Abstract
Doripenem is the latest carbapenem on the market to date. Although not an antibiotic in a new class, it offers a glimmer of hope in combating serious infections secondary to multidrug-resistant Gram-negative bacteria when we have not seen a new class of antibacterial, particularly for Gram-negative bacteria, for more than 10 years. In vitro, doripenem exhibits a broad spectrum of activity against Gram-positive and Gram-negative bacteria, including extended-spectrum β-lactamase (ESBL) and Amp-C β-lactamase producing Enterobacteriaceae and anaerobes. Doripenem also exhibits better in vitro activity against Pseudomonas aeruginosa compared to other anti-pseudomonal carbapenems. It combines the desirable activities of both imipenem and meropenem. It has similar activity to imipenem against Gram-positive pathogens and has the antimicrobial spectrum of meropenem against Gram-negative organisms. Several randomized clinical trials have demonstrated that doripenem is non-inferior to meropenem, imipenem, piperacillin/tazobactam, or levofloxacin in its efficacy and safety profile in treating a wide range of serious bacterial infections including intra-abdominal infection, complicated urinary tract infection, and nosocomial pneumonia. Due to its wide spectrum of activity and good safety profile it is susceptible to misuse leading to increasing rates of resistance. Judicious use should be considered when using doripenem as a first-line agent or drug of choice for serious infections. Doripenem is a well-tolerated drug with common adverse effects including headache, nausea and diarrhea. Caution should be used in patients with hypersensitivity to carbapenems and adverse reactions to β-lactam agents. Dosage adjustment is needed for patients with renal impairment. Doripenem has demonstrated economic and clinical benefits. It has been shown to reduce hospital length of stay and duration of mechanical ventilation for intensive care unit (ICU) patients. Therefore, doripenem is a welcome addition to our limited armamentarium of antibiotics available to treat serious bacterial infections in hospitalized patients.
Introduction
The emergence of antimicrobial resistance among Gram-negative organisms, especially extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, and resistant Pseudomonas and Acinetobacter species, has been a significant challenge for the clinician.Citation1 β-lactams are one of the most widely prescribed antimicrobial agents in the hospital setting. However, their use has resulted in a dramatic increase in the selection of β-lactamase variants, which threatens the utility of this class of antimicrobial.Citation2 The development and approval of carbapenems was a milestone in addressing this situation because of their broad-spectrum activity against Gram-positive, Gram-negative, and anaerobic bacteria.Citation3
Table 1 In vitro susceptibility of common pathogens to doripenemCitation2,Citation4,Citation10
Table 2 Pharmacokinetic properties of doripenem following a single 1-hour intravenous infusion of a 500 mg dose administered to healthy participants (N = 24)Citation10
Table 3 Manufacturer renal dosage adjustment recommendationsCitation10
Table 4 Adverse events reported with doripenem 500 mg IV every 8 hours for treatment in complicated urinary tract infections and intra-abdominal infections in three Phase 3 clinical trialsCitation20
Multi-drug resistant Gram-negative bacteria have been a significant cause of morbidity and mortality especially for hospitalized patients. There is an ongoing need for effective pharmacotherapy against these microorganisms. Among these are Pseudomonas aeruginosa, Acinetobacter spp., specifically Acinetobacter baumannii and Klebsiella pneumoniae. Colistin is an agent which has been used as empiric treatment for metallo-β-lactamase-producing K. pneumoniae. There are three other agents, that are either in development or on the market, worth mentioning that possess antimicrobial activity against multi-drug resistant Gram-negative bacteria: RO49084631/CS-023, tigecycline and CP3242.Citation4
RO49084631/CS023 is a carbapenem that has demonstrated activity against Gram-positive and Gram-negative aerobes and anaerobes such as methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus epidermidis (MRSE), penicillin-resistant Streptococcus pneumoniae (PRSP), β-lactamase-negative ampicillin-resistant Haemophilus influenzae and P. aeruginosa. Tigecycline is a bacteriostatic agent which inhibits protein translation. It possesses activity against MSSA, MRSA and MRSE, as well as vancomycin-resistant Enterococcus spp. Lastly, CP3242 is an inhibitor of metallo-β-lactamases. It demonstrated potency against MBL-producing P. aeruginosa in vivo and in vitro when combined with biapenem, imipenem, meropenem or ceftazidime.Citation4
Because the carbapenems have the broadest antibacterial spectrum of activity, there is concern that abuse or overuse of this class of antibiotic will lead to the development of resistance.Citation5 Various methods have been suggested in an effort to either delay resistance selection or to enhance activity against resistant strains including the co-administration of an aminoglycoside with doripenemCitation1 and prolonging antibiotic infusion,Citation6 respectively.
Rising rates of antibiotic-resistance among bacteria in the hospital setting highlight the need for new therapeutic options,Citation7 in particular, for the treatment of MRSA and other organisms which are resistant to doripenem. Although doripenem offers a glimmer of hope in combating serious hospital infections, the fact remains that only ten new antibiotics have been approved within the past ten years, of which only two have been truly novel. This highlights the growing concern over the present drought in the antibiotic research and development pipeline and, therefore, the need for further emphasis on drug discovery.
Chemistry
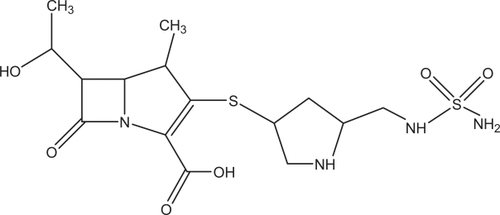
Doripenem is the newest member of the carbapenems on the US market. Human kidneys produce dehydropeptidase-1 (DHP-1), which can hydrolyze and inactivate imipenem. However, the addition of a methyl chain on the nucleus of doripenem increases its stability against DHP-1.Citation8 This methyl side chain is also found in meropenem and ertapenem. Doripenem has a sulfamoly-aminomethyl-pyrrolidinylthio side chain replacing the dimethyl-carbamoyl-pyrrolidinylthio side chain in position 2 of meropenem. This unique side chain of doripenem enhances its potency against Gram-positive bacteria, such as methicillin-sensitive S. aureus, while maintaining its activity against Gram-negative bacteria.Citation4
Mode of action
Like other β-lactams and carbapenems, doripenem exhibits its antimicrobial effects by binding penicillin binding proteins (PBP) that are necessary to maintain the bacterial cell wall leading to cell death.Citation9,Citation10 Doripenem binds to PBP-2, PBP-3, and PBP-4.Citation10 Affinities for PBPs vary by species.Citation5
Pharmacodynamics
The bactericidal effects are nonconcentration dependent; the longer the time above the minimum inhibitory concentration (T > MIC), the greater the effect.Citation5,Citation11–Citation14 The proportion of time during the dosing interval, where the concentration is greater than the MIC for non-protein bound carbapenems, correlates to in vivo activity of the drug for a specific organism.Citation15 In general, for carbapenems, a T > MIC of 20% results in a bacteriostatic effect with the maximum bactericidal effect at 40%.Citation4,Citation16 Katsube et al studied the %T > MIC for doripenem against three strains of P. aeruginosa. They showed a static effect with a T > MIC at 25%, 23.9% and 39.8% and a 2-log killing effect at 28.1%, 29.5% and 49.6% and a 90% maximum killing effect at 36.5%, 46.8% and 80.7% for respective strains.Citation17
Spectrum of activity
Doripenem has a broad spectrum of activity against Gram-positive, Gram-negative and anaerobic bacteria including multidrug-resistant strains, most notably the ESBL and Amp-C β-lactamase producing Gram-negative organisms.Citation2,Citation5,Citation9–Citation11,Citation13,Citation14,Citation18 It has comparable antimicrobial activity to meropenem and imipenem.Citation2,Citation13,Citation14 In vitro activity against P. aeruginosa, Acinetobacter spp., ceftazidime-resistant bacteria and selected ESBL-producing bacteria show lower MICs with doripenem. For example, Fritsche et al through analysis of the antimicrobial activity of 16,008 clinical isolates, demonstrated that doripenem was the most active agent (MIC90 8 mg/L) among the carbapenems tested against wild type P. aeruginosa. In this study, the MIC90 of meropenem and ertapenem were found to be 16 mg/L and >8 mg/L, respectively. However, the clinical relevance is unknown.Citation2,Citation19 Doripenem has in vitro activity against H. influenzae (including β-lactamase positive isolates) and Moraxella catarrhalis.Citation2,Citation18
As with other carbapenems, doripenem is not active against Enterococcus faecium or MRSA because of alteration of the bacteria’s PBPs leading to poor binding affinity.Citation13, Citation20
Although doripenem is stable against most β-lactamases, some carbapenemases will affect its activity. The Ambler’s class B metallo-β-lactamase is a potent cabapenemase which is intrinsically produced by Stenotrophomonas maltophilia, resulting in resistance to carbapenems, including doripenem. However, some strains of Gram-negative bacteria, such as P. aeruginosa, Acinetobacter baumannii and Enterobacteriaceae, are resistant to doripenem by producing Ambler’s class A enzymes, class B metallo-β-lactamases (IMP, VIM, SPM), class D enzymes (OXA group) and KPC enzymes.Citation2,Citation15,Citation18,Citation21, Citation22
Recently, the incidence of carbapenemase-producing Enterobacteriaceae (CRE) including Klebsiella spp. has been increasing globally. Serine β-lactamase KPC is the main carbapenemase produced by CRE. Because CRE are highly resistant to doripenem (MIC 8 to >64 μg/ml) and other carbapenems, there is no reliable antibiotic treatment for patients infected with CRE, thus, causing high morbidity and mortality in hospitalized patients.Citation23,Citation24
P. aeruginosa and other Gram-negative bacteria can confer resistance to doripenem by overexpressing multidrug efflux pump system called MexA-MexB-OprM, resulting in decreased antibiotic concentration at target sites.Citation25 These bacteria can also exhibit resistance to many antibiotics through a combination of increased permeability, efflux system and production of β-lactamases. One way of increasing permeability is through loss of OprD porin of the organisms. The sensitivity of P. aeruginosa to imipenem is reduced by loss of OprD porin; however, Mushtaq and co-workers demonstrated that a combination of loss of OprD porin and presence of efflux pump is necessary for P. aeruginosa to develop resistance against meropenem and doripenem.Citation26
Susceptibility to doripenem was defined as ≤0.5 μg/mL for Enterobacteriaceae, ≤2 μg/mL for P. aeruginosa, ≤1 μg/mL for Acinetobacter baumannii and anaerobes, and ≤ 0.12 μg/mL for Streptococcus anginosus group. However, when susceptibility reports of doripenem are not available, Jones and colleagues reported carbapenems (imipenem, meropenem, ertapenem), oxacillin and ampicillin can be used as surrogate testing agents with high absolute categorical agreement (89.1% to 100%) and negligible false-susceptible error (< 0.1%).Citation27
Pharmacokinetics
Among a group of healthy study participants given a single 1-hour intravenous infusion of 500 mg, the mean plasma concentration max (Cmax) was 23 μg/mL and the mean area under the curve (AUC) was 36.3 μg·h/mL.
Doripenem has linear kinetics when infused intravenously over one hour over a dose range of 500 mg to 1 g.Citation10 It has an 8.1% average binding to plasma proteins with a median volume of 16.8 L (8.09 tp 55.5 L) among healthy participants.Citation10 Doripenem also demonstrates significant penetration into several body fluids and tissues, including peritoneal and retroperitoneal fluids, urine, bile and the gallbladder.Citation10
Doripenem is metabolized by dehydropeptidase-I to an inactive metabolite (doripenem-M1). It does not interact with the cytochrome P450 (CYP450) enzymes as either a substrate or an inhibitor.Citation10 In healthy volunteers, 15% of the dose was recovered in urine as metabolite within 48 hours.Citation10
The approved dosing and administration of doripenem is 500 mg every 8 hours infused over one hour for patients >18 years of age for complicated intra-abdominal infections (cIAIs) or complicated urinary tract infections (cUTIs).Citation10 Doripenem is eliminated primarily by the kidneys via glomerular filtration and active tubular secretion with 70% recovered in the urine as unchanged drug.Citation10 The half-life of doripenem is approximately 1 hour among healthy adults.Citation10 Doripenem dose adjustment for renal impairment is required.
Dosage adjustments are not recommended for the elderly nor should they be based on gender.Citation10 Doripenem is hemodialyzable with systemic levels reduced by 48% to 62%.Citation4 However, the manufacturer has not given recommendations for dose adjustments in hemodialysis due to insufficient information.Citation10
Doripenem is stable in 0.9% sodium chloride for eight hours at room temperature and may exceed twelve hours.Citation4,Citation10,Citation14
Efficacy and safety of doripenem
Doripenem was approved by the United States Food and Drug Administration (FDA) in October 2007 for the treatment of cIAIs and cUTIs, including pyelonephritis.Citation4 The manufacturer (Johnson & Johnson Pharmaceutical Research and Development) of doripenem (Doribax®) has submitted an application for the additional indication of nosocomial pneumonia (NP), including ventilator-associated pneumonia, which is currently under FDA review.Citation28 Doripenem is indicated for NP in the European Union.Citation11
The following sections will discuss the clinical efficacy and safety of doripenem in treatment of cIAIs, cUTIs and NP.
Complicated intra-abdominal infections (cIAI)
Two Phase 3 trials were conducted to establish the efficacy and safety of doripenem in treating intra-abdominal infections.
Lucasti et alCitation29 conducted a prospective, multicenter, double blind trial which compared intravenous (IV) doripenem 500 mg every 8 hours (n = 237) with IV meropenem 1000 mg every 8 hours (n = 239). Patients in both groups could be switched to oral amoxicillin/clavulanate after adequate clinical improvement was achieved. The primary endpoint was to compare the clinical cure rates of doripenem versus meropenem in treatment of cIAIs in hospitalized patients at the test of cure (TOC) visit. The TOC visit could range from 21 to 60 days after completing the study drug therapy. The second endpoints were to compare (1) clinical responses at the end of IV treatment, at an early follow-up visit; (2) microbiological responses at the end of IV treatment, early follow-up and TOC visits; and (3) safety profiles.
Comparison of clinical cure rates at the TOC visit showed doripenem was non-inferior to meropenem. Microbiological eradication (ME) rates for common pathogens (eg, Escherichia coli, P. aeruginosa) were not statistically different between the treatment groups.
The most common treatment related adverse reactions were nausea, fever, diarrhea, anemia and phlebitis for both carbapenems. There was no significant difference with regard to their safety profile.
Another prospective, multicenter, double blind trial conducted by Malafaia and colleaguesCitation30 was similar in design to the study by Lucasti et al. The researchers compared IV doripenem 500 mg every 8 hours a day versus meropenem 1000 mg every 8 hours a day in treating intra-abdominal infections. Patients in both groups were allowed to switch to oral amoxicillin/clavulanate after completing a minimum of 9 doses of IV antibiotics.
Four hundred and eighty-six patients were randomized to either the doripenem group or the meropenem group. The patients were first assessed for early efficacy and safety 7 to 14 days after the end of therapy. They were then assessed for microbiologic and clinical efficacy (CE) 28 to 42 days after the TOC visit. The primary endpoint was to compare clinical cure rates in the ME group at the TOC visit and in the microbiological modified intent-to-treat (mMITT) group. Secondary endpoint was to compare clinical cure rates in the CE group and microbiologic response in the ME group at the TOC visit. Doripenem was shown to be non-inferior to meropenem in achieving both primary and secondary endpoints.
Safety profile for both carbapenems was very similar and there were no deaths during the study that were attributable to any of the antibiotics. The most common adverse reactions related to doripenem were nausea, vomiting, diarrhea and anemia.
In essence, the outcomes of Malafaia’s study were similar to Lucasti’s with respect to efficacy and adverse drug events.
Complicated urinary tract infections
A Phase 3, prospective, multicenter, randomized double-blind trial conducted by Naber et alCitation31 compared IV doripenem 500 mg every 8 hours versus IV levofloxacin 250 mg every 24 hours in the treatment of cUTI. Both antibiotics were administered as a 1-hour IV infusion. Seven hundred and fifty-three patients were enrolled, 377 in the doripenem arm and 376 in the levofloxacin arm. The primary endpoint is to determine the microbiologic response at the TOC visit, which is defined as 6 to 9 days after the completion of study drug therapy following a 10-day treatment regimen. The secondary objective is to determine the clinical response at the TOC visit. After ≥9 doses of IV study drug therapy, patients could be switched to oral levofloxacin if the patient became afebrile, and had no signs or symptoms of cUTI and negative urine culture.
Analysis of the efficacy results revealed that doripenem was non-inferior to levofloxacin in microbiological and clinical effectiveness. Doripenem was effective against major causative organisms of cUTIs including; E. coli, Proteus mirabilis, and K. pneumoniae. Analysis of safety data indicated doripenem was well tolerated by patients without demonstrating serious adverse reactions, such as seizures. The most common side effect for this study was headache.
Another randomized, controlled, double-blind trial (n = 155) was conducted in Japan by Kamidono et alCitation32 to compare the efficacy of IV doripenem 250 mg every 12 hours (n = 76) versus IV meropenem 500 mg every 12 hours (n = 79) in patients with cUTIs requiring parenteral antibiotic therapy. All subjects were adult inpatients aged 20 to 79 years of age who demonstrated both pyuria and bacteriuria. Clinical efficacy was 93.4% (71/76) in the doripenem group versus 92.4% (73/79) in the meropenem group. The bacteriologic response was 95.9% (94/98) in the doripenem group versus 96% (101/105) in the meropenem group. The authors concluded that doripenem was non-inferior to meropenem, clinically and bacteriologically. In regard to safety data, adverse drug reactions occurred in 4.3% of the doripenem group and 4.0% of the meropenem group. These results demonstrated that both doripenem and meropenem have a high safety profile.
Nosocomial pneumonia
Several clinical studies have been conducted to examine the efficacy and safety of doripenem versus other antibiotics on the market in treating NP.
The first study was a Phase 3, multicenter, prospective, randomized, open-label study conducted by Réa-Neto et alCitation33 to compare IV doripenem q8h 60-minute infusion versus intravenous piperacillin/tazobactam 4.5 g every 6 hours 30-minute infusion in non-ventilated NP patients and patients with early-onset ventilator-associated pneumonia (VAP). Four hundred forty-eight adult patients were enrolled, 225 were randomized to the doripenem group and 223 to the piperacillin/tazobactam group. Randomization was stratified by mechanical ventilation association, severity of illness, and geographic region. After 72 hours of IV study drug therapy, the patients were allowed to switch to oral levofloxacin 750 mg daily if they met all the criteria, which indicated sufficient clinical improvement.
The primary endpoint was to compare the clinical cure rate of IV doripenem versus IV piperacillin/tazobactam at the TOC visit in patients with NP. The TOC visit was conducted 7 to 14 days after completion of study drug therapy. Secondary endpoint was to compare the clinical response rate and microbiological response rate of IV doripenem versus IV piperacillin/tazobactam and to compare the safety profile of the two antibiotics.
Clinical cure rates for meropenem and piperacillin/tazobactam were 67.6% and 67.4%, respectively. Microbiological cure rate was 84.5% for doripenem versus 80.7% for piperacillin/tazobactam. The primary endpoint analysis revealed that doripenem was non-inferior to piperacillin/tazobactam in efficacy in treatment of NP. Secondary endpoint analyses demonstrated that doripenem was clinically and therapeutically noninferior to piperacillin/tazobactam.
The most common adverse drug reactions in the doripenem group were increased liver enzymes, thrombocythemia and diarrhea. Adverse events were comparable between doripenem (16.1%) and piperacillin/tazobactam (17.6%), and the authors concluded that both doripenem and piperacillin/tazobactam have high safety profiles.
The second study was a Phase 3 prospective, multicenter, randomized open-label trial conducted by Chastre et alCitation34 to compare IV doripenem 500 mg every 8 hours 4-hour infusion versus IV imipenem 500 mg every 6 hours 30-minute infusion, or 1000 mg every 8 hours 60-minute infusion in treating adult patients with VAP. Duration of treatment was 7 to 14 days for both antibiotics. Number of patients enrolled was 531, 264 in the doripenem group and 267 in the imipenem group. Randomization was stratified by duration of mechanical ventilation, severity of illness and by region.
The study design for patients in the doripenem group to receive 4-hour infusion could be based on the Psathas et al study,Citation35 which was able to demonstrate that, in vitro, doripenem was stable for up to 12 hours in 0.9% NaCl solution at room temperature.
The primary endpoint was to compare the clinical response rate of IV doripenem versus IV imipenem at the TOC. The TOC was conducted 7 to 14 days after completion of study drug therapy. The secondary endpoint was to compare per subject microbiological response rate, per pathogen microbiological outcome rate, per pathogen clinical cure rate, and the safety profile of the two antibiotics.
Clinical cure rates in the ME group for doripenem and imipenem were 69.0% and 64.5%, respectively. Favorable per-subject microbiological response rate in the ME at TOC visit for doripenem and imipenem was 73.3% and 67.3%, respectively. The results demonstrated that doripenem was not inferior to imipenem in efficacy in treating VAP.
The most common drug related adverse reactions with doripenem were increased liver enzymes (4.6%), diarrhea (1.9%), rash (1.9%), and vomiting (1.5%). The incidence of adverse events was 17.2% for doripenem versus 17.5% for imipenem. Thus, doripenem was generally well tolerated and the safety profiles of doripenem and imipenem were comparable.
A Japanese study examined the clinical efficacy and safety of IV doripenem 250 mg every 12 hours versus IV meropenem 500 mg every 12 hours in a randomized, double-blind trial in treatment of patients with respiratory infections.Citation36 The majority of the patients in both groups were patients with bacterial pneumonia (131/193), and the rest were patients with concomitant chronic respiratory tract and other lung diseases (eg, chronic bronchitis, bronchietasis, bronchial asthma or emphysema) (62/193). Both groups were treated for 7 days. One hundred and ninety-three patients were evaluated for clinical efficacy. Clinical efficacy was 92.7% and 90.7% in the doripenem group and the meropenem group, respectively. The authors concluded that doripenem demonstrated noninferiority to meropenem in clinical efficacy. For safety profile, 218 patients were available for data analysis. The incidence of nonlaboratory adverse drug reactions was 8.1% in the doripenem group and 6.5% in the meropenem group. For laboratory adverse drug reactions, the incidence was 23.4% and 25.5% in the doripenem group and meropenem group, respectively. The authors found no significant difference between nonlaboratory and laboratory adverse drug reaction incidence.
In summary, although doripenem has not yet been approved by the FDA for treating NP, the prospective clinical trials mentioned above found that doripenem was not inferior to other antibacterial agents on the market for treating cIAIs, cUTIs and NP in terms of efficacy and adverse effects.
Safety, drug interactions, and tolerability
Adverse reactions
In general, doripenem is well tolerated.Citation4,Citation10,Citation37 The most common adverse reactions reported in Phase 3 clinical trials included headache, nausea, diarrhea, rash, and phlebitis.Citation20
Rare, but serious, doripenem postmarket adverse events reported included anaphylaxis, neutropenia, Stevens-Johnson Syndrome, toxic epidermal necrolysis, interstitial pneumonia, and seizure.Citation10 Doripenem was noted to cause a mild elevation in alanine aminotransferase and aspartate aminotransferase enzymes.Citation33 Doripenem has also caused pneumonitis when administered via inhalation, therefore, this route should be avoided.Citation10
Previous carbapenems have been noted to induce seizures primarily via inhibition of γ-aminobutyric acid (GABA) receptor binding.Citation20,Citation29,Citation34,Citation38 Doripenem has low affinity to the GABA receptor, resulting in low potential for seizure induction.Citation34 In an experiment by Horiuchi et al utilizing rats and dogs, doripenem did not induce seizure-like behavior or activity on electroencephalogram (EEG). In addition, doripenem did not induce convulsive activities post intra-cerebroventricular injection.Citation4,Citation38 These findings are consistent with other Phase 3 clinical trials of doripenem.Citation33
Safety
Doripenem should be used with caution or avoided in patients with previous hypersensitivity to β-lactam antibiotics. Severe anaphylaxis and serious skin reactions have developed in those patients with hypersensitivity to β-lactam antibiotics.Citation4,Citation10,Citation39 Structural similarity of the bi-cyclic core of β-lactam antibiotics is thought to be the underlying cause of cross-reactivity.Citation37,Citation39 A retrospective chart review concluded that carbapenem hypersensitivity reaction occurred in 9% to 11% of patients with a history of penicillin allergy and 3% to 4% in patients without penicillin allergy.Citation4,Citation37,Citation39
Due to lack of safety and efficacy data in the pediatric population, doripenem is only recommended for patients ≥18 years of age.Citation10,Citation20 Imipenem has been approved for use in neonates; ertapenem and meropenem in infants ≥3 months of age.Citation20
In pregnancy, doripenem is classified as a category B medication as it did not produce teratogenic effects, ossification, or developmental delays. Animal reproduction studies have failed to demonstrate a fetal risk; however, there have been no controlled studies in pregnant women or animal studies demonstrating adverse effects. Doripenem should be used with caution in pregnant women and should be administered only if there are clear indications.Citation10
Drug interactions
Doripenem should be used with caution when administered concomitantly with valproic acid due to the inhibition of valproic acid glucuronide hydrolysis leading to sub-therapeutic valproic serum levels resulting in possible seizure activity.Citation4,Citation10 Valproic levels should be monitored carefully with concomitant doripenem use.Citation10,Citation20 A different antibiotic or anticonvulsant should be considered if the valproic level is not readily maintained in therapeutic range.Citation10,Citation20
Concomitant use of doripenem and probenecid should be avoided.Citation10 Probenecid interferes with the active tubular secretion of doripenem resulting in an increased doripenem plasma concentration causing a 75% increase AUC and prolonged plasma elimination half life by 53%.Citation10,Citation20,Citation33
Place in therapy
Generally, doripenem is well tolerated in patients with serious bacterial infections. The most common side effects include headache, nausea and diarrhea, while the most commonly reported laboratory abnormalities included increased levels of ALT and AST.Citation14 There is less seizure potential as it has the lowest GABA receptor binding affinity among the carbapenems.Citation34,Citation38 Caution should be used in patients with known hypersensitivity to carbapenems or β-lactam agents.
Doripenem is used as a single agent for the treatment of cIAIs or complicated cUTIs in adult patients.Citation18 Doripenem’s dosing schedule makes it fairly easy to administer in hospitals, nursing homes, or other skilled nursing facilities. In limited circumstances, doripenem may even be used for qualified patients requiring outpatient (home) intravenous antibiotics.
Doripenem has demonstrated a decrease in the length of hospital stay and time of mechanical ventilation in a Phase 3, randomized, open-label, noninferiority study.Citation40 It combines the desirable attributes of imipenem and meropenem, which makes it a favorable drug against serious bacterial infections. It is well-tolerated and proven to be cost-effective leading to greater patient and hospital satisfaction. These factors make doripenem a broad-spectrum antibiotic that can be used to treat a wide range of infections while minimizing negative effects on patients.
Since carbapenems have time-dependent bactericidal effects, the optimal method to maximize the %T > MIC is to give the drug as a continuous infusion.Citation16,Citation41 Alternative dosing strategies such as continuous or prolonged infusions of previous carbapenems (ie, imipenem and meropenem) have had limited feasibility due to their stability at room temperature for 4 to 6 hours.Citation16 The prolonged stability of doripenem at room temperature may allow for prolonged infusions in an effort to extend the T > MIC and, therefore, enhance antimicrobial activity. Ikawa et al reported that prolonging the infusion time (4 hours) was more effective in increasing the pharmacokinetic and pharmacodynamic breakpoint to achieve a T > MIC of 40%.Citation6 Van Wart et al used pharmacokinetic-pharmacodynamic modeling to show that a higher dose of doripenem (1000 mg) infused over 4 hours every 8 hours was predicted to be effective for Gram-negative bacilli with MIC values of 8 mcg/mL, which would currently be considered carbapenem nonsusceptible (MICs, ≥ 8 μg/mL).Citation42 Ongoing clinical trials are evaluating the use of this higher dose and prolonged infusion time for hospital-acquired pneumonia.Citation42
Conclusions
Doripenem is a valuable drug that is being used more frequently in antibiotic therapy. It has proven its efficacy against NP, cIAIs and cUTIs. It is currently the latest FDA approved carbapenem and combines the antimicrobial activity of meropenem against Gram-negative bacteria and imipenem’s action against Gram-positive organisms. Doripenem offers further solution to the treatment of nosocomial infections given its efficacy, spectrum, and β-lactamase stability compared to other carbapenems.Citation2 Present data suggests that doripenem can play an important role in patients with serious nosocomial infections in the setting of multidrug resistant Gram-negative organisms where P. aeruginosa is prevalent.Citation4
The efficacy and tolerability of doripenem in adults with cIAIs, cUTIs, and VAP, has been shown in several early trials.Citation18,Citation29,Citation34
A recent study comparing resource utilization with doripenem versus imipenem from a hospital perspective among patients with VAP has demonstrated a significantly shorter length of stay and time on mechanical ventilation with doripenem. This study suggests that doripenem use may be economically and clinically beneficial to hospitals and patients.Citation40 These desirable effects make doripenem a key drug to treat serious bacterial infections.
Doripenem’s broad-spectrum of activity, its reported efficacy, and cost-effectiveness may make this the drug of choice for serious bacterial infections. Current clinical trials and in vitro susceptibilities have shown that doripenem may be useful for adult patients with serious infections that require broad spectrum antibiotics against multidrug resistant pathogens.Citation43
In vitro testing demonstrated that doripenem exhibits low potential for selecting for resistance.Citation14 One recent study examined 34 carbapenem-resistant P. aeruginosa isolates and found that doripenem displayed the lowest rates of resistance with an MIC50 value of 8 mg/mL.Citation19 Doripenem may soon be widely used in the hospital setting to treat serious polymicrobial infections.
However, doripenem’s broad spectrum of activity and safety profile make it susceptible to misuse and overuse.Citation44 This can result in higher resistance rates to common Gram-negative bacteria such as P. aeruginosa. Care should, therefore, be taken when considering doripenem as a first-line drug. Development of in vivo resistance against doripenem should be further investigated.Citation4 Local hospital epidemiology and bacterial resistance patterns should be key factors in making clinical use decisions.
Disclosures
The authors declare no conflicts of interest.
References
- VergidisPIFalagasMEShimadaJYamaguchiKShibaTMultidrug-resistant Gram-negative bacterial infections: the emerging threat and potential novel treatment optionsCurr Opin Investig Drugs200892176183
- FritscheTRStilwellMGJonesRNAntimicrobial activity of doripenem (S-4661): a global surveillance report (2003)Clin Microbiol Infect2005111297498416307551
- ShimadaJYamaguchiKShibaT[A new carbapenem antibiotic for injection: characteristics of doripenem]Jpn J Antibiot200558648950616521342
- PoulakouGGiamarellouHDoripenem: an expected arrival in the treatment of infections caused by multidrug-resistant Gram-negative pathogensExpert Opin Investig Drugs2008175749771
- LoTSWelchJMAlontoAMVicaldo-AlontoEAA review of the carbapenems in clinical use and clinical trialsRecent Pat Antiinfect Drug Discov20083212313118673125
- IkawaKMorikawaNUeharaSPharmacokinetic-pharmacodynamic target attainment analysis of doripenem in infected patientsInt J Antimicrob Agents200933327627919095418
- WeigeltJAEmpiric treatment options in the management of complicated intra-abdominal infectionsCleve Clin J Med200774Suppl 4S29S3717847176
- FukasawaMSumitaYHarabeETStability of meropenem and effect of 1 beta-methyl substitution on its stability in the presence of renal dehydropeptidase IAntimicrob Agents Chemother1992367157715791510457
- ZhanelGGWiebeRDilayLComparative review of the carbapenemsDrugs20076771027105217488146
- Package insert. Doribax® (doripenem for injection)Raritan, NJOrtho-McNeil Pharmaceuticals, Inc2008 Available from www.doribax.com/doribax/shared/pi/doribax.pdf Accessed: January 1, 2009.
- HagermanJKKnechtelSAKlepserMEDoripenem: A new extended-spectrum carbapenem antibioticFormulary200742676688
- NicolauDPCarbapenems: a potent class of antibioticsExpert Opin Pharmacother200891233718076336
- GreerNDDoripenem (Doribax): the newest addition to the carbapenemsProc (Bayl Univ Med Cent)200821333734118628935
- Bulletin. New Product, Doribax® (Doripenem for Injection)Washington, DCAmerican Pharmacists Association2008
- MatthewsSJLancasterJWDoripenem monohydrate, a broad-spectrum carbapenem antibioticClin Ther2009311426319243706
- KotapatiSNicolauDPNightingaleCHKutiJLClinical and economic benefits of a meropenem dosage strategy based on pharmacodynamic conceptsAm J Health Syst Pharm200461121264127015259757
- KatsubeTYanoYYamanoYPharmacokinetic-pharmacodynamic modeling and simulation for bactericidal effect in an in vitro dynamic modelJ Pharm Sci20089794108411718314887
- KeamSJDoripenem: a review of its use in the treatment of bacterial infectionsDrugs200868142021205718778123
- JonesRNHuynhHKBiedenbachDJActivities of doripenem (S-4661) against drug-resistant clinical pathogensAntimicrob Agents Chemother20044883136314015273134
- CadaDLevienTMistryBBakerDDoripenem for InjectionHospital Pharmacy2008432210218
- NishioHKomatsuMShibataNMetallo-beta-lactamase-producing gram-negative bacilli: laboratory-based surveillance in cooperation with 13 clinical laboratories in the Kinki region of JapanJ Clin Microbiol200442115256526315528723
- JonesRNSaderHSFritscheTRComparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various beta-lactamase resistance mechanismsDiagn Microbiol Infect Dis2005521717415878447
- SchwaberMJCarmeliYCarbapenem-resistant Enterobacteriaceae: a potential threatJAMA2008300242911291319109119
- MushtaqSGeYLivermoreDMComparative activities of doripenem versus isolates, mutants, and transconjugants of Enterobacteriaceae and Acinetobacter spp. with characterized beta-lactamasesAntimicrob Agents Chemother20044841313131915047535
- LiXZNikaidoHEfflux-mediated drug resistance in bacteriaDrugs200464215920414717618
- MushtaqSGeYLivermoreDMDoripenem versus Pseudomonas aeruginosa in vitro: activity against characterized isolates, mutants, and transconjugants and resistance selection potentialAntimicrob Agents Chemother20044883086309215273124
- JonesRNSaderHSFritscheTRJanechekMJSelection of a surrogate beta-lactam testing agent for initial susceptibility testing of doripenem, a new carbapenemDiagn Microbiol Infect Dis200759446747217997070
- PRNewswire. “FDA requires additional information on Doribax for treatment of hospital-acquired pneumonia”. Johnson & Johnson Pharmaceuticals Research and Development L.L.C.; August 21, 2008 Available at: www.drugs.com/nda/fda-requires-additional-doribax-hospital-acquired-pneumonia-1610.html Accessed: January 14, 2009.
- LucastiCJasovichAUmehOEfficacy and tolerability of IV doripenem versus meropenem in adults with complicated intra-abdominal infection: a phase III, prospective, multicenter, randomized, double-blind, noninferiority studyClin Ther200830586888318555934
- MalafaiaOUmehOJiangJDoripenem versus meropenem for the treatment of complicated intra-abdominal infections [Abstract No L-1564b plus poster]47th Interscience Conference on Antimicrobial Agents and Chemotherapy2006San Francisco, CA, USA2006
- NaberKRedmanRKoteyPLlorensLKanigaKIntravenous therapy with doripenem versus Levofloxacin with an option for oral step down therapy in the treatment of complicated urinary tract infections and pyelonephritis [Abstract No. 833 plus poster]Proceedings of the 17th European Congress of Clinical Microbiology and Infectious Diseases and the 25th International Congress of Chemotherapy2007 March 31–April 3Munich, Germany2007
- KamidonoSArakawaSHiroseTDouble-blind, controlled study to evaluate safety and efficacy of doripenem and meropenem in patients with complicated urinary tract infection [in Japanese]Jpn J Chemother200553Suppl 1244259
- Rea-NetoANiedermanMLoboSMEfficacy and safety of doripenem versus piperacillin/tazobactam in nosocomial pneumonia: a randomized, open-label, multicenter studyCurr Med Res Opin20082472113212618549664
- ChastreJWunderinkRProkocimerPEfficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized studyCrit Care Med20083641089109618379232
- PsathasPAKuzmissionAIkedaKYasuoSStability of doripenem in vitro in representative infusion solutions and infusion bagsClin Ther200830112075208719108795
- SaitoAWatanabeANakataKComparative study of doripenem and meropenem in respiratory infections. Phase III double-blind comparative study [in Japanese]Jpn J Chemother200553Suppl. 1185204
- ListerPDCarbapenems in the USA: focus on doripenemExpert Rev Anti Infect Ther20075579380917914914
- HoriuchiMKimuraMTokumuraMAbsence of convulsive liability of doripenem, a new carbapenem antibiotic, in comparison with beta-lactam antibioticsToxicology20062221–211412416549226
- PrescottWAJrKusmierskiKAClinical importance of carbapenem hypersensitivity in patients with self-reported and documented penicillin allergyPharmacotherapy200727113714217192167
- MerchantSGastCNathwaniDHospital resource utilization with doripenem versus imipenem in the treatment of ventilator-associated pneumoniaClin Ther200830471773318498921
- KutiJLMaglioDNightingaleCHNicolauDPEconomic benefit of a meropenem dosage strategy based on pharmacodynamic conceptsAm J Health Syst Pharm200360656556812659058
- Van WartSAAndesDRAmbrosePGBhavnaniSMPharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patientsDiagn Microbiol Infect Dis200963440941419249182
- AndersonDLDoripenemDrugs Today (Barc)200642639940416845443
- VollesDFBrananTNAntibiotics in the intensive care unit: focus on agents for resistant pathogensEmerg Med Clin North Am200826381383418655946