years showed that BP decreased more sharply over 24 hours with telmisartan 40–80 mg (+HCTZ 12.5 mg) treatment than with amlodipine 5–10 mg (+HCTZ 12.5 mg) treatment.Citation31 In this trial, the percentage of patients with controlled systolic BP was higher in the telmisartan group than in the amlodipine group (65.9% vs 58.3%, P = 0.02).
Finally, a recent analysis of 24-hour ambulatory BP data from the ONTARGET showed that telmisartan was more effective in controlling nocturnal BP than ramipril.Citation32
These positive results with telmisartan are due not only to its BP-lowering efficacy but also to its long duration of action.
Telmisartan’s efficacy against end-organ damage
Renal disease
CV risk factors underlie arterial, myocardial, cerebral/ocular, and renal lesions. Among these risk factors, hypertension and diabetes are key factors, particularly in the development of nephropathy. It is therefore essential not only to prevent existing renal lesions from worsening (secondary prevention), but also to prevent the formation of lesions in the first place (primary prevention). Recommendations on treating patients with hypertension and/or diabetes emphasize the potential benefit of RAAS inhibitors, in particular, when the patients have renal failure and/or proteinuria.Citation1,Citation33 Among the RAAS inhibitors, several trials have shown that ARBs merit a special place, particularly in patients with type 2 diabetes.Citation34–Citation36 Telmisartan is one of the drugs that have proven their worth in this area.
The INNOVATION trial, conducted on 514 hypertensive or normotensive subjects with type 2 diabetes and microal-buminuria but no renal failure, showed that both doses of telmisartan 80 mg and 40 mg slowed down the appearance of overt nephropathy when compared with placebo (16.7%, 22.6%, and 49.9%, respectively, after a mean follow-up period of 1.3 years).Citation37 This positive effect of telmisartan has been observed in patients with hypertension, regardless of their BP.
The DETAIL trial of 250 patients with type 2 diabetes and incipient nephropathy showed that telmisartan 40–80 mg and enalapril 20 mg had similar effects on the progressive loss of glomerular filtration function over a 5-year period.Citation38
The AMADEO trial of 860 patients with type 2 diabetes with overt nephropathy (morning spot urine protein-to-creatinine ratio of 700 or more) demonstrated that telmisartan 40 mg preserved kidney function more effectively than losartan 50 mg.Citation39 In this trial, proteinuria reduced after 52 months by 29% with telmisartan compared with only 20% with losartan (P < 0.05) treatment, independently of the decrease in BP.
The VIVALDI trial found similar reductions in proteinuria with telmisartan 80 mg and valsartan 160 mg in 885 patients with hypertension and type 2 diabetes (proteinuria ≥900 mg/24 hour and serum creatinine ≤3.0 mg/dL) over the 52 weeks of the trial.Citation40
The ARAMIS trial of 614 patients, who did not necessarily have diabetes, with isolated systolic hypertension and albuminuria >2.2 mg/L showed that the reduction in urinary albumin excretion was greater in the telmisartan 20–80 mg group than in the HCTZ 12.5 mg group.Citation41
A recent meta-analysis indicated that the combination of an ACE inhibitor and an ARB reduces proteinuria to a greater extent than does either drug alone.Citation42 With respect to the efficacy of this combination vis-à-vis renal function and CV events, one of the aims of the ONTARGET (this trial will be explained later in greater detail) was to investigate the long-term nephrological outcome in 25,620 subjects at high vascular risk, taking telmisartan 80 mg vs ramipril 10 mg or a combination of these 2 drugs.Citation17 After a follow-up period of 56 months, the primary renal end point (a composite parameter of dialysis, doubling of serum creatinine, and death) was similar for both telmisartan (13.4%) and ramipril (13.5%), but was superior with the combination therapy (14.5%, P = 0.037).Citation43 The estimated glomerular filtration rate decreased less with ramipril than with telmisartan (–2.82 vs −4.12 mL/min/1.73 m², P < 0.0001) or combination therapy (–6.11, P < 0.0001), but urinary albumin excretion increased less with telmisartan (25%, P = 0.033) and combination therapy (22%, P = 0.0028) than it did with ramipril (32%). In light of these results, we can conclude first that renal protection is identical with ARBs and ACE inhibitors in the high vascular risk population, and second that a serious renal event, as well as hypotensive symptoms and syncope, is more likely to occur with the combination of ARB and ACE inhibitor. This combination should therefore be prescribed only to patients with heart failure that is not controlled by ACE inhibitorsCitation44,Citation45 (it is the only licensed indication).
Left ventricular hypertrophy
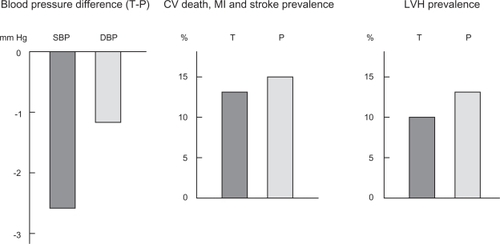
Although LVH does not have a direct effect on vascular risk, it is important to be aware of the impact of any given antihypertensive agents on this pathological process. Some trials have shown that telmisartan significantly reduces LVH in patients with hypertension. In fact, telmisartan induces greater LVH regression than carvedilol, ramipril, and HCTZ, despite comparable BP reductions.Citation46–Citation48 In the ONTARGET and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trial (see design later), the effect of telmisartan on LVH (electrocardiogram criteria) was studied in patients at high vascular risk without known heart failure.Citation17,Citation49 In TRANSCEND trial, telmisartan 80 mg was more effective than placebo in reducing LVH (P = 0.0017, ).Citation50 Moreover, new-onset LVH decreased by 37% in the telmisartan group. In the ONTARGET, LVH prevalence was slightly lower with telmisartan than with ramipril (P = 0.07). It is also important to be aware of this positive effect, given that LVH is involved in the development of heart failure and arrhythmia.
Figure 1 Beneficial effects of telmisartan compared with placebo at the end of the TRANSCEND study: blood pressure (difference between both groups: −2.6/−1.1 mmHg),Citation49 cardiovascular events (13.0% vs 14.8%, P = 0.048),Citation49 and electric left ventricular hypertrophy (9.9% vs 12.8%, P = 0.0017).Citation50
Arterial wall damage
Angiotensin II plays a key role in the initiation and exacerbation of atherosclerosis, and RAAS inhibitors seem to occupy a special place among vascular protective treatments. Several vascular parameters are used as CV risk markers, including the presence of vascular hypertrophy (increased intima-media thickness, IMT) and plaques. Interestingly, it has been shown in patients with hypertension that telmisartan significantly reduces carotid IMT and carotid wall cross-sectional area than does ramipril.Citation48
Arterial wall stiffness, which can be assessed using carotid-to-femoral pulse wave velocity (PWV), is another CV risk marker. It is responsible for an increase in systolic BP and a relative decrease in diastolic BP. Reduced arterial stiffness on treatment is associated with decreases in morbidity and mortality, independently of BP reduction. In TRANSCEND trial, the patients taking telmisartan 80 mg tended to have a lower PWV (difference of 0.5 m/s) than those taking placebo after 2 years of treatment (Roland Asmar, pers comm).
Using telmisartan to prevent CV events
CV prevention
The beneficial role of RAAS inhibition in the secondary prevention of CV and renal diseases, and in patients with diabetes at high CV risk, has been clearly demonstrated. However, what is the best therapeutic strategy in this type of patient: ACE inhibitors, ARBs, or a combination of both? The HOPE trial compared the effect of ramipril 10 mg with placebo for a mean follow-up period of 5 years in 9,297 patients at high CV risk who had evidence of vascular disease or diabetes plus another CV risk factor.Citation18 In this trial, a primary outcome (myocardial infarction, stroke, or death from CV causes) was reached less often in patients receiving ramipril (–22%, P < 0.001). The recent ONTARGET included 25,620 patients (mean age, 66.4 years; 73% men and 38% patients with diabetes) who were at high risk for vascular events.Citation17 On inclusion, 85% of patients had a CV pathology, 49% had already experienced myocardial infarction, but none had presented heart failure, 21% were had a stroke, and 69% had hypertension. These patients were being well managed (62% were taking a statin and 76% were taking aspirin). The subjects were randomized to receive either telmisartan up to the 80 mg or ramipril up to 10 mg per day, or combination therapy. The aim of this trial was 2-fold: (1) to demonstrate that telmisartan was more effective against the incidence of CV events (primary composite outcome: death from CV causes, myocardial infarction, stroke, or hospitalization for heart failure) and tolerated better than ramipril; and (2) to determine whether the combination of telmisartan and ramipril was more effective than monotherapy with ramipril against the incidence of CV events. The main results of the ONTARGET showed that, after a mean follow-up period of 56 months, the overall incidence of CV events was identical with telmisartan alone (16.7%), with ramipril alone (16.5%), and with the combination of telmisartan and ramipril (16.3%). In this trial, the patients in the telmisartan group and the combination-therapy group had slightly lower BP levels throughout the trial period (mean reductions of 0.9/0.6 mmHg and 2.4/1/4 mmHg, respectively) than the patients in the ramipril group. This is the first trial to show that an ARB, telmisartan, is as effective as an ACE inhibitor, ramipril, in terms of reducing the risk of all types of CV complications occurring in patients at high vascular risk. Interestingly, the same trial showed that the main benefit of reducing clinical systolic BP to below 130 mmHg was the reduction in the incidence of stroke, but the incidence of myocardial infarction was unaffected.Citation51
Although ACE inhibitors are indicated in many clinical situations, around 20% of patients cannot tolerate them due to coughing, hypotension, and impaired renal function. As ACE inhibitor-intolerant patients at high vascular risk could not be included in the ONTARGET, a separate trial was created for them, namely the TRANSCEND trial.Citation49 A total of 5,926 patients were included in this trial, and the main result showed that, after a median follow-up period of 56 months with telmisartan 80 mg and placebo, there was a similar incidence of CV events (15.7% vs 17.0%). However, in the TRANSCEND trial, telmisartan significantly reduced the risk of the secondary composite outcome (CV death, myocardial infarction, or stroke, ie, the HOPE trial end point) (P = 0.048, ).
Atrial fibrillation
Atrial fibrillation is associated with increased cardiac and vascular risks, particularly stroke. A meta-analysis by Healey et al demonstrates that ACE inhibitors and ARBs reduce the relative risk of atrial fibrillation by 38% (P = 0.0002), with both classes inducing a similar reduction.Citation52 This positive effect of RAAS inhibitors is seen, in particular, in patients with heart failure (–44%). In the ONTARGET, which was conducted on patients without known heart failure, both drugs (telmisartan and ramipril) had similar effects on new-onset atrial fibrillation.Citation17
Cerebrovascular prevention
As mentioned earlier, both ACE inhibitors and ARBs have a positive effect at the vascular level. But what about the secondary prevention of stroke? The PROGRESS trial of 6,105 patients showed that a combination of perindopril and indapamide reduces the risk of recurrent stroke by 28% compared with placebo after a 4-year follow-up period.Citation53 The PROFESS trial compared telmisartan 80 mg with placebo over 2.5 years in 20,332 patients who had recently experienced an ischemic stroke.Citation54 Stroke recurrence (the primary outcome) was similar in both groups (P = 0.23), and the same was true for all major CV events. We should however remember that, in this trial, 37% of patients in the placebo group received an ACE inhibitor, 47% received a statin, and 100% received aspirin.
Safety and tolerance
Besides efficacy, safety and tolerance are also the essential properties of a drug, especially when it is used in the long term, as is the case with antihypertensive medications. Several trials have shown that ARBs are well tolerated and are probably even the best-tolerated class of antihypertensive drugs.Citation55 Several trials have shown that telmisartan is well tolerated, in particular, compared with placebo and an ACE inhibitor.
The PRISMA trial showed that the tolerance of both ramipril and telmisartan was good, although coughing was reported less frequently with the ARB than with the ACE inhibitor.Citation24 In the ONTARGET, patients discontinued telmisartan less often than ramipril (21.0% vs 23.7%, P = 0.02).Citation17 Similarly, fewer patients had experienced coughing (1.1% vs 4.2%, P < 0.001) or angioedema (0.1% vs 0.3%, P = 0.01) on treatment with telmisartan. However, although the incidence of syncope did not increase, the rate of hypotensive symptoms with telmisartan (2.6% vs 1.7%, P < 0.001) was higher. An important fact to note is that there were as many renal events with telmisartan (10.6%) as with ramipril (10.2%), and in particular, a similar number of patients presented with doubled creatinine levels. Likewise, the number of patients whose potassium levels increased by more than 5.5 mmol/L was similar in both monotherapy groups, but higher with the combination-therapy groups.
The tolerance of telmisartan was also compared with that of amlodipine. In subjects older than 60 years, telmisartan + HCTZ was better tolerated than the calcium channel blocker + HCTZ.Citation31
The consequences of good tolerance of telmisartan are essentially improved safety of use and compliance.
In the ONTARGET, a combination of telmisartan and ramipril induced more side effects and laboratory abnormalities than ramipril alone.Citation17 In particular, syncope was more common (0.3% vs 0.2%, P = 0.03), as were episodes of renal failure (1.1% vs 0.7%, P < 0.001) and hyperkalemia.
Conclusion: telmisartan’s place in the therapeutic arsenal
The modern approach to treating CV diseases should take account not only of individual CV risk factors but also of the patient’s overall CV risk. Furthermore, it is essential to have access to drugs that have been proven effective for both primary and secondary preventions. This is why telmisartan warrants a special place in the therapeutic arsenal. It is effective in reducing BP, it has a favorable metabolic profile, it has been proven effective in patients at high vascular risk, and it is well tolerated. These data, mostly derived from the ONTARGET and TRANSCEND trial, were taken into account when the European Society of Hypertension recently reappraised its guidelines on hypertension management.Citation33 Finally, in 2010, telmisartan is indicated for essential hypertension in adults and for the prevention of CV disease in patients with (1) manifest atherothrombotic CV disease (a history of coronary heart disease, stroke, or peripheral arterial disease) or (2) type 2 diabetes mellitus with documented target-organ damage.
Disclosures
The authors do not have any financial interests or conflicts related to this manuscript.
References
- ManciaGDe BackerGDominiczakAfor Management of Arterial Hypertension of the European Society of Hypertension and European Society of Cardiology2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens2007251105118717563527
- De BackerGAmbrosioniEBorch-JohnsenKfor Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical PracticeEuropean guidelines on cardiovascular disease prevention in clinical practice. Third joint task force of European and other societies on cardiovascular disease prevention in clinical practiceEur Heart J2003241601161012964575
- MazzagliaGAmbrosioniEAlacquaMAdherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patientsCirculation20091201598160519805653
- ChapmanRHFerrufinoCPKowalSLClassiPRobertsCSThe cost and effectiveness of adherence-improving interventions for antihypertensive and lipid-lowering drugsInt J Clin Pract20106416918120089007
- BurnierMBrunnerHRAngiotensin II receptor antagonistsLancet200035563764510696996
- VanderheydenPMFierensFLVauquelinGAngiotensin II type 1 receptor antagonists. Why do some of them produce insurmountable inhibitionBiochem Pharmacol2000601557156311077037
- SongJCWhiteCMOlmesartan medexomil (CS-866) an angiotensin II receptor blocker for treatment of hypertensionFormulary2001236487499
- KakutaHSudohKSasamataMYamagishiSTelmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockersInt J Clin Pharmacol Res200525414615864875
- GoebelMClemenzMUngerTEffective treatment of hypertension by AT(1) receptor antagonism: the past and future of telmisartanExpert Rev Cardiovasc Ther2006461562917081084
- MaillardMPPerregauxCCentenoCIn vitro and in vivo characterization of the activity of telmisartan: an insurmountable angiotensin II receptor antagonistJ Pharmacol Exp Ther20023021089109512183667
- RemkováAKratochvíl’ováHDurinaJImpact of the therapy by renin-angiotensin system targeting antihypertensive agents perindopril versus telmisartan on prothrombotic state in essential hypertensionJ Hum Hypertens20082233834518305548
- BensonSCPershadsinghHAHoCIIdentification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activityHypertension200443993100215007034
- PershadsinghHAKurtzTWInsulin-sensitizing effects of telmisartan: implications for treating insulin-resistant hypertension and cardiovascular diseaseDiabetes Care200427101515047668
- MiuraYYamamotoNTsunekawaSReplacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes: metabolic and antiatherogenic consequencesDiabetes Care20052875775815735228
- VitaleCMercuroGCastiglioniCMetabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndromeCardiovasc Diabetol200515615892894
- AbuissaHJonesPGMarsoSPO’KeefeJHAngiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trialsJ Am Coll Cardiol20054682182616139131
- YusufSTeoKKPogueJfor ONTARGET InvestigatorsTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med20083581547155918378520
- YusufSSleightPPogueJBoschJDaviesRDagenaisGEffects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study InvestigatorsN Engl J Med200034214515310639539
- DellesCRaffUMimranAFauvelJPRuilopeLMSchmiederREEffects of telmisartan and ramipril on adiponectin and blood pressure in patients with type 2 diabetesAm J Hypertens2008211330133618989258
- HopkinsTAOuchiNShibataRWalshKAdiponectin actions in the cardiovascular systemCardiovasc Res200774111817140553
- SchmiederREDellesCMimranAFauvelJPRuilopeLMImpact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetesDiabetes Care2007301351135617337492
- BaguetJPLegallicierBAuquierPRobitailSUpdated meta-analytical approach to the efficacy of antihypertensive drugs in reducing blood pressureClin Drug Investig200727735753
- LacourcièreYNeutelJMDavidaiGKovalSA multicenter, 14-week study of telmisartan and ramipril in patients with mild-to-moderate hypertension using ambulatory blood pressure monitoringAm J Hypertens20061910411216461201
- WilliamsBGossePLoweLHarperRfor PRISMA I Study GroupThe prospective, randomized investigation of the safety and efficacy of telmisartan versus ramipril using ambulatory blood pressure monitoring (PRISMA I)J Hypertens20062419320016331118
- GossePNeutelJMSchumacherHLacourcièreYWilliamsBDavidaiGThe effect of telmisartan and ramipril on early morning blood pressure surge: a pooled analysis of two randomized clinical trialsBlood Press Monit20071214114717496463
- WhiteWBMurwinDChrysantSGKovalSEDavidaiGGuthrieREffects of the angiotensin II receptor blockers telmisartan versus valsartan in combination with hydrochlorothiazide: a large, confirmatory trialBlood Press Monit200813212718199920
- MallionJSichéJLacourcièreYABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertensionJ Hum Hypertens19991365766410516734
- BakrisGComparison of telmisartan vs valsartan in the treatment of mild to moderate hypertension using ambulatory blood pressure monitoringJ Clin Hypertens (Greenwich)20024 Suppl 1S26S31
- NeutelJMLittlejohnTWChrysantSGSinghAfor Telmisartan Study GroupTelmisartan/hydrochlorothiazide in comparison with losartan/hydrochlorothiazide in managing patients with mild-to-moderate hypertensionHypertens Res20052855556316335883
- SharmaAMDavidsonJKovalSLacourcièreYTelmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH studyCardiovasc Diabetol200762817910747
- NeldamSEdwardsCfor ATHOS Study GroupTelmisartan plus HCTZ vs amlodipine plus HCTZ in older patients with systolic hypertension: results from a large ambulatory blood pressure monitoring studyAm J Geriatr Cardiol20061515116016687967
- ManciaGParatiGBiloGEffects of ramipril, telmisartan and their combination on ambulatory blood pressure in an ONTARGET substudy [abstract]J Hypertens200927Suppl 4S168
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension task force documentJ Hypertens2009272121215819838131
- BrennerBMCooperMEde ZeeuwDfor RENAAL Study InvestigatorsEffects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathyN Engl J Med200134586186911565518
- LewisEJHunsickerLGClarkeWRfor Collaborative Study GroupRenoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetesN Engl J Med200134585186011565517
- ParvingHHLehnertHBröchner-MortensenJGomisRAndersenSArnerPfor Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study GroupThe effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetesN Engl J Med200134587087811565519
- MakinoHHanedaMBabazonoTfor INNOVATION Study GroupPrevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetesDiabetes Care2007301577157817389334
- BarnettAHBainSCBouterPfor Diabetics Exposed to Telmisartan and Enalapril Study GroupAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med20043511952196115516696
- BakrisGBurgessEWeirMDavidaiGKovalSfor AMADEO Study InvestigatorsTelmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathyKidney Int20087436436918496508
- GalleJSchwedhelmEPinnettiSBögerRHWannerCfor VIVALDI investigatorsAntiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathyNephrol Dial Transplant2008233174318318450829
- VogtLNavisGKösterJManolisAJReidJLde ZeeuwDfor Angiotensin II Receptor Antagonist telmisartan Micardis in Isolated Systolic hypertension (ARAMIS) study groupThe angiotensin II receptor antagonist telmisartan reduces urinary albumin excretion in patients with isolated systolic hypertension: results of a randomized, double-blind, placebo-controlled trialJ Hypertens2005232055206116208149
- KunzRFriedrichCWolbersMMannJFMeta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal diseaseAnn Intern Med2008148304817984482
- MannJFSchmiederREMcQueenMfor ONTARGET investigatorsRenal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multi-centre, randomised, double-blind, controlled trialLancet200837254755318707986
- CohnJNTognoniGfor Valsartan Heart Failure Trial InvestigatorsA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med20013451667167511759645
- McMurrayJJOstergrenJSwedbergKfor CHARM Investigators and CommitteesEffects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trialLancet200336276777113678869
- GalzeranoDTammaroPCercielloAFreehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre studyJ Hum Hypertens200418535914688811
- GalzeranoDTammaroPdel ViscovoLThree-dimensional echocardiographic and magnetic resonance assessment of the effect of telmisartan compared with carvedilol on left ventricular mass a multicenter, randomized, longitudinal studyAm J Hypertens2005181563156916364826
- PetrovicIPetrovicDVukovicNVentricular and vascular remodelling effects of the angiotensin II receptor blocker telmisartan and/or the angiotensin-converting enzyme inhibitor ramipril in hypertensive patientsJ Int Med Res200533Suppl 139A49A
- YusufSTeoKAndersonCfor Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) InvestigatorsEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trialLancet20083721174118318757085
- VerdecchiaPSleightPManciaGfor ONTARGET/TRANSCEND InvestigatorsEffects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the ongoing telmisartan alone and in combination with ramipril global end point trial and the telmisartan randomized assessment study in ACE intolerant subjects with cardiovascular diseaseCirculation20091201380138919770395
- SleightPRedonJVerdecchiaPfor ONTARGET investigatorsPrognostic value of blood pressure in patients with high vascular risk in the ongoing telmisartan alone and in combination with ramipril global endpoint trial studyJ Hypertens2009271360136919506526
- HealeyJSBaranchukACrystalEPrevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysisJ Am Coll Cardiol2005451832183915936615
- PROGRESS Collaborative GroupRandomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attackLancet20013581033104111589932
- YusufSDienerHCSaccoRLfor PRoFESS Study GroupTelmisartan to prevent recurrent stroke and cardiovascular eventsN Engl J Med20083591225123718753639
- GerthWCCompliance and persistence with newer antihypertensive agentsCurr Hypertens Rep2002442443312419170