Abstract
Hypertension remains a significant health burden in the United States, with almost one in three adults affected, and is an independent risk factor for cardiovascular and renal disease. The goal of antihypertensive treatment is to reduce cardiovascular and renal morbidity and mortality by reducing blood pressure (BP). Guidelines recommend a target BP of <140/90 mmHg, with a more stringent goal of <130/80 mmHg for patients with diabetes and chronic renal disease. However, BP goal attainment rates remain low and most patients require therapy with two or more antihypertensive agents. Combination antihypertensive therapy usually employs agents from different classes, thus benefitting from complementary mechanisms of action to achieve greater BP control with fewer side effects. Patient adherence to therapy is enhanced by formulating treatments as fixed-dose (single-pill) combinations. One example is the combination of amlodipine, a dihydropyridine calcium channel blocker (CCB), with olmesartan medoxomil, an angiotensin receptor blocker (ARB). Here, the rationale for the use of CCB/ARB combination therapy is discussed, as well as the pharmacology and tolerability of the amlodipine/olmesartan medoxomil combination and its efficacy in terms of achieving BP goal in patients with hypertension. Advantages of its use from the patient’s perspective are also discussed.
Introduction
Hypertension affects nearly 1 in 3 adults in the United States,Citation1–Citation3 and is an important modifiable risk factor for coronary artery disease, heart failure, renal failure, and stroke.Citation4,Citation5 An analysis of global data suggests that the overall prevalence of hypertension is similar in men and women and increases with age.Citation6 According to a recent estimate, hypertension was responsible for around 7.6 million premature deaths per year, contributing 6.0% to the total global disease burden.Citation7
Hypertension control correlates with a significant reduction in cardiovascular (CV) events.Citation2 Indeed, over the last 50 years, reductions observed in CV event-related morbidity and mortality have been attributed to the increased availability and use of antihypertensive treatments.Citation2 In particular, blood pressure (BP) reductions in patients with diabetes mellitus are linked with reductions in disease events.Citation8 However, the United States National Health and Nutrition Examination Survey (NHANES) findings for 2003 to 2004 showed that adequate BP control was achieved in only 37% of patients with hypertension and in only 57% of those who received antihypertensive therapy.Citation1 Furthermore, because hypertension can be asymptomatic, many patients go undiagnosed and thus it remains a major public health challenge in the United States.Citation1
American guidelines for the management of hypertension recommend that all individuals should achieve a BP target of at least <140/90 mmHg, with a target of <130/80 mmHg for patients with diabetes or chronic renal disease.Citation4,Citation9 Recently revised guidelines from the European Society of Hypertension (ESH) recommend lowering BP to values within the range of 130–139/80–85 mmHg in all patients with hypertension.Citation10 However, despite agreement over the benefits of hypertension control, there is no consensus as to the optimal choice of antihypertensive agents.Citation4 Additionally, recent guidelines recommend different antihypertensive protocols depending on the underlying morbidities and patient characteristics.Citation4 For example, in patients with stable angina, a beta (β)-blocker and an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB) are recommended, whereas in patients with severe left ventricular (LV) dysfunction, the addition of an aldosterone antagonist and a thiazide or loop diuretic are recommended. In Black patients with severe LV dysfunction, the combination of hydralazine/isosorbide dinitrate is recommended as an adjunct to diuretic, ACE inhibitor or ARB, and β-blocker regimens.Citation4 In all cases of hypertension, lifestyle modifications including weight loss, diet changes, exercise, smoking cessation, and alcohol moderation are recommended.Citation4,Citation11
Concept of combination therapy to attain BP goals
Hypertension treatment guidelines emphasize the importance of starting treatment with combination therapy, especially in patients whose BP exceeds the goal by more than 20/10 mmHg.Citation9 Guidelines recommend combining different classes of antihypertensive agents that have complementary mechanisms of action, an antihypertensive effect that is greater than that of either component alone, and a favorable tolerability profile.Citation8 Furthermore, patient adherence appears to be greater with single-pill combination treatment because of greater efficacy and a reduced pill burden.Citation10,Citation12
Recently published results from a randomized, double-blind study showed effective BP lowering with combination therapy.Citation13 Each of the three dual-therapy combinations produced significant (P < 0.0001 vs baseline) reductions from baseline in both mean seated systolic BP (SeSBP) and diastolic BP (SeDBP) in patients with moderate or severe hypertension (N = 2,271). The therapies were all possible dual combinations of a dihydropyridine calcium channel blocker (DHP-CCB; amlodipine), a thiazide diuretic (hydrochlorothiazide [HCTZ]), and an ARB (valsartan). The DHP-CCB/ARB combination produced numerically greater BP reductions than the other combinations.Citation13 A triple combination of these agents enabled even greater reductions than the dual therapies and has been approved by the United States Food and Drug Administration (FDA).
Current antihypertensive drug options
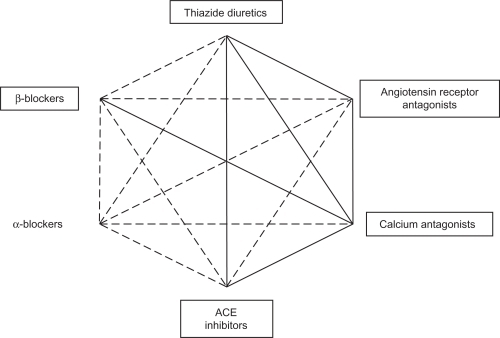
Several effective classes of antihypertensive drugs are currently available, including ACE inhibitors, ARBs, a direct renin inhibitor, β-blockers, CCBs, and thiazide-type diuretics. Two-drug combinations found to be effective and well tolerated include: ACE inhibitor/thiazide diuretic, ARB/thiazide diuretic, DHP-CCB/ACE inhibitor, DHP-CCB/ARB, DHP-CCB/thiazide diuretic, DHP-CCB/β-blocker, direct renin inhibitor/HCTZ,Citation8 and a very recently FDA-approved direct renin inhibitor/ARB combination.Citation14 shows possible combinations of some of these classes of agents; preferred combinations recommended by treatment guidelines are indicated with a thick line.
Figure 1 Possible combinations between some classes of antihypertensive agents. Framed agents have shown clinical benefit in interventional trials. Reproduced with permission from Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25(6):1105–1187. Copyright © 2007 Lippincott, Williams & Wilkins.
The rationale for fixed-dose (single-pill) combinations of DHP-CCBs and ARBs
Single-pill DHP-CCB/ARB combinations are emerging as convenient and rational options for antihypertensive treatment.Citation15 As previously demonstrated, combination antihypertensive therapy provides greater BP-lowering effects than single-agent therapy, and the added benefit of a DHP-CCB/ARB combination is a reduction in the incidence of adverse events.Citation15 For example, when an ARB and DHP-CCB are administered together, the complementary BP-reducing mechanism of action of an ARB appears to offset DHP-CCB–induced edema.Citation15
Furthermore, ARBs can be used at increasingly higher doses without compromising tolerability,Citation16 have organ-protective effects,Citation17 and are associated with a lower risk of cough and angioedema compared with ACE inhibitors.Citation18 In addition to a mechanism of action complementary to ARBs, CCBs also provide CV benefits.Citation19 An amlodipine-based treatment regimen prevented more CV events in the BP-lowering arm of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) compared with atenolol–based therapy.Citation20 In patients with coronary artery disease and diastolic BP (DBP) <100 mmHg, treatment with amlodipine reduced the incidence of CV events compared with placebo in the CAMELOT (Comparison of Amlodipine vs Enalapril to Limit Occurrences of Thrombosis) study.Citation21 Administration of CCBs has been shown to be associated with stroke prevention relative to other antihypertensive agents, as well as a reduction in all-cause mortality.Citation19
In the ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension) trial, a DHP-CCB/ACE inhibitor (benazepril) combination provided a greater reduction in CV events in patients with hypertension at high risk for such events than was provided with a diuretic (HCTZ)/ACE inhibitor combination.Citation22 BP control (<140/90 mmHg) was achieved by 74.5% and 72.4% of patients in the DHP-CCB/ACE inhibitor and diuretic/ACE inhibitor groups, respectively. It is possible to speculate that by extrapolating these data to DHP-CCB/ARB combinations similar benefits may be observed because both ACE inhibitors and ARBs act on the renin-angiotensin-aldosterone system (RAAS).
Results from the large ONTARGET (Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) study in patients with vascular disease or high-risk diabetes showed that treatment with an ARB (telmisartan) was associated with a reduction in death from CV causes, myocardial infarction or stroke, or hospitalization for heart failure (primary endpoint) that was equivalent to the risk reduction observed with an ACE inhibitor.Citation18 Of note, the rates of angioedema (P < 0.01) and cough (P < 0.001) were significantly lower in the ARB group than in the ACE inhibitor group.Citation18 In the LIFE (Losartan Intervention For Endpoint reduction in hypertension) study, losartan prevented more death and CV morbidity than atenolol and was better tolerated.Citation23
In the United States, a fixed-dose combination of amlodipine and olmesartan medoxomil (AZOR®; Daiichi Sankyo, Inc.; Parsippany, NJ; 5/20, 5/40, 10/20, and 10/40 mg) is indicated for first-line treatment in patients unlikely to reach their BP goal with monotherapy.Citation24 This article will review the mechanism of action, pharmacology, and pharmacokinetics of amlodipine/olmesartan medoxomil, and evaluate the efficacy of this combination for achieving BP goals in patients with hypertension. The tolerability of the combination will also be discussed, along with advantages of its use from the patient’s perspective.
Amlodipine/olmesartan medoxomil: pharmacokinetics and mechanism of action
Amlodipine pharmacokinetics
Following oral administration of therapeutic dosages, amlodipine is slowly absorbed from the gastrointestinal (GI) tract, and the time to peak plasma concentrations is 10 to 14 hours.Citation25 Steady-state concentrations are reached after 7 to 8 days. Amlodipine does not undergo significant first-pass metabolism, but is slowly metabolized to inactive metabolites in the liver.Citation26 Primarily excreted in the urine, amlodipine has an elimination half-life of 35 to 45 hours.Citation25
Olmesartan medoxomil pharmacokinetics
Olmesartan medoxomil is a prodrug that undergoes ester hydrolysis in the GI tract to form its active metabolite (olmesartan), which, once absorbed, does not undergo further metabolism and is excreted in the urine.Citation27 After rapid absorption from the GI tract, peak plasma concentrations are achieved in 1 to 2 hours, followed by an elimination half-life of 13 hours.Citation24 Steady-state concentrations are reached within 3 to 5 days, and accumulation in the plasma does not occur with once-daily dosing.Citation24
Pharmacokinetics of amlodipine and olmesartan medoxomil administered as a combination
No significant pharmacokinetic drug–drug interactions occur when olmesartan medoxomil and amlodipine are co-administered in separate dosage forms.Citation28,Citation29 When orally administered as a fixed-dose combination, amlodipine/olmesartan medoxomil is bioequivalent to single-entity dosage forms of both drugs.Citation28
Bioequivalence was demonstrated during single- and multi-dose phase I trials in healthy volunteers who received concomitant amlodipine and olmesartan medoxomil in separate dose forms and together in a fixed-dose amlodipine/olmesartan medoxomil 10/40 mg tablet formulation ().Citation28 For example, following a single dose of the combination tablet, peak plasma concentrations of amlodipine and olmesartan were 7.6 and 833.3 ng/mL, and values for the area under the plasma concentration-time curve were 424.8 and 5,374.2 ng·h/mL, respectively.Citation28 Corresponding values following administration of amlodipine 10 mg and olmesartan medoxomil 40 mg as two separate dosage forms were 7.4 and 810.3 ng/mL and 410.9 and 5,418.6 ng·h/mL, respectively.Citation28
Figure 2 Mean plasma concentration profiles of amlodipine (upper panel) and olmesartan (lower panel) after administration of single-pill amlodipine/olmesartan medoxomil 10/40 mg combination therapy and concomitant administration of the two drugs and single tablets. Reproduced with permission from Rohatagi S, Lee J, Shenouda M, et al. Pharmacokinetics of amlodipine and olmesartan after administration of amlodipine besylate and olmesartan medoxomil in separate dosage forms and as a fixed-dose combination. J Clin Pharmacol. 2008;48(11):1309–1322. Copyright © 2008 Sage Publications.
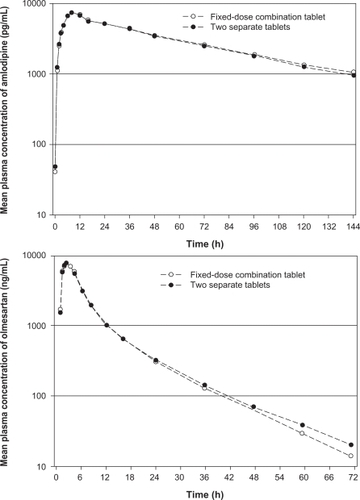
Other pharmacokinetic parameters remain similar when amlodipine and olmesartan medoxomil are administered in either a fixed-dose combination or as individual components. For instance, peak plasma concentrations in the fixed-dose combination were achieved in about 8 hours for amlodipine and 2 hours for olmesartan, and terminal elimination half-lives in the fixed-dose combination were about 40 to 55 hours for amlodipine and 11 to 16 hours for olmesartan.Citation28
Mechanism of action
ARBs
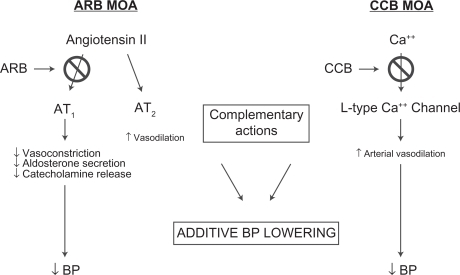
Angiotensin receptor blockers act by selectively binding to the AT1 receptor, blocking the effects of angiotensin II, and therefore suppressing vasoconstriction and other adverse CV effects of angiotensin II.Citation30–Citation32 Angiotensin II is the predominant effector peptide of the RAAS, which plays a key role in fluid and electrolyte balance and ultimately, BP regulation.Citation30
Compared with most other ARBs, olmesartan medoxomil exhibits a high degree of selectivity for the AT1 receptor, to which it binds with high affinity. In vitro findings showed that olmesartan was second only to telmisartan in binding affinity for the AT1 receptor; the other comparators were candesartan, valsartan, and losartan.Citation33 Like other ARBs, olmesartan medoxomil has a low affinity for the AT2 receptor, which is also activated by angiotensin II, but is believed to have a vasodilatory effect and a protective role in BP regulation and sodium excretion ().Citation34
Figure 3 Complementary mechanisms by which ARBs and CCBs lower BP. ARBs block the effects of angiotensin II at the AT1 receptor thus suppressing vasoconstriction. CCBs block the entry of calcium into cells allowing arterial smooth muscle to relax causing peripheral vasodilation. These complementary activities cause reductions in blood pressure. Reproduced with permission from Neutel JM. Complementary mechanisms of angiotensin receptor blockers and calcium channel blockers in managing hypertension. Postgrad Med. 2009;121(2):40–48. Copyright © 2009 JTE Multimedia, LLC.
CCBs
Calcium channel blockers decrease the entry of calcium ions into cells by blocking L-type calcium channels, leading to relaxation of arterial smooth muscle, peripheral vasodilation, and lowered BP.Citation35 Peripheral vasodilation is achieved through greater effects on calcium channels in arteries and arterioles than on cardiac muscle cells; CCBs do not affect serum calcium levels ().
Efficacy of amlodipine and olmesartan medoxomil combination therapy
COACH study
The efficacy of an amlodipine + olmesartan medoxomil free combination was assessed in patients (N = 1,940) with mild-to-severe hypertension in the COACH (Combination of Olmesartan Medoxomil and Amlodipine Besylate in Controlling High Blood Pressure) study, which was a randomized, double-blind, placebo-controlled study conducted over eight weeks.Citation36 Patients were aged ≥18 years with an SeDBP of 95 to 120 mmHg. The 12 treatment groups were: amlodipine 5 or 10 mg monotherapy, olmesartan medoxomil 10, 20, or 40 mg monotherapy, each possible amlodipine + olmesartan medoxomil combination, and placebo.
The primary endpoint was the change from baseline in mean SeDBP at the end of the 8-week double-blind treatment period using the last observation carried forward (LOCF) method. Secondary variables included the proportions of patients achieving prespecified JNC 7 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure)Citation9 recommended BP goals. The safety analysis also included an active assessment of edema, with specific ratings for the incidence and severity of peripheral edema at scheduled visits. Edema was recorded as an adverse event when its severity increased following randomization.
The mean seated BP (SeBP) of the 1,940 patients who were randomized was 164/102 mmHg, and 79.3% had Stage 2 hypertension. The majority of patients were white (71.4%), 19.8% were aged ≥65 years, and 54.3% were male. In the study cohort, there were 13.5% of patients with diabetes and 64.6% with a body mass index (BMI) ≥ 30 kg/m2.
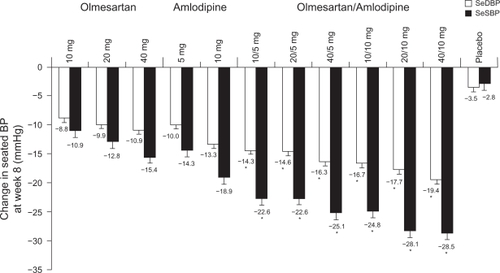
At 8 weeks, significant reductions from baseline in SeDBP (primary endpoint) were observed in all active treatment groups and in those receiving placebo (P < 0.001 for all groups). Reductions from baseline in SeSBP were also significant in all active treatment groups (P < 0.001) and in placebo recipients (P < 0.05). Of note, SeDBP reductions with combination treatment were significantly greater than those observed with equivalent doses of monotherapy with either amlodipine or olmesartan medoxomil (). Mean changes from baseline in mean SeDBP with amlodipine + olmesartan medoxomil ranged from −14.0 to −19.0 mmHg with 5 + 20 mg and 10 + 40 mg, respectively, whereas changes with monotherapy dosages of amlodipine were −9.4 mmHg (5 mg) and −12.7 mmHg (10 mg), and with olmesartan medoxomil were −8.3 mmHg (10 mg), −9.2 mmHg (20 mg), and −10.2 mmHg (40 mg) (P < 0.001 for all comparisons vs combination treatment).
Figure 4 Least-squares mean (standard error) reduction in seated blood pressure (BP) after 8 weeks of treatment with placebo, amlodipine and olmesartan medoxomil monotherapy, and amlodipine + olmesartan medoxomil combination therapy in the COACH study. Amlodipine/olmesartan medoxomil is available as 5/20, 5/40, 10/20, and 10/40 mg fixed-dose combinations in the United States. Reproduced with permission from Chrysant S, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30(4):587–604. Copyright © 2008 Elsevier.
Similarly, dose-dependent reductions in SeSBP were greater with combination amlodipine + olmesartan medoxomil therapy than with equivalent dosages of the individual monotherapies () and were significant for all active treatment groups (P < 0.001) and placebo (P = 0.024).
An antihypertensive effect was evident within the first 2 weeks of active treatment, during which time the greatest mean SeBP reduction occurred. Reductions in BP plateaued at Week 4 and were maintained until study end without further notable reductions.
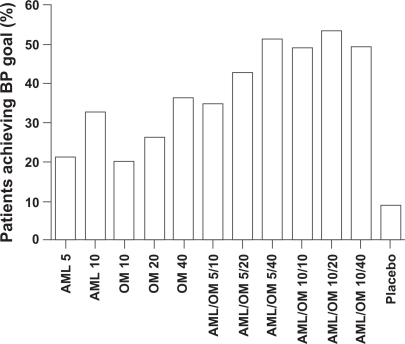
The prespecified BP goal of <140/90 mmHg (<130/80 mmHg for patients with diabetes) was attained by a significantly greater proportion of combination therapy recipients than by monotherapy recipients. After 8 weeks of treatment, the proportion of amlodipine + olmesartan medoxomil recipients achieving their BP goal ranged from 35.0% to 53.2%, compared with 20.0%, 26.4%, and 36.3% receiving olmesartan medoxomil 10, 20, or 40 mg/day, and 21.1% and 32.5% receiving amlodipine 5 or 10 mg/day (P < 0.005 combination vs monotherapies) ().
Figure 5 Percentage of patients achieving BP goal in the COACH study. (Adapted with permission from Table III in Chrysant et al).Citation36
Prespecified subanalyses of the COACH patient cohort
Prespecified subgroup analyses showed that the amlodipine + olmesartan medoxomil combination produced significant reductions from baseline in mean SeDBP and SeSBP, irrespective of the severity of hypertension (Stage 1 or 2) or prior antihypertensive treatment.Citation37
Most amlodipine + olmesartan medoxomil combinations were associated with significantly greater SeBP reductions than component monotherapies in patients with Stage 1 hypertension and all in Stage 2 patients.Citation37 Furthermore, of patients with Stage 1 hypertension, 65.6% to 80.0% who received amlodipine + olmesartan medoxomil combination therapy achieved the prespecified BP goal, compared with 40.5% to 66.7% of monotherapy component recipients (P < 0.0001 across treatment groups) (). Among those with Stage 2 hypertension, combination therapy that included amlodipine 10 mg resulted in 40.5% to 49.2% of patients achieving BP goal, compared with 13.1% to 29.2% of monotherapy component recipients ().Citation37 In a post hoc analysis of patients with baseline SeSBP ≥ 180 mmHg, combination amlodipine + olmesartan medoxomil therapy produced even greater SeSBP reductions from baseline of 43.5 and 40.8 mmHg for the 10 + 20 and 10 + 40 mg dosages, respectively.Citation37
Figure 6 Achievement of BP goal in patients with Stage 1 and 2 hypertension (upper panel) and based on prior antihypertensive exposure (lower panel) in the COACH study. Reproduced with permission from Oparil S, Lee J, Karki S, Melino M. Subgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: evaluation by baseline hypertension stage and prior antihypertensive medication use. J Cardiovasc Pharmacol. 2009;54:427–436. Copyright © 2009 Lippincott, Williams & Wilkins.
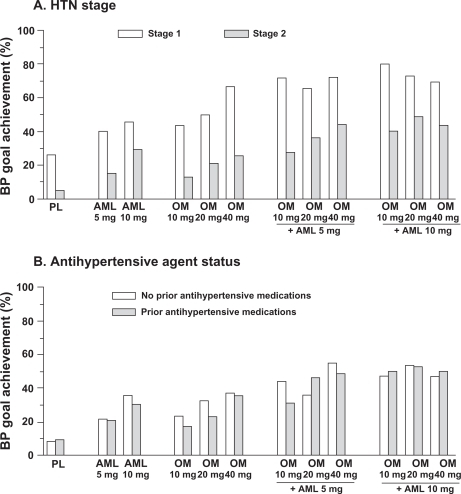
Among antihypertensive treatment-naive and non-naive patients, all combination therapy dosages resulted in significantly greater SeBP reductions compared with monotherapy.Citation37 Proportionally, more patients (both treatment naive [36.2%–55.0%] and non-naive [31.3%–52.9%]) who received combination therapy achieved prespecified BP goals than their counterparts who received monotherapies (21.8%–37.7% and 18.0%–35.5%, respectively; P < 0.0001 across all treatment groups for combination vs monotherapy).Citation37
Prespecified subgroup analyses of the COACH study based on age (≥65 or <65 years), race (Black or non-Black), or absence of diabetes, and BMI (≥30 or <30 kg/m2) showed that combination therapy enabled greater SeBP reductions than monotherapy.Citation38 Changes from baseline in mean SeBP for amlodipine + olmesartan medoxomil 10 + 40 mg were −33.9/−20.9 mmHg in patients aged ≥65 years, −28.7/−15.7 mmHg for Blacks, −30.3/−18.4 mmHg in patients with diabetes, and −29.7/−17.9 mmHg in patients with a BMI ≥ 30 kg/m2. Combination therapy generally allowed more patients to achieve BP goal than monotherapy. The rate of BP goal achievement was lower in patients with diabetes due to the aggressive target of <130/80 mmHg.Citation38
Amlodipine/olmesartan medoxomil following amlodipine or olmesartan medoxomil monotherapy
The efficacy of amlodipine/olmesartan medoxomil combination therapy has also been assessed in clinical studies where patients initially received amlodipineCitation39 or olmesartan medoxomilCitation40 monotherapy (). The efficacy of amlodipine/olmesartan medoxomil following amlodipine monotherapy was assessed in a randomized, double-blind, parallel-group, multicenter study.Citation39 The study comprised of 24 weeks of active treatment. In Period I (Weeks 0 to 8) patients received open-label amlodipine 5 mg. At the end of the open-label period, non-responders, with mean SeDBP ≥ 90 mmHg, mean SeSBP ≥ 140 mmHg, and mean 24-hour DBP ≥ 80 mmHg (with ≥30% daytime DBP > 85 mmHg), entered Period II, where patients were randomized to 1 of 4 double-blind treatment groups: amlodipine 5 mg plus placebo or a combination of amlodipine/olmesartan medoxomil 5/10, 5/20, or 5/40 mg for 8 weeks. At the end of Period II, patients with SeDBP < 90 mmHg or SeSBP < 140 mmHg remained on their current therapy for a further 8 weeks (Period III). For patients with SeDBP ≥ 90 mmHg and SeSBP ≥ 140 mmHg, study medication was further up-titrated ().
Table 1 Randomized double-blind, parallel-group, multicenter studies assessing efficacy of AML/OM following AML or OM monotherapy
The primary efficacy endpoint was change in mean SeDBP from the end of Period I (after the amlodipine run-in) to the end of Period II and the secondary endpoint was change in mean SeSBP values for the same period. All SeBP changes for combination therapy were significant compared with amlodipine monotherapy (P < 0.05 vs amlodipine/olmesartan medoxomil 5/10 mg; P < 0.0001 vs amlodipine/olmesartan medoxomil 5/20 and 5/40 mg).Citation39
Up-titration of medication for patients not at the SeBP goal at the end of Period II for a further 8 weeks of therapy enabled additional SeBP reductions and allowed more patients to achieve their BP goal (). Overall, at the end of the 24-week duration of the study, 63% of patients achieved a SeBP threshold of <140/90 mmHg.Citation39
In a similar study, the efficacy of amlodipine/olmesartan medoxomil following olmesartan medoxomil monotherapy was examined.Citation40 The primary efficacy endpoint was the change in mean SeDBP from the end of the olmesartan medoxomil monotherapy period to the end of the 8-week randomized phase. All treatments significantly reduced SeDBP from baseline in a dose-dependent manner (). The achievement of BP goal (SeBP <140/90 mmHg; <130/80 mmHg for patients with diabetes) was also assessed. Treatment with amlodipine/olmesartan medoxomil 5/20 or 10/20 mg combination therapy led to significantly greater proportions of patients achieving their BP goal, compared with olmesartan medoxomil monotherapy; P < 0.01 for both combinations vs monotherapy ().Citation40 It is worth noting that the highest United States FDA-approved dosage of olmesartan medoxomil monotherapy (40 mg/day) was not used in this European study.Citation24
Long-term efficacy of amlodipine/olmesartan medoxomil
The long-term efficacy of the amlodipine + olmesartan medoxomil combination was investigated in a 44-week open-label extension (OLE) of the eight-week COACH study.Citation41 At the start of the OLE, 1,684 patients were switched to amlodpine/olmesartan medoxomil 5/40 mg. Patients who were not at the SeBP goal (<140/90 mmHg; <130/80 for patients with diabetes) were up-titrated as necessary to amlodipine/olmesartan medoxomil 10/40 mg, followed by the addition of HCTZ 12.5 and 25 mg. Back titration and dose adjustment were permitted. Approximately one-quarter of patients (n = 419) were up-titrated to amlodipine/olmesartan medoxomil 10/40 mg + HCTZ 25 mg. Mean SeBP changes from baseline (measured at randomization into the 8-week study) ranged from −30.0/−19.3 mmHg for amlodipine/olmesartan medoxomil 5/40 mg to −36.1/−19.8 mmHg for amlodipine/olmesartan medoxomil 10/40 mg + HCTZ 25 mg. Overall, 66.7% of patients in the OLE achieved the SeBP goal.Citation41
An extension of the Volpe et alCitation39 study was also conducted. Out of the patients who completed Period III, 692 were enrolled into a 28-week OLE conducted to assess the effectiveness of long-term treatment with amlodipine/olmesartan medoxomil with or without the addition of HCTZ.Citation42 Patients initially received amlodipine/olmesartan medoxomil 5/40 mg (one patient received 10/40 mg), and most patients (63%) remained on this dosage. If SeDBP was >90 mmHg and SeSBP was >140 mmHg, patients were up-titrated to amlodipine/olmesartan medoxomil 10/40 mg followed by the addition of HCTZ 12.5 and 25 mg as needed. Of the patients who completed the OLE phase, 436 remained on amlodipine/olmesartan medoxomil 5/40 mg, 142 were up-titrated to amlodipine/olmesartan medoxomil 10/40 mg, and 68 and 27 required the addition of HCTZ 12.5 mg and 25 mg, respectively. Up-titration from amlodipine/olmesartan medoxomil 5/40 mg enabled additional SeBP changes of −8.8/−5.5, −10.2/−6.3, and −3.8/−3.7 mmHg with each titration step. The overall rate of goal achievement (<140/90 mmHg; <130/80 mmHg for patients with diabetes) was 66.9%.Citation42
A post hoc analysis of this OLE determined the magnitude of BP reductions observed in patients who received the amlodipine/olmesartan medoxomil 5–10/40 mg combination.Citation43 The proportions of patients with categorical SeSBP reductions from baseline of ≥15 mmHg, >15 to ≤30 mmHg, >30 to ≤45 mmHg, and >45 mmHg were 12.8%, 36.0%, 35.3%, and 15.9%, respectively. Unsurprisingly, the greatest SeSBP reductions were associated with the highest rates of achievement of a target of <140 mmHg, with 97.8%, 89.7%, 77.4%, and 55.4% from the >45 mmHg, >30 to ≤45 mmHg, >15 to ≤ 30 mmHg, and ≥15 mmHg groups, respectively.Citation43
Efficacy of amlodipine and olmesartan medoxomil over the 24-hour dosing interval
Cardiovascular events appear to occur more frequently in the morning, a phenomenon that has been linked with activation of the sympathetic nervous system that results in hormonal and physiological changes, including an increase in heart rate and BP elevation.Citation44 These morning BP elevations differ from BP alterations observed with normal changes in position.
Ambulatory BP monitoring (ABPM) is an effective and accurate method of hypertension diagnosis and a valuable tool for optimizing hypertension management.Citation45 Ambulatory devices are particularly useful for the detection of white-coat hypertension or masked hypertension and for monitoring patients who are receiving complex antihypertensive treatment protocols.Citation46 The use of ABPM also allows the efficacy of antihypertensive medications to be monitored over a 24-hour dosing interval. However, there is a lack of current consensus guidance for ambulatory BP treatment goals.
Two recent studies have indicated that amlodipine/olmesartan medoxomil provides effective BP lowering over a 24-hour period in patients not adequately controlled with amlodipine 5 mg/day monotherapy.Citation47,Citation48
24-Hour BP control in patients treated with an amlodipine/olmesartan medoxomil titration regimen
At the American Society of Hypertension (ASH) 2009 Annual Scientific Meeting, data were presented describing the efficacy of an amlodipine/olmesartan medoxomil-based titration regimen.Citation47 Seated BP inclusion criteria were SeSBP ≥ 140 and ≤199 mmHg and SeDBP ≥ 90 and ≤109 mmHg.Citation49 Patients with hypertension received monotherapy with amlodipine 5 mg/day and were up-titrated at 3-week intervals to combination amlodipine/olmesartan medoxomil dosages of 5/20, 5/40, and 10/40 mg if SeBP was ≥120/80 mmHg. Of the 185 patients who entered the study, 56.8% were male and the mean age was 56.8 years. Baseline ambulatory BP was 144.8/85.7 mmHg for those patients with baseline and end-of-study ABPM readings (n = 172).Citation47 Baseline SeBP for the efficacy cohort (n = 185) was 158.0/92.8 mmHg.Citation50 The primary efficacy endpoint was the change from baseline in mean 24-hour SBP as assessed by ABPM at Week 12. At Week 12, the titration regimen enabled a change from baseline in mean 24-hour ambulatory BP of =21.4/=12.7 mmHg, and ambulatory BP was reduced from baseline throughout the dosing interval including the last 6, 4, and 2 hours.Citation47,Citation51 A cumulative SeBP target of <140/90 mmHg was achieved by 76.8% of patients.Citation50
24-Hour BP control in patients receiving amlodipine/olmesartan medoxomil who previously were non-responders to amlodipine monotherapy
Data recently presented at the ESH 2009 Annual Scientific Meeting showed that reductions in mean 24-hour, daytime, and nighttime ambulatory BP were significantly greater with amlodipine/olmesartan medoxomil combination therapy than with amlodipine monotherapy in patients who had not responded adequately to amlodipine (P < 0.0001 for each combination dosage vs amlodipine monotherapy).Citation48 Furthermore, patients who did not achieve BP goals had further reductions in BP following 8 weeks of dose up-titration.Citation48 Changes in mean 24-hour ambulatory BP values from baseline at the end of the double-blind period associated with amlodipine monotherapy were −3.4/−2.8 mmHg, compared with changes of up to −10.1/−7.2 mmHg (for the maximum combination therapy dosage of 5/40 mg). Dose up-titration for a further 8 weeks occurred at the end of the double-blind period in patients who were still inadequately controlled (DBP ≥ 90 mmHg and SBP ≥ 140 mmHg) and provided additional clinically relevant ambulatory BP reductions, eg, further mean 24-hour ambulatory BP changes from baseline of −8.8/−6.6 mmHg for patients up-titrated from amlodipine/olmesartan medoxomil 5/40 to 10/40 mg.Citation48 These data represent a subanalysis of data from the study conducted by Volpe et al.Citation39
Safety and tolerability of amlodipine/olmesartan medoxomil
In large controlled studies, the amlodipine/olmesartan medoxomil combination of 5–10/10–40 mg was generally well tolerated.Citation36,Citation39,Citation40 In all studies, the incidences of adverse events were comparable among the different amlodipine/olmesartan medoxomil dose combinations, the olmesartan medoxomil or amlodipine monotherapy groups, and placebo.Citation36,Citation39,Citation40 Overall, most adverse events were of a mild nature and were consistent with the tolerability profile of either CCB or ARB therapy. Across three clinical trialsCitation36,Citation39,Citation40 in which patients received combination amlodipine/olmesartan medoxomil dosages of 5–10/10–40 mg, commonly occurring drug-related adverse events were headache (0.0%–6.9%) and dizziness (0.0%–5.0%); two trials indicated rates of peripheral edema of 0.5% to 2.3%,Citation39,Citation40 whereas in the third trial (COACH),Citation36 reported rates for edema were between 18.0% and 26.5%, which is probably due to the fact that this aspect of drug safety was actively assessed in this study.
In 2 trials that compared combination amlodipine/olmesar tan medoxomil therapy with amlodipine monotherapy, the incidence of peripheral edema was lower for combination therapy compared with amlodipine monotherapy when the DHP-CCB dosage was 5Citation39 or 10 mg.Citation36 In the COACH trial, among patients receiving amlodipine 10 mg, the frequency of edema was 36.8%, but this was reduced to 25.6% and 23.5% in patients who received olmesartan medoxomil 20 mg or 40 mg, respectively, in combination with amlodipine (P < 0.05 for both combination dosages vs amlodipine monotherapy).Citation36 Similarly, in the Volpe et al study, the lower dosage of amlodipine monotherapy (5 mg/day) was associated with rates of peripheral edema that were 2- to 4-fold higher than in those who received amlodipine/olmesartan medoxomil 5/10–40 mg combinations (2.1% vs 0.5%–1.1%; descriptive data only).Citation39
In the ABPM study presented at ASH 2009, the most common drug-related adverse events reported in patients were peripheral edema (4/185) and dizziness (2/185), and there were no reports of headache, orthostatic hypotension, or hypotension.Citation47
In the OLE of the COACH study, no major safety issues emerged as a consequence of extended therapy.Citation41 The incidence of edema continued to be monitored in the OLE and increases in severity were reported as adverse events. Incidences of edema determined to be related to the study drug ranged from 7.0% to 11.1% in patients who received amlodipine/olmesartan medoxomil 5/40 and 10/40 mg, respectively.Citation41
In the 28-week extension of the Volpe et al study,Citation39 drug-related incidences of edema were low in patients who received amlodipine/olmesartan medoxomil 5/40 mg (0.7%) and 10/40 mg (1.6%). No edema was reported in patients who were administered HCTZ as a component of the combination regimen.Citation42
Patient perspectives
Fixed-dose combination therapy provides a number of potential advantages, including better adherence, simplified dosing regimens and titration, reduced likelihood of adverse events, lower cost, and improved quality of life.Citation5,Citation15
The simplified dosing schedule associated with combination therapy is linked to improved adherence. Multiple medications and complex treatment regimens lead to poor patient adherence.Citation5 In particular, adherence to antihypertensive treatment decreases as dosing frequency increases.Citation52 In a Canadian study (N = 98), significantly more patients with hypertension randomized to a once-daily amlodipine regimen took their medication regularly, compared with patients receiving a twice-daily diltiazem regimen.Citation53 In another study of patients with diabetes, the average percentage of doses taken decreased by more than 50% when dosing frequency of an oral antidiabetic agent was increased to 3 times daily from a once-daily regimen (79% vs 38%).Citation54 These results are supported by findings from 2 more recent studies in patients with hypertension, where observed adherence was greater with fixed-dose combinations than with free combination therapy.Citation55,Citation56 It is worth noting that in the earlier study in patients with diabetes, the investigators found that, for the most part, the major nonadherence event was dose omission.Citation54 However, more than 33% of patients took more doses than were prescribed, indicating that decreasing the dose frequency may increase the risk of overmedication.Citation54
Because antihypertensive combinations like amlodipine/olmesartan medoxomil are more effective than single-drug treatment, BP control can be achieved potentially more quickly.Citation57 Titration of dose is simplified with fixed-dose combination therapy, which increases convenience for the patient,Citation5,Citation15 and may therefore increase compliance and adherence.Citation12
Patients are less likely to experience adverse effects with combination therapy because lower doses of the individual agents can be used.Citation16 In addition, one agent can attenuate adverse effects caused by the other agent;Citation5,Citation15 for example, as discussed in the tolerability section of this paper, olmesartan medoxomil ameliorates the dose-related peripheral edema that is associated with amlodipine. A further illustration of the adverse effect neutralizing properties of one agent over another is the attenuation of thiazide-induced hypokalemia with concomitant use of ARBs, ACE inhibitors, or potassium-sparing diuretics.Citation5
Fixed-dose combination therapy may incur lower overall costs than treatment with the component agents separately because of lower prescription costs and fewer regimen modifications, leading to fewer physician visits.Citation57,Citation58 Modifications (not including discontinuations) to drug therapy, regardless of the antihypertensive drug class, have been associated with significantly higher health service costs in the first 12 months of therapy.Citation57
In 2 retrospective studies, the annual cost of treatment was significantly lower for patients with hypertension receiving fixed-dose benazepril/amlodipine than for those receiving the free combination.Citation55,Citation56 Results from a retrospective database analysis showed that recipients of a once-daily, single-capsule, fixed-dose combination of amlodipine/benazepril required fewer medical resources and that annual per-patient CV-related medical costs were less than those associated with a similar regimen comprised of separate components.Citation55 Similarly, in a longitudinal cohort analysis of South Carolina Medicaid claims, fixed-combination antihypertensive therapy was associated with a significant reduction in average total costs of 12.5%, compared with free-combination therapy (P < 0.003).Citation56 Furthermore, according to a simulation model, CCB/ARB combination therapy may be more cost effective than monotherapy with either agent alone for lifetime treatment of hypertension in Japan.Citation59
Combination antihypertensive therapy may also improve health related quality of life outcomes over monotherapy. A study in patients with poorly controlled hypertension on low-dose amlodipine showed that the combination of amlodipine and the β-blocker betaxolol significantly improved health-related quality of life, whereas increasing the dose of amlodipine had no significant effect.Citation60
Conclusion
The prevalence of hypertension is increasing worldwide and is a powerful, independent risk factor for CV and renal disease, placing considerable burden on health care resources. In addition, the majority of patients with hypertension have inadequately controlled BP. It is now recognized that most patients with hypertension will require combination therapy with two or more antihypertensive drugs to achieve BP goals. Extensive evidence shows that a combination of antihypertensive agents with complementary mechanisms of action have a number of additive benefits over monotherapy including a greater BP response and percentage of responders, a reduction in side effects, simplification of dose titration, and improved adherence rates.
The combination of a DHP-CCB (amlodipine) with an ARB (olmesartan medoxomil) is effective in patients with mild-to-severe hypertension, and significantly greater proportions of patients achieve BP goals with this combination treatment compared with the component monotherapies. The efficacies of amlodipine/olmesartan medoxomil combination therapy regimens are maintained over a 24-hour dosing interval. Furthermore, in large clinical trials, amlodipine/olmesartan medoxomil combinations exhibit favorable tolerability profiles. Of note, the incidence of peripheral edema, an adverse event commonly associated with DHP-CCB montherapy, was significantly less frequent in amlodipine/olmesartan medoxomil combinations compared with high-dose amlodipine monotherapy, where this event was specifically evaluated in a clinical trial.
The combination of amlodipine/olmesartan medoxomil is effective for achieving BP goals in a wide range of patients with hypertension, including patients who have not responded to monotherapy with either agent. The clinical evidence discussed in this review provides a strong rationale for the use of this combination as an antihypertensive treatment strategy, particularly in a single-pill formulation, regardless of patient age, gender, or ethnicity or those with common comorbid conditions such as diabetes.
Acknowledgements
Editorial support was provided by Stephanie Blick and Christopher J Jones, PhD, of in Science Communications, a part of the Wolters Kluwer organization. Funding for the preparation of the manuscript was provided by Daiichi Sankyo, Inc.
Disclosures
Jan Basile, MD, receives grant/research support from the NHLBI and Novartis, serves as a consultant for Abbott Laboratories, Daiichi Sankyo, Inc., GlaxoSmithKline, Novartis, and Takeda Pharmaceuticals, and is on the Speakers’ Bureau for Abbott Laboratories, AstraZeneca, Daiichi Sankyo, Inc., Forest Laboratories, GlaxoSmithKline, and Novartis.
References
- OngKLCheungBMManYBLauCPLamKSPrevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004Hypertension2007491697517159087
- HajjarIKotchenTATrends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000JAMA2003290219920612851274
- Lloyd-JonesDAdamsRCarnethonMHeart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics SubcommitteeCirculation20091193e21e18119075105
- RosendorffCBlackHRCannonCPTreatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and PreventionCirculation2007115212761278817502569
- PimentaEOparilSFixed combinations in the management of hypertension: patient perspectives and rationale for development and utility of the olmesartan-amlodipine combinationVasc Health Risk Manag20084365366418827915
- KearneyPMWheltonMReynoldsKMuntnerPWheltonPKHeJGlobal burden of hypertension: analysis of worldwide dataLancet2005365945521722315652604
- LawesCMVander HoornSRodgersAGlobal burden of blood-pressure-related disease, 2001Lancet200837196231513151818456100
- ManciaGDe BackerGDominiczakA2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC)J Hypertens20072561105118717563527
- ChobanianAVBakrisGLBlackHRThe seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 reportJAMA2003289192560257212748199
- ManciaGLaurentSAgabiti-RoseiEReappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force documentJ Hypertens20091015 [E-pub ahead of print].
- AppelLJGilesTDBlackHRASH position paper: dietary approaches to lower blood pressureJ Clin Hypertens (Greenwich)200911735836819583632
- MoserMRationale for combination therapy in the management of hypertensionJ Clin Hypertens (Greenwich)200356Suppl 4172514688490
- CalhounDALacourciereYChiangYTGlazerRDTriple antihypertensive therapy with amlodipine, valsartan, and hydrochlorothiazide: a randomized clinical trialHypertension2009541323919470877
- Novartis Pharmaceuticals CorporationNovartis receives FDA approval for Valturna®, a single-pill combination of valsartan and aliskiren, to treat high blood pressure2009917 Available from: http://www.pharma.us.novartis.com/newsroom/press-release.jsp?PRID=2261 Accessed Apr 24, 2010.
- OparilSWeberMAngiotensin receptor blocker and dihydropyridine calcium channel blocker combinations: an emerging strategy in hypertension therapyPostgrad Med20091212253919332960
- LawMRWaldNJMorrisJKJordanREValue of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trialsBMJ20033267404142712829555
- TurnbullFEffects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336293951527153514615107
- YusufSTeoKKPogueJTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med2008358151547155918378520
- CostanzoPPerrone-FilardiPPetrettaMCalcium channel blockers and cardiovascular outcomes: a meta-analysis of 175,634 patientsJ Hypertens20092761136115119451836
- DahlofBSeverPSPoulterNRPrevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trialLancet2005366948989590616154016
- NissenSETuzcuEMLibbyPEffect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trialJAMA2004292182217222515536108
- JamersonKWeberMABakrisGLBenazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patientsN Engl J Med2008359232417242819052124
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet20023599311995100311937178
- Daiichi Sankyo, IncAZOR (amlodipine and olmesartan medoxomil tablets) Prescribing Information2009 Available from: http://www.azor.com/hcp/utilities/prescribing_information.html. Accessed Apr 24, 2010.
- PrisantLMCalcium antagonistsOparilSWeberMHypertension: A Companion to Brenner and Rector’s the Kidney2nd edPhiladelphiaElsevier2005683704
- MeredithPAElliottHLClinical pharmacokinetics of amlodipineClin Pharmacokinet199222122311532771
- SchwochoLRMasonsonHNPharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjectsJ Clin Pharmacol200141551552711361048
- RohatagiSLeeJShenoudaMPharmacokinetics of amlodipine and olmesartan after administration of amlodipine besylate and olmesartan medoxomil in separate dosage forms and as a fixed-dose combinationJ Clin Pharmacol200848111309132218974285
- BolbrinkerJHuberMScholzeJKreutzRPharmacokinetics and safety of olmesartan medoxomil in combination with either amlodipine or atenolol compared to respective monotherapies in healthy subjectsFundam Clin Pharmacol200923676777419659504
- GoodfriendTLAngiotensins: actions and receptorsIzzoJLJrBlackHRGoodfriendTLHypertension Primer: The Essentials of High Blood Pressure3rd edPhiladelphiaLippincott, Williams & Wilkins2003811
- MireDESilfaniTNPugsleyMKA review of the structural and functional features of olmesartan medoxomil, an angiotensin receptor blockerJ Cardiovasc Pharmacol200546558559316220064
- WeberMAAngiotensin II receptor blockersIzzoJLJrBlackHRHypertension Primer, The Essentials of High Blood PressurePhiladelphiaLippincott, Williams & Wilkins2003430432
- KakutaHSudohKSasamataMYamagishiSTelmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockersInt J Clin Pharmacol Res2005251414615864875
- CareyRMWangZQSiragyHMRole of the angiotensin type 2 receptor in the regulation of blood pressure and renal functionHypertension2000351 Pt 215516310642292
- KrumHCritical assessment of calcium antagonistsAust Fam Physician19972678418459232924
- ChrysantSMelinoMKarkiSLeeJHeyrmanRThe combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety studyClin Ther200830458760418498909
- OparilSLeeJKarkiSMelinoMSubgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: evaluation by baseline hypertension stage and prior antihypertensive medication useJ Cardiovasc Pharmacol20095442743619730391
- ChrysantSGLeeJMelinoMKarkiSHeyrmanREfficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult-to-treat hypertensionJ Hum Hypertens2010218 [E-pub ahead of print].
- VolpeMBrommerPHaagUMieleCEfficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig20092911125
- BarriosVBrommerPHaagUCalderonAEscobarCOlmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: a randomized, double-blind, parallel-group, multicentre StudyClin Drug Investig2009297427439
- ChrysantSGOparilSMelinoMKarkiSLeeJHeyrmanREfficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertensionJ Clin Hypertens (Greenwich)200911947548219751459
- VolpeMMieleCHaagUEfficacy and safety of a stepped-care regimen using olmesartan medoxomil, amlodipine and hydrochlorothiazide in patients with moderate-to-severe hypertension: an open-label, long-term studyClin Drug Investig2009296381391
- MouradJJLe JeuneSEffective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy: post hoc analysis of data from a randomized, double-blind, parallel-group, multicentre studyClin Drug Investig2009296419425
- GossePLasserreRMinifieCLemetayerPClementyJBlood pressure surge on risingJ Hypertens20042261113111815167445
- ChavanuKMerkelJQuanAMRole of ambulatory blood pressure monitoring in the management of hypertensionAm J Health Syst Pharm200865320921818216005
- PickeringTGWhiteWBASH position paper: home and ambulatory blood pressure monitoring. When and how to use self (home) and ambulatory blood pressure monitoringJ Clin Hypertens (Greenwich)2008101185085519128274
- NeutelJMKereiakesDJPunziHEfficacy and safety of an amlodipine/olmesartan medoxomil-based titration regimen on blood pressure (BP) assessed by mean 24-hr ambulatory BP monitoring in patients with hypertensionJ Clin Hypertens200911Suppl 4A129
- HeagertyAMLaeisPHaagUOlmesartan medoxomil/amlodipine (OLM/AML) provides 24-hour antihypertensive efficacy – additional effect by uptitration in patients with moderate-to-severe hypertensionJ Hypertens200927Suppl 4S283
- Daiichi Sankyo, Inc, ClinicalTrials.govBlood Pressure Lowering Ability and Safety of an Olmesartan and Amlodipine Based Treatment Regimen in Patients With Stage I and Stage II Hypertension2009114 Available from: http://clinicaltrials.gov/ct2/show/NCT00527514 Accessed Apr 24, 2010.
- PunziHLewinAShojaeeAWaverczakWFDubielRXuJEfficacy of an amlodipine/olmesartan medoxomil-based titration regimen on blood pressure goal achievement in patients with hypertensionJ Clin Hypertens200911Suppl 4A133
- NeutelJMLittlejohnTIIIShojaeeAWaverczakWFDubielRXuJ24 hour efficacy of an amlodipine/olmesartan medoxomil-based titration regimen on blood pressure at daytime, nighttime and last 6, 4, 2 hours of dosing intervalJ Clin Hypertens200911Suppl 4A130
- SicaDAFixed-dose combination antihypertensive drugs. Do they have a role in rational therapy?Drugs199448116247525192
- LeenenFHWilsonTWBolliPPatterns of compliance with once versus twice daily antihypertensive drug therapy in primary care: a randomized clinical trial using electronic monitoringCan J Cardiol199713109149209374947
- PaesAHBakkerASoe-AgnieCJImpact of dosage frequency on patient complianceDiabetes Care19972010151215179314626
- TaylorAAShoheiberOAdherence to antihypertensive therapy with fixed-dose amlodipine besylate/benazepril HCl versus comparable component-based therapyCongest Heart Fail20039632433214688505
- DicksonMPlauschinatCACompliance with antihypertensive therapy in the elderly: a comparison of fixed-dose combination amlodipine/benazepril versus component-based free-combination therapyAm J Cardiovasc Drugs200881455018303937
- SalehSSSzebenyiSCarterJAZacherCBellettiDPatterns and associated health services costs of antihypertensive drug modificationsJ Clin Hypertens (Greenwich)2008101435018174770
- RabbaniAAlexanderGCOut-of-pocket and total costs of fixed-dose combination antihypertensives and their componentsAm J Hypertens200821550951318437141
- SaitoIKobayashiMMatsushitaYMoriAKawasugiKSarutaTCost-utility analysis of antihypertensive combination therapy in Japan by a Monte Carlo simulation modelHypertens Res20083171373138318957808
- TakaseBTakeishiYHiraiTComparative effects of amlodipine monotherapy and combination therapy with betaxolol on cardiac autonomic nervous activity and health-related quality of life in patients with poorly controlled hypertensionCirc J200872576476918441457
- NeutelJMComplementary mechanisms of angiotensin receptor blockers and calcium channel blockers in managing hypertensionPostgrad Med20091212404819332961