Abstract
Nasal congestion is a common symptom in rhinitis (both allergic and nonallergic), rhinosinusitis and nasal polyposis. Congestion can also be caused by physical obstruction of nasal passages and/or modulation of sensory perception. Mucosal inflammation underlies many of the specific and interrelated factors that contribute to nasal congestion, as well as other symptoms of both allergic rhinitis and rhinosinusitis. A wide range of biologically active agents (eg, histamine, tumor necrosis factor-α, interleukins, cell adhesion molecules) and cell types contribute to inflammation, which can manifest as venous engorgement, increased nasal secretions and tissue swelling/edema, ultimately leading to impaired airflow and the sensation of nasal congestion. Inflammation-induced changes in the properties of sensory afferents (eg, expression of peptides and receptors) that innervate the nose can also contribute to altered sensory perception, which may result in a subjective feeling of congestion. Increased understanding of the mechanisms underlying inflammation can facilitate improved treatment selection and the development of new therapies for congestion.
Introduction
Nasal congestion or obstruction is one of the most frequent symptoms encountered in primary care and specialist clinics, and it is often the predominant symptom in upper respiratory tract disorders, such as allergic rhinitis, rhinosinusitis, nonallergic rhinitis, and nasal polyposis. Additionally, nasal congestion is also a common symptom in otitis media and asthma, and it can contribute to the onset or worsening of sleep disturbances, including obstructive sleep apnea.Citation1
The pathophysiology of nasal congestion, which may be best described as a perception of reduced nasal airflow or a sense of facial fullness, involves a number of underlying mechanisms. These include mucosal inflammation, often involving increased venous engorgement, increased nasal secretions, and tissue swelling/edema; physical problems affecting the structure of the nasal passage; and/or modulation of sensory perception. Many inflammatory and neurogenic mediators contribute to plasma exudation and vasodilatation, with resultant edema and swelling of the nasal mucosa. Various technological advancements including rhinomanometry and acoustic rhinometry, offer complementary tools to qualitatively and quantitatively study the nasal airway, providing greater insight into the physiological fluctuations and pathophysiological mechanisms that influence nasal patency.Citation2,Citation3
The purpose of this article is to review the various pathophysiological mechanisms that contribute to objective nasal congestion or the perception of nasal congestion.
Mucosal inflammation
Mucosal inflammation is the central pathophysiological mechanism that underlies many of the specific and interrelated factors that contribute to congestion, including increased venous engorgement, increased nasal secretions and tissue swelling/edema.Citation4 In the following sections, we will provide examples of parts of the inflammatory process, rather than encyclopedic coverage of the pathophysiological processes associated with each disease.
Inflammation associated with allergic rhinitis and rhinosinusitis can reduce the physical size of the nasal passages by inducing vasodilatation, increasing blood flow and increasing vascular permeability. The result is engorgement of nasal venous sinusoids, swelling of the anterior and inferior turbinates and obstruction of nasal airflow, ultimately contributing to nasal congestion.Citation5 In addition, some patients are unable to adequately control sinusoid venous engorgement, which may be due to conditions such as Horner’s syndrome, nasal reflex sympathetic dystrophy, rhinitis medicamentosa, and treatment with α-adrenergic antagonists.Citation6–Citation8 More frequently, however, sinonasal venous engorgement and inflammation are associated with common upper respiratory tract disorders, such as allergic rhinitis and rhinosinusitis.
Allergic rhinitis
The prevalence of allergic rhinitis is increasing worldwide, occurring in 10% to 30% of adults and up to 45% of children.Citation9–Citation11 Nearly 50% of patients with allergic rhinitis experience symptoms for >4 months of the year, and nasal congestion is frequently the predominant symptom. Other symptoms of allergic rhinitis include nasal itching, rhinorrhea, and sneezing, as well as ocular itching, redness, and tearing.Citation12
Although inflammation and tissue swelling/edema are frequent components of other common upper respiratory tract disorders, such as rhinosinusitis, the underlying inflammatory mechanisms have been primarily studied in the setting of allergic rhinitis. Symptoms of allergic rhinitis, including nasal congestion, are primarily due to a combination of the early and late-phase allergic inflammatory response.Citation13–Citation18 In a sensitized host, an antigen comes into contact with the nasal mucosa, leading to crosslinking of immunoglobulin E (IgE) receptors on mast cells. This results in degranulation of these cells and the release of histamine and proteases from preformed granules.Citation13–Citation15 In addition, an array of early-phase proinflammatory molecules are synthesized and released, most notably leukotrienes, prostaglandins, tumor necrosis factor (TNF)-α, and interleukin (IL)-4.Citation13,Citation19,Citation20 Release of these inflammatory mediators leads to swelling/edema and fluid secretion, resulting in congestion as well as other nasal symptoms.Citation19
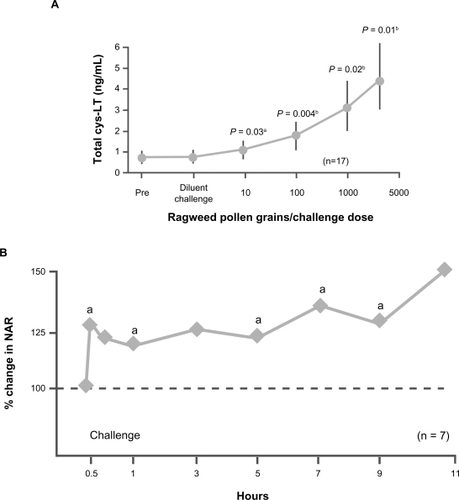
A large body of literature supports a role for leukotrienes as mediators in allergic rhinitis. Cysteinyl leukotrienes can be recovered in nasal secretions after exposure to natural allergensCitation21 and at elevated concentrations in allergic rhinitis with increased allergen dose exposure ().Citation22 Challenge with cysteinyl leukotriene also increases nasal airway resistance ().Citation23 In addition, cysteinyl leukotrienes may facilitate the maturation of eosinophil precursors and act as eosinophil chemoattractants, promoters of eosinophil adhesion, and inhibitors of eosinophil apoptosis.Citation20
Figure 1 Clinical data support a role for leukotrienes as mediators of congestion in allergic rhinitis. A) Cysteinyl leukotrienes (cys-LT) can be recovered at elevated levels in nasal secretions with increased allergen dose exposure in patients with allergic rhinitis. aVersus baseline. bVersus previous pollen dose and baseline. Adapted with permission from Creticos PS, Peters SP, Adkinson NF Jr, Naclerio RM, Hayes EC, Norman PS. Peptide leukotriene release after antigen challenge in patients sensitive to ragweed. N Engl J Med. 1984;310(25):1626–1630.Citation22 Copyright © 1984 Massachusetts Medical Society. All rights reserved. B) Challenge with cysteinyl leukotriene increases nasal airway resistance (NAR). aP < 0.05 vs baseline. Adapted with permission from Okuda M, Watase T, Mezawa A, Liu CM. The role of leukotriene D4 in allergic rhinitis. Ann Allergy. 1988;60(6):537–540.Citation23 Copyright © 1988 American College of Allergy, Asthma and Immunology.
Like leukotrienes, thromboxanes are arachidonic acid derivatives, released from mast cells and other inflammatory cells, that are found in nasal lavage fluid samples following nasal allergen challenge.Citation24,Citation25 In animal models, TXA2 agonists increase nasal airway resistance and vascular permeability. Citation25 TXA2 receptor antagonists have also been shown to improve congestion in animals and reduce nasal mucosal swelling in AR patients following allergen challenge.Citation24,Citation25
Prostaglandin D2 (PGD2) is the major prostanoid produced in the acute phase of allergic reactions, and it is thought to be associated with hypertrophic inflammation in the nose and recruitment of eosinophils.Citation26 A number of other biomarkers of inflammation, including tryptase, N-alpha-tosyl L-arginine methyl ester (TAME)-esterase and eosinophil cationic protein (ECP), are also detectable in the nasal mucosa within minutes to hours after allergen challenge. These mediators stimulate the early phase response and also lead to increased venous engorgement, which results in concomitant rhinorrhea and nasal congestion.Citation19
The chronic, late-phase inflammatory response involves cellular infiltration, which sustains tissue swelling and edema, further exacerbating congestion.Citation13–Citation15 As a result of cytokine or mediator release, the nasal mucosa becomes infiltrated with inflammatory cells including eosinophils, neutrophils, basophils, mast cells, and lymphocytes, that sustain and exacerbate the nasal mucosal inflammatory reaction.Citation20
Eosinophils are the predominant cell type in the chronic inflammatory processes that characterize the late-phase allergic response, and they release a broad array of pro-inflammatory mediators, including cysteinyl leukotrienes, ECP, eosinophil peroxidase, and major basic protein.Citation15,Citation20 These cells may also serve as a major source of IL-3, IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-13.Citation20 IL-5 is an eosinopoietic cytokine that promotes eosinophil differentiation and maturation within the bone marrow.Citation27 Circulating eosinophils are increased in number in subjects with allergic disorders, and infiltration at the site of provocation has been generally attributed to influx of mature cells. However, there appears to be a subset of eosinophil progenitor cells that undergo local maturation in the nasal mucosa, also in an IL-5-dependent fashion.Citation27 Eosinophil infiltration has been demonstrated to have a significantly negative correlation with nasal airflow in patients with allergic rhinitis.Citation28 The cellular infiltration of the late-phase response also primes the mucosa for additional antigen exposure and increases the response to it, thus further exacerbating symptoms upon continued exposure (eg, as the allergy season progresses).Citation29
In addition to eosinophils, other inflammatory cells, eg, basophils, mast cells, T cells, also accumulate within the nasal epithelium during the late-stage response.Citation13,Citation15 Leukocyte activation, with subsequent migration to sites of inflammation, leads to changes in the cell membrane (eg, increased integrin expression) that result in adhesion to the endothelial surface.Citation30 Survival of cells that have been recruited and migrated to sites of inflammation, particularly eosinophils and mast cells, is enhanced by epithelial generation of GM-CSF, IL-5, and stem cell factor (SCF).Citation30,Citation31 Cultured nasal epithelial cells have been shown to generate SCF in vitro, and levels of this growth factor are increased in nasal lavage fluids from patients with seasonal allergic rhinitis.Citation30
TNF-α is a key inflammatory mediator of the late-phase response, and its levels have been shown to be dramatically increased beginning at about 1 hour after allergen challenge.Citation19 Plasma exudation and preferential upregulation of neutrophils over eosinophils during the late phase are characteristic of the response to TNF-α.Citation32 This cytokine has been demonstrated to activate T cells, endothelial cells, fibroblasts, and macrophages to express cell surface receptors and to release additional inflammatory cytokines.Citation13,Citation14,Citation19 TNF-α also increases the expression of cell adhesion molecules (intercellular adhesion molecule 1 [ICAM-1] and vascular cell adhesion molecule 1 [VCAM-1]).Citation19 Proinflammatory interleukins (IL-1β, IL-6, and IL-8) are elevated in patients with allergic rhinitis and have been shown to promote the activation of immune cells as well as to enhance expression of receptors for cell adhesion molecules (eg, selectins, integrins).Citation19 These events, along with IgE synthesis and eosinophil/basophil priming, collectively contribute to inflammation, venous engorgement, nasal hyperreactivity, and symptoms of allergic rhinitis, including congestion.Citation19,Citation33
Nonallergic rhinitis (that which is not mediated by an IgE response) includes infectious rhinitis, vasomotor rhinitis, nonallergic rhinitis with eosinophilia syndrome (NARES), and hormonal rhinitis (that precipitated by pregnancy and menstrual irregularities). In particular, significant nasal congestion may be present with pregnancy-related rhinitis.Citation34
Rhinosinusitis
Rhinosinusitis is now the accepted term for a group of disorders characterized by inflammation of the mucosa of the nasal passages and paranasal sinuses. Although the term itself is specific, rhinosinusitis may be due to an array of etiologic agents, including microorganisms; noninfectious, nonimmunologic causes; and allergic and nonallergic immunologic inflammation.Citation34,Citation35
The most common cause of rhinosinusitis is viral infection (often referred to as the common cold).Citation36 A large body of research supports the view that symptoms of the common cold are not due to direct cytopathic effects of the viral infection. Rather, it appears that viral infection stimulates inflammatory pathways that, once activated, tend to prolong symptoms even after viral replication has been ablated.Citation37 In addition, an estimated 0.5% to 2% of cases of viral rhinosinusitis are complicated by secondary bacterial infections.Citation35
Regardless of etiology, rhinosinusitis is typically classified as acute or chronic, depending on duration of symptoms. Citation38 Although microorganisms play a predominant role in the etiology of acute rhinosinusitis, the role of infection in patients with chronic rhinosinusitis is controversial (although in a subset of chronic rhinosinusitis patients, fungi may play a role).Citation35 Furthermore, allergic and immunologic factors have also been shown to be associated with the development of rhinosinusitis; eg, perennial allergic rhinosinusitis is a documented predisposing condition for acute bacterial rhinosinusitis.Citation39
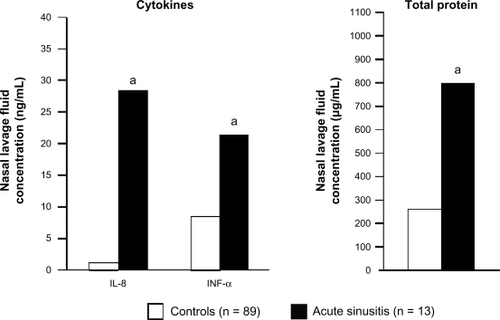
The pathogenesis of rhinosinusitis, like that of allergic rhinitis, includes secretion of proinflammatory cytokines. In patients with acute rhinosinusitis, levels of inflammatory cytokines and total protein are significantly increased in nasal lavage fluid compared with healthy controls ().Citation40 Kinin levels have also been found to be markedly increased in the nasal secretions of patients with acute viral rhinosinusitis, and elevated levels of IL-1, IL-6, and IL-8 have also been detected in the nasal secretions of these patients.Citation4,Citation41 Kinins can act on blood vessels to cause vascular leakage and/or engorgement, and they also stimulate afferent nerve fibers in the nasal mucosa, leading to hyperresponsiveness.Citation4,Citation42,Citation43 In addition, TNF-α and other proinflammatory cytokines are elevated during the course of naturally acquired acute viral upper respiratory tract infection.Citation44 Similar to allergic rhinitis, acute rhinosinusitis is also associated with significantly increased infiltration of inflammatory cells, including neutrophils and T cells, in the nasal epithelium and lamina propria.Citation45
Figure 2 Increased levels of inflammatory cytokines and total protein are found in nasal lavage fluids from patients with acute rhinosinusitis compared with healthy controls. aP ≤ 0.011 vs controls. Drawn from data of Repka-Ramirez et al.Citation40
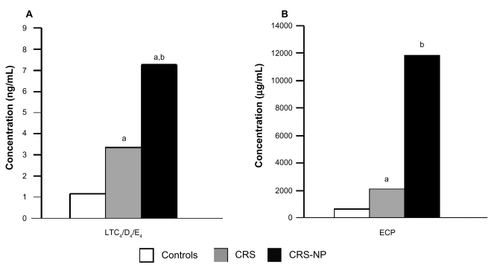
The pathophysiology of chronic rhinosinusitis is not fully understood; however, the cytokine and mediator profile in this condition closely resembles the profile found in acute rhinosinusitis, with the exception of a small but significant increase in ECP.Citation38 Nasal tissue samples taken from patients with chronic rhinosinusitis have been shown to have increased levels of leukotrienes C4, D4, and E4 and higher levels of markers of eosinophilic inflammation, such as ECP ().Citation46 In addition, a number of studies have reported that markers of atopy are more prevalent in populations with chronic rhinosinusitis.Citation47–Citation49 Although the role of allergy in chronic rhinosinusitis remains controversial, it has been postulated that swelling of the nasal mucosa in allergic rhinitis may restrict ventilation and obstruct sinus ostia, leading to mucus retention and infection.Citation47
Figure 3 Inflammation associated with chronic rhinosinusitis (CRS) and chronic rhinosinusitis with nasal polyps (CRS-NP). A) Levels of eicosanoid leukotrienes C4, D4, and E4 (LTC4/D4/E4) were significantly higher in nasal tissue taken from CRS and CRS-NP patients compared with healthy controls. aP < 0.05 vs controls. bP < 0.05 vs CRS. B) Levels of eosinophil cationic protein (ECP), a marker of eosinophilic inflammation, were significantly higher in nasal tissue taken from CRS and CRS-NP patients compared with healthy controls. aP < 0.05 vs controls. bP < 0.02 vs controls. Drawn from data of Pérez-Novo et al.Citation46
Nasal polyposis
Nasal polyposis is a chronic inflammatory disease of the upper airway characterized histologically by the infiltration of inflammatory cells, most notably eosinophils.Citation50 The cause or causes of nasal polyposis are not clear but may involve chronic infection, aspirin intolerance, alteration in aerodynamics with trapping of pollutants, epithelial disruptions, epithelial cell defects, or inhalant or food allergies.Citation51,Citation52 This condition may affect as much as 4% of the population,Citation53 and its symptoms include nasal obstruction, nasal discharge, and impairment of sense of smell.Citation47
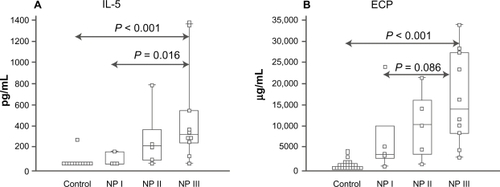
Congestion in nasal polyposis is related to edema formation secondary to inflammatory processes similar to those described above for allergic rhinitis and rhinosinusitis. Numerous studies have demonstrated the presence of eosinophils and related mediators in patients with allergic or nonallergic nasal polyposis. These mediators include IL-4, IL-5, IL-6, IL-8, IL-10, TNF-α, RANTES, GM-CSF, granulocyte colony-stimulating factor, ECP, eotaxin, and interferon (IFN)-γ ().Citation54–Citation57 Increased levels of metalloproteinases and destruction of extracellular matrix due to chronic inflammation are also thought to play a significant role in the pathobiology of nasal polyposis.Citation58
Figure 4 Inflammatory mediators and markers of nasal congestion in inferior turbinate tissue of control (those without polyps) and nasal polyposis (NP) patients. A) Patients with NP show significantly increased levels of interleukin (IL)-5, a proinflammatory cytokine, compared with controls. B) Patients with NP have increased levels of eosinophil cationic protein (ECP), a marker of eosinophilic inflammation, compared with controls. NP patients were grouped on the basis of the presence of specific immunoglobulin E (IgE) antibodies in tissue: NP I, undetectable specific IgE; NP II, selected specific IgE; and NP III, multiclonal IgE. The box-and-whisker plot represents the median, the lower to upper quartile, and the minimum to the maximum value, excluding outside and far-out values, which are displayed as separate points. Adapted from J Allergy Clin Immunol, Vol 107, Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P, Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. Pages 607–614.Citation57 Copyright © 2001, with permission from Elsevier.
The pattern of inflammatory markers in nasal polyposis differentiates this disease from chronic rhinosinusitis. T-cell response in CRS exhibits a Th1 pattern, in which interferon (IFN)-g, transforming growth factor-b1 (50) and IL-3 (51) are key players. A Th2 pattern predominates in nasal polyposis, characterized by IgE production, infiltration of eosinophils and upregulation of cytokines and mediators, including IL-5, ECP and eotaxin, that drive eosinophil response.Citation59 Staphylococcus aureus enterotoxins may play a role in the pathophysiology of NP. S. aureus colonization is higher in patients with NP compared to those with CRS, and IgE antibodies to S. aureus enterotoxins have been associated with higher levels of interleukin (IL)-5, eotaxin and eosinophil cationic protein (ECP).Citation59 Nasal exposure to S. aureus enterotoxin B was shown to augment eosinophilic inflammation, IgE production, and cytokine production in mice,Citation60 and recent data demonstrated that the stimulation of human NP tissue with S. aureus enterotoxin B significantly induced production of proinflammatory cytokines, with a shift toward a Th2 pattern of expression.Citation61
Structural problems
Nasal congestion can also occur secondary to structural causes, such as septal deviation, choanal atresia, concha bullosa, cleft palate, adenoid hypertrophy, and neoplasia.Citation34,Citation62–Citation66 The anterior nasal valve is the narrowest part of the airway, and inspiratory airflow through the nose can be compromised by the size of this nasal opening and the shape/structure of the nasal passages. Septal deviation may also cause impaired airflow and the symptom/perception of nasal congestion. However, significant anatomic variance exists across individuals; anterior deflections affecting the nasal valve have the greatest impact on airflow, while those in the middle and inferior part of the nasal cavity have little effect on airflow resistance.Citation67 Adenoid hypertrophy is another example of physical obstruction that can affect airflow, particularly in children, and it may also contribute to otitis media.Citation63
The recumbent position can influence both the perception of nasal obstruction and objective measurements of nasal volume and nasal cross-sectional area in normal subjects, as well as in patients with rhinitis.Citation68 It has been suggested that the nasal mucosa reaction to venous changes that alter local blood flow, secondary to compression of the neck veins or hydrostatic pressures, may give rise to this phenomenon. The perception of nasal obstruction induced by lying down seems to be greater in subjects with symptoms of rhinitis.Citation68
Secondary inflammation may result from neurologic responses that involve a wide range of neurotransmitter systems. The nasal mucosa is invested with sensory, parasympathetic and sympathetic nerves, and they may all contribute to reflex activation of glands or neurogenic inflammation. Sensory nerves generate sensations, including pruritus, and provide the afferent limb for motor reflexes, such as sneezing. Parasympathetic and sympathetic reflexes can affect both glandular and vascular function in the nose. Neural function can be chronically upregulated in the presence of mucosal inflammation. This may lead to neural hyperresponsiveness and neurogenic inflammation, which is thought to result from the release of peptides (eg, substance P, calcitonin gene-related peptide [CGRP], neurokinin A) from the peripheral terminals of nociceptive sensory nerve fibers. The molecular mechanisms underlying hyperresponsiveness are not fully understood but are thought to involve actions of neurotrophins on sensory afferents.Citation43,Citation69 The interplay of these molecules and the pathological processes they stimulate induce or exacerbate many typical upper respiratory symptoms, including congestion ().Citation43
Figure 5 Generation of nasal symptoms through neural pathways. Sensory nerves can be stimulated by products of allergic reactions and by external physical and chemical irritants. Signals are transmitted to the central nervous system (CNS), where they can trigger sensations (pruritus) and can further travel through secondary synapses to activate efferent motor (sneezing) and autonomic neurons. Action potentials traveling through parasympathetic efferent nerves can lead to glandular activation and rhinorrhea, as well as to some vasodilatation. Suppression of sympathetic neural output, on the other hand, results in vasodilatation and nasal congestion. Antidromic stimulation of sensory nerves with release of tachykinins and other neuropeptides at the nasal mucosa contributes to symptom development with glandular activation, vasodilatation, and plasma extravasation. Neuropeptide release can also lead to leukocyte recruitment and activation. Collectively, events generated by the antidromic stimulation of sensory nerves constitute the phenomenon of “neurogenic inflammation”. Reprinted from J Allergy Clin Immunol, Vol 118, Sarin S, Undem B, Sanico A, Togias A, The role of the nervous system in rhinitis, Pages 999–1016, Copyright 2006, with permission from American Academy of Allergy, Asthma and Immunology.Citation43
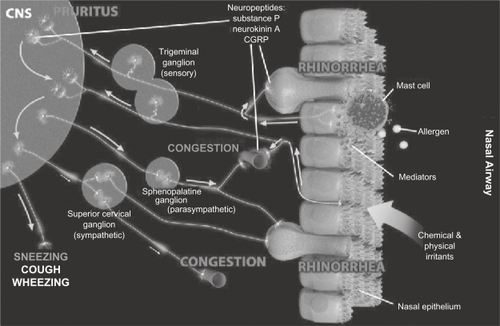
Modulation of sensory perception
The sensory nerves of the nose arise from the olfactory nerve, as well as from the ophthalmic and maxillary branches of the trigeminal nerve. Nonolfactory sensory nerves consist of both myelinated and unmyelinated (primarily nociceptive) fibers.Citation43 Physical and chemical stimuli, as well as endogenous biochemical products, can stimulate sensory afferents in the nasal mucosa to carry sensations (eg, pruritus) to the central nervous system and also activate reflexes (eg, sneezing).Citation43 It should be noted that symptoms typical of rhinitis can be produced through neural mechanisms without any demonstrable mucosal abnormality.Citation43 In addition, mentholated vapors can influence the perception of relief from congestion without actually altering airflow. This effect is thought to be due to activation of cold receptors by menthol, and this cool sensation creates the impression of increased airflow.Citation70 Conversely, patients with complete turbinectomy (“empty nose”) may still complain of the perception of nasal congestion.Citation66,Citation71 The importance of events initiated by the nervous system is further underscored by the many patients with perennial nonallergic rhinitis who complain of nasal congestion in the absence of any demonstrable abnormalities in the mucosa.Citation72
Role of specific neural pathways in rhinitis symptoms
Specific symptoms of rhinitis are mediated by the actions of distinct neural pathways. Sensory axons can be classified according to size, conduction velocity, the neurotransmitters they release, and the different types of stimuli to which they are sensitive. Small unmyelinated fibers (C fibers) conduct action potentials slowly and are generally responsive to noxious mechanical and chemical stimuli. Thinly myelinated Aδ fibers are also nociceptors. Larger myelinated Aβ fibers have more rapid conduction velocities and may convey non-nociceptive information. Pruritus is a tactile sensation that is conveyed to the central nervous system via trigeminal fibers that have their cell bodies in the trigeminal ganglion.Citation43,Citation69
Trigeminal neuronal activation caused by mast cell mediators may also contribute to sneezing and itching. Calcitonin gene-related peptide (CGRP), a potent vasodilator that may play a role in congestion, is also associated with trigeminal activation and is increased in nasal lavage fluids following allergen challenge. Support for the role of trigeminal activation in the development of AR symptoms is seen in a recent study in which inhalation of CO2, a known inhibitor of both neuronal activation and CGRP release, significantly improved nasal allergy symptoms, including congestion.Citation73
Abnormalities in parasympathetic reflex arcs may also contribute to the development of rhinorrhea and congestion. It has been suggested that vasomotor, idiopathic, or “irritant” rhinitis may result from increased sensitivity of afferent fibers to irritant stimuli and/or augmented glandular responses to activation by parasympathetic axons.Citation69
Increased understanding of the neurotransmitters and other modulators released by different primary afferents has led to the realization that these neurons can also be classified on the basis of the molecules they use to communicate with other cells. Primary afferents can also be differentiated on the basis of the specific receptors they express and thus the substances to which they are sensitive. A small subset of C fibers that express histamine H1, and perhaps also H4, receptors convey information that gives rise to the sensation of itch.Citation74,Citation75 Another subset of C fibers expresses transient receptor potential vanilloid-1 (TRPV1) receptors. Primary afferents expressing these receptors are thought to be involved in detection of painful heat stimuli.Citation76 These receptors are also involved in the development of thermal hyperalgesia that may occur secondary to inflammation.Citation77 In addition to sensing thermal stimuli, primary afferents with TRPV receptors also convey information about mechanical stimuli and changes in local osmolarity.Citation78
A growing body of evidence has indicated that the neurotransmitter phenotypes of primary afferent neurons are highly plastic and may change rapidly as a function of exposure to inflammatory stimuli. This inflammatory neuroplasticity is the consequence of a combination of activity-dependent changes in the neurons and specific molecules that initiate particular signal transduction pathways.Citation79 An inflammation-induced release of mediators can change the properties of primary sensory neurons, producing alterations in sensitivity and changes in transmitter phenotypes. For example, inflammation results in a nerve growth factor–dependent increase in substance P expression in C fibers and novel expression of this neuropeptide in some large Aβ fibers, which do not normally contain this peptide.Citation79 Alterations of this type may contribute to the sensation of congestion in the absence of impaired breathing or blocked nasal passages. This suggestion is supported by the observation that nasal responsiveness to histamine and capsaicin (a highly specific stimulus for C fibers) is increased in subjects with allergic rhinitis.Citation80 It has also been noted that patient perception of idiopathic rhinitis improves with long-term exposure to capsaicin, an agent that desensitizes nerves but has no effect on inflammatory mediators. These data support the view that alterations in sensory processing may play a role in the pathophysiology of congestion in some cases.Citation81,Citation82
Summary
Nasal obstruction or congestion is one of the most common symptoms encountered in primary care and specialist clinics, and it is the symptom that is most bothersome to patients. Mucosal inflammation is the primary pathophysiological mechanism leading to congestion in common upper respiratory diseases, such as allergic rhinitis, rhinosinusitis, and nasal polyposis. Mucosal inflammation in these conditions is responsible for many of the distinct and interrelated factors that contribute to congestion, including increased venous engorgement, elevated nasal secretions, and tissue swelling/edema. In addition, mechanical and structural features of the sinonasal passages (eg, septal deviation, choanal atresia, concha bullosa and adenoid hypertrophy) can result in blockage/obstruction/congestion. Importantly, neurogenic mechanisms also contribute significantly to the pathophysiological changes underlying nasal congestion, and abnormal primary afferent signaling may give rise to the sensation of congestion even in the absence of inflammation and impaired airflow. A greater understanding of the pathophysiological mechanisms underlying congestion, in particular the mucosal inflammation associated with common conditions such as allergic rhinitis and rhinosinusitis, has the potential to help clinicians and researchers optimize treatment with existing therapies and develop new treatments for these conditions.
Acknowledgements
Editorial assistance was provided by Henry Hamilton, PhD, former employee of Health Science Communications, Inc., and Joyce O’Connor, MS of Health Science Communications, Inc. This assistance was funded by Schering-Plough Corporation, now Merck & Co., Whitehouse Station, NJ, USA.
Disclosures
Dr Naclerio: grant support from Merck, GlaxoSmithKline, Schering-Plough Corporation, now Merck & Co., Whitehouse Station, NJ, USA.
Dr Bachert: study funding from Schering-Plough Corporation, now Merck & Co., Whitehouse Station, NJ, USA, lecturer for Schering-Plough Corporation, now Merck & Co., Whitehouse Station, NJ, USA.
Dr Baraniuk: none.
References
- CoreyJPHouserSMNgBANasal congestion: a review of its etiology, evaluation, and treatmentEar Nose Throat J200079969069811011488
- WangDYRazaMTGordonBRControl of nasal obstruction in perennial allergic rhinitisCurr Opin Allergy Clin Immunol20044316517015126936
- RomeroFACasaleTBCorrelation between objective and subjective measures of nasal congestion in a nasal allergen challenge modelJ Allergy Clin Immunol2009123S204S204Abstract 784.
- ProudDNaclerioRMGwaltneyJMHendleyJOKinins are generated in nasal secretions during natural rhinovirus coldsJ Infect Dis199016111201232295843
- CorbozMRMutterJCRivelliMAAlpha2-adrenoceptor agonists as nasal decongestantsPulm Pharmacol Ther200720214915616809058
- StaevskaMTBaraniukJNDifferential diagnosis of persistent nonallergic rhinitis and rhinosinusitis syndromesClin Allergy Immunol200719355317153006
- RameyJTBailenELockeyRFRhinitis medicamentosaJ Investig Allergol Clin Immunol2006163148155
- GrafPRhinitis medicamentosa: aspects of pathophysiology and treatmentAllergy19975240 Suppl28349353558
- BousquetJKhaltaevNCruzAAAllergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen)Allergy200863Suppl 86816018331513
- MeltzerEOBlaissMSDereberyMJBurden of allergic rhinitis: results from the Pediatric Allergies in America surveyJ Allergy Clin Immunol20091243S43S7019592081
- AsherMMontefortSBjorkstenBthe ISAAC Phase Three Study GroupWorldwide trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveysLancet200636873374316935684
- NathanRAPharmacotherapy for allergic rhinitis: a critical review of leukotriene receptor antagonists compared with other treatmentsAnn Allergy Asthma Immunol200390218219012602664
- PearlmanDSPathophysiology of the inflammatory responseJ Allergy Clin Immunol19991044 Pt 1S132S13710518809
- WhiteMMediators of inflammation and the inflammatory processJ Allergy Clin Immunol1999103 Pt 2S378S38110069896
- QuraishiSADaviesMJCraigTJInflammatory responses in allergic rhinitis: traditional approaches and novel treatment strategiesJ Am Osteopath Assoc2004104Suppl 5S7S1515176523
- BascomRPipkornULichtensteinLMNaclerioRMThe influx of inflammatory cells into nasal washings during the late response to antigen challenge. Effect of systemic steroid pretreatmentAm Rev Respir Dis198813824064123195836
- BascomRWachsMNaclerioRMPipkornUGalliSJLichtensteinLMBasophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatmentJ Allergy Clin Immunol19888135805892450113
- MinshallEGhaffarOCameronLAssessment by nasal biopsy of long-term use of mometasone furoate aqueous nasal spray (Nasonex) in the treatment of perennial rhinitisOtolaryngol Head Neck Surg199811856486549591864
- BachertCWagenmannMHoltappelsGCytokines and adhesion molecules in allergic rhinitisAm J Rhinol1998121389513652
- GelfandEWInflammatory mediators in allergic rhinitisJ Allergy Clin Immunol20041145 SupplS135S13815536444
- WangDClementPSmitzJDerdeMPConcentrations of chemical mediators in nasal secretions of patients with hay fever during natural allergen exposureActa Otolaryngol199411455525557825440
- CreticosPSPetersSPAdkinsonNFJrNaclerioRMHayesECNormanPSPeptide leukotriene release after antigen challenge in patients sensitive to ragweedN Engl J Med198431025162616306328300
- OkudaMWataseTMezawaALiuCMThe role of leukotriene D4 in allergic rhinitisAnn Allergy19886065375403382059
- KishiYNakanoYJiangSParticipation in cysteinyl leukotrienes and thromboxane A2 in nasal congestion model in Brown Norway ratsInt Immunopharmacol200771483148717761352
- ShirasakiHKikuchiMSekiNKanaizumiEWatanabeKHimiTExpression and localization of the thromboxane A2 receptor in human nasal mucosaProstaglandins Leukot Essent Fatty Acids200776631532017513100
- OkanoMFujiwaraTSugataYPresence and characterization of prostaglandin D2-related molecules in nasal mucosa of patients with allergic rhinitisAm J Rhinol200620334234816871941
- CameronHLYangPCPerdueMHGlucagon-like petide-2-enhanced barrier function reduces pathophysiology in a model of food allergyAm J Physiol Gastrointest Liver Physiol20032846G905G91212736145
- CiprandiGCirilloIVizzaccaroAMilaneseMToscaMANasal obstruction in patients with seasonal allergic rhinitis: relationships between allergic inflammation and nasal airflowInt Arch Allergy Immunol20041341344015051938
- WachsMProudDLichtensteinLMKagey-SobotkaANormanPSNaclerioRMObservations on the pathogenesis of nasal primingJ Allergy Clin Immunol1989844 Pt 14925012477429
- HowarthPHSalageanMDokicDAllergic rhinitis: not purely a histamine-related diseaseAllergy200055Suppl 6471611291780
- SimonHUMolecular mechanisms of defective eosinophil apoptosis in diseases associated with eosinophiliaInt Arch Allergy Immunol19971131–32062089130524
- WidegrenHErjefaltJKorsgrenMEffects of intranasal TNF-alpha on granulocyte recruitment and activity in healthy subjects and patients with allergic rhinitisRespir Res200891518234086
- HowarthPHABC of allergies. Pathogenic mechanisms: a rational basis for treatmentBMJ199831671337587619529415
- WallaceDVDykewiczBernsteinDIfor the Joint Task Force on Practice Parameter for Allergy and ImmunologyThe diagnosis and management of rhinitis: an updated practice parameterJ Allergy Clin Immunol2008122Suppl 2S1S8418662584
- MeltzerEOHamilosDLHadleyJARhinosinusitis: establishing definitions for clinical research and patient careOtolaryngol Head Neck Surg20041316 SupplS1S6215577816
- KalinerMAOsguthorpeJDFiremanPSinusitis: bench to bedside. Current findings, future directionsOtolaryngol Head Neck Surg19971166 Pt 2S1S209212028
- GwaltneyJMClinical significance and pathogenesis of viral respiratory infectionsAm J Med2002112Suppl 6A13S18S11955455
- European Academy of Allergology and Clinical ImmunologyEuropean position paper on rhinosinusitis and nasal polypsRhinol Suppl2005Suppl 1818715847064
- BerrettiniSCarabelliASellari-FranceschiniSPerennial allergic rhinitis and chronic sinusitis: correlation with rhinologic risk factorsAllergy199954324224810321560
- Repka-RamirezSNaranchKParkYJClauwDBaraniukJNCytokines in nasal lavage fluids from acute sinusitis, allergic rhinitis, and chronic fatigue syndrome subjectsAllergy Asthma Proc200223318519012125506
- RöselerSHoltappelsGWagenmannMBachertCElevated levels of interleukins IL-1 beta, IL-6 and IL-8 in naturally acquired viral rhinitisEur Arch Otorhinolaryngol1995252Suppl 1S61S637734976
- RennéTSchuhKMuller-EsterlWLocal bradykinin formation is controlled by glycosaminoglycansJ Immunol200517553377338516116231
- SarinSUndemBSanicoATogiasAThe role of the nervous system in rhinitisJ Allergy Clin Immunol20061185999101617088122
- BachertCvan KempenMJHopkenKHoltappelsGWagenmannMElevated levels of myeloperoxidase, pro-inflammatory cytokines and chemokines in naturally acquired upper respiratory tract infectionsEur Arch Otorhinolaryngol2001258840641211724263
- BergerGKattanABernheimJOphirDFinkelsteinYAcute sinusitis: a histopathological and immunohistochemical studyLaryngoscope2000110122089209411129027
- Pérez-NovoCAClaeysCVan CauwenbergePBachertCExpression of eicosanoid receptors subtypes and eosinophilic inflammation: implication on chronic rhinosinusitisRespir Res200677516689996
- FokkensWLundVBachertCEAACI position paper on rhinosinusitis and nasal polyps executive summaryAllergy200560558360115813802
- SavolainenSAllergy in patients with acute maxillary sinusitisAllergy19894421161222497653
- EmanuelIAShahSBChronic rhinosinusitis: allergy and sinus computed tomography relationshipsOtolaryngol Head Neck Surg2000123668769111112958
- MygindNDahlRBachertCNasal polyposis, eosinophil dominated inflammation, and allergyThorax200055Suppl 2S79S8310992568
- PawankarRNasal polyposis: an update: editorial reviewCurr Opin Allergy Clin Immunol2003311612582307
- StjarnePMosgesRJorissenMA randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposisArch Otolaryngol Head Neck Surg2006132217918516490876
- HedmanJKaprioJPoussaTNieminenMMPrevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based studyInt J Epidemiol199928471772210480701
- BachertCGevaertPHoltappelsGCuvelierCvan CauwenbergePNasal polyposis: from cytokines to growthAm J Rhinol200014527929011068652
- BachertCGevaertPHoltappelsGvan CauwenbergePMediators in nasal polyposisCurr Allergy Asthma Rep20022648148712359119
- GevaertPBachertCHoltappelsGNovoCPVan der HeydenJFransenLEnhanced soluble interleukin-5 receptor alpha expression in nasal polyposisAllergy200358537137912752323
- BachertCGevaertPHoltappelsGJohanssonSGvan CauwenbergePTotal and specific IgE in nasal polyps is related to local eosinophilic inflammationJ Allergy Clin Immunol2001107460761411295647
- WateletJBBachertCClaeysCVan CauwenbergePMatrix metalloproteinases MMP-7, MMP-9 and their tissue inhibitor TIMP-1: expression in chronic sinusitis vs nasal polyposisAllergy2004591546014674934
- BachertCZhangNPatouJvan ZeleTGevaertPRole of staphylococcal superantigens in upper airway diseaseCurr Opin Allergy Clin Immunol200881343818188015
- OkanoMFujiwaraTHarunaTProstaglandin E2 suppresses staphylococcal enterotoxin-induced eosinophilia-associated cellular responses dominantly through an E-prostanoid 2-mediated pathway in nasal polypsJ Allergy Clin Immunol2009123486887419254809
- PatouJGevaertPVan ZeleTHoltappelsGvan CauwenbergePBachertCStaphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polypsJ Allergy Clin Immunol2008121111011517980412
- FarmerSEEcclesRChronic inferior turbinate enlargement and the implications for surgical interventionRhinology200644423423817216738
- WrightEDPearlAJManoukianJJLaterally hypertrophic adenoids as a contributing factor in otitis mediaInt J Pediatr Otorhinolaryngol19984532072149865437
- KemkerBLiuXGungorAMoinuddinRCoreyJPEffect of nasal surgery on the nasal cavity as determined by acoustic rhinometryOtolaryngol Head Neck Surg1999121556757110547471
- Bar-SelaSLevyMWestinJBLasterRRichterEDMedical findings in nickel-cadmium battery workersIsr J Med Sci1992288–95785831428813
- Warwick-BrownNPMarksNJTurbinate surgery: how effective is it? A long-term assessmentORL J Otorhinolaryngol Relat Spec19874963143203431839
- ColePChabanRNaitoKOpryskDThe obstructive nasal septum. Effect of simulated deviations on nasal airflow resistanceArch Otolaryngol Head Neck Surg198811444104122450553
- RoithmannRDemeneghiPFaggianoRCuryAEffects of posture change on nasal patencyRev Bras Otorrinolaringol (Engl Ed)2005714478484
- TaiCFBaraniukJNUpper airway neurogenic mechanismsCurr Opin Allergy Clin Immunol200221111911964745
- EcclesRMenthol: effects on nasal sensation of airflow and the drive to breatheCurr Allergy Asthma Rep20033321021412662469
- MooreGFFreemanTJOgrenFPYonkersAJExtended follow-up of total inferior turbinate resection for relief of chronic nasal obstructionLaryngoscope1985959 Pt 1109510994033334
- EnbergRNPerennial nonallergic rhinitis: a retrospective reviewAnn Allergy1989636 Pt 15135162480728
- CasaleTBRomeroFASpieringsELHIntranasal noninhaled carbon dioxide for the symptomatic treatment of seasonal allergic rhinitisJ Allergy Clin Immunol2008121110510918028997
- ReesJMurrayCSItching for progressClin Exp Dermatol200530547147316045669
- TaiCFBaraniukJNA tale of two neurons in the upper airways: pain versus itchCurr Allergy Asthma Rep20033321522012662470
- SteenlandHWKoSWWuLJZhuoMHot receptors in the brainMol Pain200623417092351
- LiedtkeWKimCFunctionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon!Cell Mol Life Sci200562242985300116314934
- MontellCPhysiology, phylogeny, and functions of the TRP superfamily of cation channelsSci STKE2001200190RE111752662
- WoolfCJCostiganMTranscriptional and posttranslational plasticity and the generation of inflammatory painProc Natl Acad Sci U S A199996147723773010393888
- TogiasAUnique mechanistic features of allergic rhinitisJ Allergy Clin Immunol20001056 Pt 2S599S60410856164
- BlomHMSeverijnenLAVan RijswijkJBMulderPGVan WijkRGFokkensWJThe long-term effects of capsaicin aqueous spray on the nasal mucosaClin Exp Allergy19982811135113589824407
- BlomHMVan RijswijkJBGarreldsIMMulderPGTimmermansTGerth van WijkRIntranasal capsaicin is efficacious in non-allergic, non-infectious perennial rhinitis. A placebo-controlled studyClin Exp Allergy19972777968019249272