Abstract
Ciclesonide is a novel inhaled corticosteroid used in the continuous treatment of mild-to-severe asthma. Its formulation and mechanism of action yield a low oral and systemic bioavailability, and high pulmonary deposition. In multiple clinical trials, ciclesonide is at least as effective as either fluticasone propionate or budesonide at symptom control, while in many cases having improved safety outcomes and tolerability. The improved safety and comparable efficacy profiles of ciclesonide demonstrated in current studies could potentially yield a treatment option that may lead to improved adherence and outcome.
Keywords:
Introduction
Asthma poses a significant burden to society in terms of morbidity, mortality, quality of life, and healthcare costs.Citation1–Citation3 Among children, asthma rates in the United States are currently at record highs, with a nationwide prevalence of approximately 9% of children aged 1 to 17 years.Citation3 Its burden on the economy is estimated at between US$4 to 6 billion annually, considering healthcare costs and lost work days for caregivers and patients alike.Citation4 Inhaled corticosteroids (ICS) are recommended as first-line therapy for the treatment of asthma,Citation5,Citation6 and can improve both asthma symptomatology and the markers of airway inflammation.Citation7–Citation9 However, despite being demonstrated as an efficacious controller therapy, concerns remain regarding the potential for adverse side effects associated with chronic ICS treatment. Specifically, some ICS molecules have been demonstrated to cause reductions in growth velocityCitation9–Citation11 and bone mineral density,Citation12,Citation13 HPA-axis suppression,Citation14,Citation15 and oral candidiasis.Citation16,Citation17
Ciclesonide (Alvesco®; Sepracor, Inc., Nycomed, Inc.) is a novel, new corticosteroid developed for the treatment of mild to severe persistent asthma. It is delivered by metered-dose inhaler (MDI) once daily or twice daily (dosing depends on country). This review will focus on the safety and efficacy profile of ciclesonide, as well as to establish its mechanism of action.
Mechanism of action
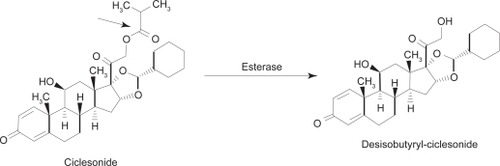
Ciclesonide ([R]-11β, 16α, 17, 21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with cyclohexanecarboxaldehyde 21-isobutyrate; CIC) is inhaled into the lungs via hydrofluoroalkane-MDI (HFA-MDI), where it is converted by local esterases to its active metabolite, desisobutyryl-ciclesonide (des-CIC, ). Relative to dexamethasone (100), CIC has a low glucocorticoid receptor binding affinity of 12, while des-CIC has a significantly higher binding affinity of 1212.Citation18,Citation19 Studies demonstrate no conversion of R-CIC or des-CIC to the S-epimer in vivo, which has different physicochemical properties, and a markedly lower receptor affinity.Citation20 Pharmacokinetic profiles of des-CIC were similar in comparative healthy and asthma patients, likely indicating that bronchial narrowing and airway inflammation do not affect the distribution of CIC and its subsequent activation to des-CIC in the lungs.Citation21 An additional study also demonstrated equivalent PK/PD profiles of CIC-HFA when administered with and without a spacer.Citation22
Figure 1 Molecular structure of ciclesonide and its activated metabolite, desisobutyryl-ciclesonide (des-CIC). Christie P. Ciclesonide: A novel inhaled corticosteroid for asthma. Drugs Today (Barc). 2004; 40(7):569–576.Citation19 Copyright © 2004 Prous Science, S.A.U. All rights reserved.
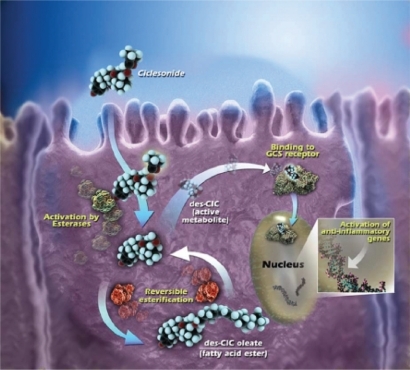
In vitro data indicate metabolism of des-CIC was different in precision-cut human lung and liver tissue slices.Citation23 After 24 hours incubation with [14C]-CIC, 7.2 times more radioactivity was present in the lung tissue, as compared with the liver. Furthermore, in the lung tissue [14C]-CIC was converted to des-CIC and subsequently conjugated with fatty acid metabolites, a reversible process which increases lipophilicity of des-CIC and may result in prolonged drug retention and anti-inflammatory activity in the lung ().Citation23,Citation24 Alternatively, [14C]-CIC was catabolically inactivated in liver tissue into at least 5 different polar compounds, with dihydroxylated des-CIC being the major metabolite.Citation23 Additionally, other findings demonstrated that orally and intravenous-administered [14C]-CIC resulted in a negligible serum concentration of des-CIC and no accumulation in red blood cells, indicating a low absorption and almost complete first-pass metabolism (systemic bioavailability of des-CIC < 1%).Citation25
Figure 2 Intracellular activation of ciclesonide and reversible esterification of desisobutyryl-ciclesonide (des-CIC). Ciclesonide is activated by intracellular esterases to active metabolite, des-CIC, which has high affinity to the glucocorticosteroid (GCS) receptor. Desisobutyryl-ciclesonide can undergo reversible esterification to des-CIC oleate (fatty acid ester). Reprinted with permission from Nave R, Meyer W, Fuhst R, Zech K. Formation of fatty acid conjugates of ciclesonide active metabolite in the rat lung after 4-week inhalation of ciclesonide. Pulm Pharmacol Ther. 2005;18:390–396.Citation62 Copyright © 2005 Elsevier.
The demonstrated mean lung deposition of CIC is 52%.Citation26 The internal diameter of the smallest airways in adults are typically ∼2 μm, thus, it can be inferred that smaller ICS particles lead to greater pulmonary deposition and more even distribution throughout the lungs (). Accordingly, the HFA-MDI formulation of CIC contains a majority of ICS particles which range between 1.1 and 2.1 μm.Citation27,Citation28 This particle size is likely related to the high observed pulmonary deposition of CIC.Citation28 Furthermore, uptake of CIC, budesonide (BUD), and fluticasone propionate (FP) in human alveolar type II epithelial cells (A549) was measured, and at all incubation timepoints, intracellular concentration of CIC was higher than that of BUD and FP.Citation29 This indicates a more rapid uptake of CIC molecules into target tissue, and at a higher concentration. Additionally, separate in vitro data indicate intracellular concentration of des-CIC in A549 cells to be maintained for >20 hours.Citation29
Table 1 Characteristics of ciclesonide
Efficacy
Placebo-controlled
The therapeutic action of ciclesonide is achieved after the inhaled parent compound (CIC) is cleaved by esterases in the lungs to its active metabolite (des-CIC), a corticosteroid with high receptor affinity and anti-inflammatory activity.Citation18,Citation19,Citation30 In a randomized, double-blind trial, early (EAR) and late (LAR) phase allergen-induced asthmatic reactions were significantly inhibited (p < 0.05) by treatment with CIC, versus placebo (as evaluated by decrease in FEV1 following allergen challenge).Citation31 These anti-imflammatory properties were also exhibited in vitro, as CIC attenuated EAR/LAR, infiltration of inflammatory cells into bronchoalveolar lumen, and airway hyperresponsiveness in sensitized Brown Norway rats.Citation32 These effects were observed in a dose-dependent manner.
These anti-inflammatory properties have been noted in patients with mild, persistent asthma, treated with CIC 160 μg (all doses noted in this review are ex-actuator) once-daily over a period of 4 weeks.Citation33 Measurements were made before and after treatment in this double-blind study for adenosine monophosphate (AMP) bronchial challenge, and exhaled nitric oxide (eNO). Mean AMP challenge PC20 following ciclesonide treatment was significantly increased (p < 0.001) compared to placebo, and decreases in eNO (ppb) and induced sputum eosinophil cell counts were also noted.Citation33 Additionally, Bateman et alCitation34 demonstrated the effectiveness of CIC 320 μg and 640 μg in reducing oral corticosteroid use in adults with severe, persistent asthma, versus placebo.
Three double-blind studies demonstrated that treatment with CIC improves symptom control in patients who were previously treated with another ICS. O’Connor, et alCitation35 demonstrated significant improvement in FEV1 in moderate-to-severe asthmatics treated with either CIC 800 μg or 1600 μg daily, compared to placebo (12-week treatment, pretreatment with 800–2000 μg/day BDP). A similarly designed study by Langdon et alCitation36 noted improvements in baseline FEV1 and morning PEF in subjects treated with CIC over a period of 12 weeks, but at more clinically-relevant doses (CIC 80 μg, 320 μg daily, versus placebo). Subjects were also previously treated with lower, constant doses of BDP or its equivalent (400–800 μg daily) for at least 4 weeks prior to randomization. Asthma control in previously treated subjects was maintained by CIC 160 μg or 640 μg once daily in another study by Chapman.Citation37
In a large, double-blind, 12-week study (n = 1031), GelfandCitation38 studied the effect of multiple strength doses of CIC (40 μg, 80 μg, or 160 μg) on children aged 4 to 11 with mild to severe persistent asthma. Following run-in, subjects were randomized to one of three once-daily CIC treatments, or placebo. All CIC doses were associated with significant increases in FEV1 compared to placebo at study endpoint (CIC 40 μg, 11.91; CIC 80 μg, 13.58; CIC 160 μg, 14.17). Reductions in rescue medication use within study treatment cohorts were also reported.
Comparative studies
Once-daily dosing of CIC (which is approved in some, but not all countries) is a significant distinction between it and other common ICS for treatment of asthma, such as BUD and FP. Currently, there have not been any comparative efficacy trials between CIC and mometasone furoate, another commonly used ICS which is approved for once-daily dosing; future studies should directly compare efficacy of these two agents. CIC has demonstrated efficacy with a single daily dose,Citation33–Citation42 as well as when administered either in the morning or evening.Citation39 The effectiveness of a single daily dose could lead to improved compliance and symptom control in patients using CIC as asthma control therapy.
CIC comparative efficacy trials are summarized in . UkenaCitation43 demonstrated CIC 320 μg once daily to be superior to BUD 200 μg twice-daily in increasing FEV1 from baseline (416 mL in CIC versus 321 mL in BUD; p = 0.019, 95% CI). While improvements were seen in FVC for both cohorts, a significantly larger difference was seen in CIC-treated subjects. Additionally, significant improvement versus baseline in morning PEF was seen after Day 2 in the CIC cohort (p = 0.039 versus baseline) and Day 7 in the BUD cohort (p = 0.047 versus baseline), indicating a more rapid onset of action for CIC.
Table 2 Ciclesonide comparative efficacy trials
However, these findings appear to be an outlier, as the majority of trials have found CIC at least as effective as BUD (Turbuhaler®/DPI) in controlling asthma, in varying subject populations and disease severities.Citation44–Citation48 FEV1 improvement was noted in these studies with both BUD and CIC, and no significant between-group differences were noted. Pediatric Asthma Quality of Life Questionnaire (PAQLQ) and Pediatric Asthma Caregiver’s Quality of Life Questionnaire (PACQLQ) scores were similarly improved over a 12-week study comparing CIC 160 μg once daily to BUD 400 μg once daily.Citation47 Some modest benefit was seen for CIC, with BouletCitation45 finding a greater percentage of symptom-free days for CIC (43%) than BUD (34%; p = 0.0288), but overall, both ICS seem to be comparably efficacious. Similarly, comparative studies between CIC and FP show clear non-inferiority of CIC.Citation40–Citation42,Citation49–Citation51 No difference in comparative efficacy was observed when comparing CIC to both FP-DPICitation49 and FP-MDI.Citation40–Citation42,Citation50,Citation51 FP and CIC were equally effective at maintaining asthma control in subjects who were on continuous ICS therapy prior to randomization.Citation40,Citation50
Safety and tolerability
Oral candidiasis
Considering CIC is relatively inactive, and is converted to active des-CIC in the lungs versus some conversion in the oral cavity, this profile would suggest a lower oropharyngeal deposition and frequency of side effects (ie, candidiasis). Indeed, comparative studies indicate low deposition and activated des-CIC within the oropharynx. Two similarly designed studies compared CIC to FPCitation52 and BUDCitation53, using mouthwash solutions containing 50% ethanol at 5 time points (immediate, 15, 30, 45, and 60 minutes after inhalation) to compare deposition. The sum of CIC and des-CIC molar AUCs0–60 min was 53% (95% CI: 40%–69%) of FP depositionCitation52 and 47% of the BUD deposition.Citation53 Furthermore, oral deposition of des-CIC was less than 10% that of FP and BUD.
Incidence of candidiasis from continuous ICS treatment is a concern, and a common reason for poor adherence, and in some cases, not beginning an asthma patient on an ICS treatment regimen.Citation16 For example, in one of the aforementioned efficacy studies, 9 cases of candidiasis were noted (over a 12-week treatment period) among subjects receiving FP 200 μg twice-daily, compared to no reported cases in subjects treated with CIC 320 once-daily.Citation49 Likewise, GelfandCitation38 found the incidence of oral candidiasis and pharyngitis over a 12-week treatment period with CIC 40 μg, 80 μg, and 160 μg to be not significantly different from placebo. This finding is consistent with that of Pearlman,Citation54 who found incidences of oropharyngeal side effects similar to placebo in patients treated with CIC 80 μg, 160 μg, or 320 μg over the same duration.
HPA-axis function
Concerns of ICS acting as endogenous glucocorticoids, thereby suppressing the HPA-axis, have resulted in reluctance of some physicians to prescribe such a treatment continuously. CIC has high protein binding and high systemic clearance, a profile that would seem to minimize interference with normal HPA-axis function. In one double-blind, randomized, crossover trial, AgertoftCitation55 studied children aged 6 to 12 years who were given placebo, CIC 40 μg, 80 μg, or 160 μg once daily during four 2-week treatment periods, followed by 2-week washouts. Analysis of 12 hours urinary cortisol at the end of each treatment period yielded no significant between-group differences or dose-response effects. This is of interest because these subjects were given clinically relevant doses of CIC for their age and disease severity.
Vermeulen et alCitation48 found that over 12 weeks of treatment in children, CIC 320 μg once-daily was not associated with a significant decrease in 24 urinary free cortisol (Δ = +1.05; nmol/mmol), while BUD 800 μg once daily was (Δ = −2.63). This between-group difference was significant (p = 0.003). Adult patients (n = 60, ≥18 years) in a separate study were randomized to receive CIC 320 μg or 640 μg twice daily, FP 440 μg or 880 μg twice daily (CFC-MDI), or placebo. Neither CIC nor FP treatment was associated with a significant change in mean serum cortisol AUC0–24 h at these clinically relevant doses.Citation56 Alternatively, a study by Lipworth et al using the same comparative doses of FP and CIC, found evidence of adrenal suppression by FP, using cosyntropin-stimulated peak serum cortisol levels to analyze HPA-axis function.Citation57
Noteworthy is that this pattern of adrenal safety continued even when CIC was administered in doses higher than would normally be used in clinical practice. Derom et alCitation58 concluded that doses as high as CIC 640 μg twice daily had no significant effect on mean urinary cortisol levels (AUC0–24 h), while FP 440 μg and 880 μg twice daily suppressed them by 29% (95% CI, 15–41), compared with placebo. Decreases in PC20 hyperresponsiveness were similar in all cohorts.Citation58 In the previously mentioned study by Szefler,Citation56 serum and urinary cortisol suppression was associated with high-dose FP 2000 μg daily, but not with CIC 1600 μg daily.Citation56 Again, airway outcomes (PC20 hyperresponsiveness, exhaled nitric oxide) were improved with both treatments.
Growth effects
The effects of continuous CIC treatment on childhood growth velocity have been studied. Knemometry, though not a predictor of long-term growth velocity or final adult height, is an extremely sensitive measure of short-term changes in lower-leg growth. This method was utilized by AgertoftCitation55 to determine if 2-week treatment with CIC 40 μg, 80 μg, and 160 μg once daily resulted in any short-term changes in growth velocity. Lower leg growth rates for CIC were not significantly different from placebo, and no dose-response effects were noted (placebo: 0.412 mm/week; CIC 40: 0.425 mm/week; CIC 80: 0.397 mm/week; CIC 160: 0.370 mm/week).
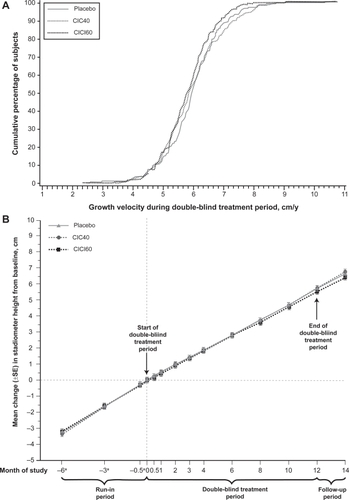
To date, the only long-term assessment of growth velocity in children treated with CIC was completed by Skoner et alCitation59 where height was assessed by stadiometry. Children (n = 661) aged 5 to 8½ years were randomized to receive placebo, CIC 40 μg, or CIC 160 μg once daily, for a treatment period of 1 year. Mean differences in yearly height from placebo (5.75 cm/year) were nonsignificant (−0.02 cm/year for CIC 40 and −0.15 cm/year for CIC 160), illustrating the noninferiority of CIC for growth velocity, when compared to placebo ().Citation59 Ideally, a study similar to that of Agertoft and Pedersen,Citation60 which determined children treated with BUD eventually reached normal adult height despite some initial reduction in growth velocity, should be performed with CIC.
Figure 3 Subjects in all cohorts (CIC40, CIC160, placebo) achieved virtually the same growth velocity during one year of continuous CIC treatment. Reprinted with permission from Skoner DP, Maspero J, Banerji D, and the Ciclesonide Pediatric Growth Study Group. Assessment of the Long-term Safety of Inhaled Ciclesonide on Growth in Children with Asthma. Pediatrics. 2008 Jan;121(1):e1–14.Citation59 Copyright © 2008 American Academy of Pediatrics.
Conclusion
From the evidence currently available, CIC appears to be a novel, safe, and efficacious ICS for use in the continuous treatment of asthma. The low oral bioavailability can likely be attributed to the low affinity of CIC to glucocorticoid receptors, as compared to its active metabolite, des-CIC, which is activated in the lungs. des-CIC is highly lipid conjugated in the lungs, allowing for greater retention in target tissues, and clinically, once-daily dosing (in at least some patients). While these pharmacokinetic properties result in an efficacious ICS, they directly contribute to the noticeably enhanced safety profile of CIC, especially in comparison with other ICS molecules, such as BUD and FP. High protein binding and the aforementioned receptor affinity of CIC result in low systemic bioavailability, and potentially explain the low occurrence of adverse events such as candidiasis, adrenal suppression, and growth velocity disturbance. Comparative studies indicate the effectiveness of CIC to be similar to that of FP and BUD, but with an improved safety profile, indicating the potential of this alternative treatment option in patients concerned about the risks of continuous ICS treatment. Minimizing risk of treatment while maintaining efficacy is a top clinical priority to improve treatment adherence and gain optimal outcome of therapy.
Disclosures
Speakers’ Bureau: AstraZeneca, Sanofi-Aventis, GlaxoSmithKline, Merck, Inc., Schering Plough Laboratories, Inc., Novartis Pharmaceuticals Corp. Grant/Research Support: AstraZeneca, Sanofi-Aventis, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Merck, Inc., Greer Laboratories, Inc., Alcon Laboratories, Inc., Schering-Plough. Consultant: Merck, Inc., Nycomed, Schering-Plough.
References
- FuhlbriggeALAdamsRJGuillbertTWThe burden of asthma in the United StatesAm J Repir Crit Care Med200216610441049
- KivitySShochatZBresslerRWienerMLermanYThe characteristics of bronchial asthma among a young adult populationChest199510824277606964
- AkinbamiLThe state of childhood asthma, United States 1980–2005 Advance data from vital and health statistics: no 381Hyattsville, MDNational Center for Health Statistics2006
- ManninoDMHomaDMAkinbamiLJSurveillance for asthma – United States 1980–1999MMWR Surveill Summ2002329511113
- National Asthma Education and Prevention ProgramNAEPP Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma – Full Report 2007Bethesda, MDNational Institutes of Health, National Heart, Lung and Blood Institute82007
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA)2007 Available from: http://www.ginasthma.org.
- PedersenSHansenORBudesonide treatment of moderate and severe asthma in children: a dose-response studyJ Allergy Clin Immunol1995951pt 129337822661
- ShapiroGMendelsonLKraemerMCruz-RiveraMWalton-BowenKSmithJAEfficacy and safety of budesonide inhalation suspension in young children with inhaled steroid-dependent, persistent asthmaJ Allergy Clin Immunol19981027897969819296
- SkonerDPBalancing safety and efficacy in pediatric asthma managementPediatrics20021092 suppl38139211826254
- BeckerABKuznetsovaOVermeulenJPediatric Montelukast Linear Growth Study GroupLinear growth in prepubertal asthmatic children treated with montelukast, beclomethasone, or placebo: a 56-week randomized double-blind studyAnn Allergy Asthma Immunol200696680080716802767
- SimonsFEA comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate-Salmeterol Xinafoate Study GroupN Engl J Med199733723165916659385125
- HananiaNAChapmanKRSturtridgeWCDose-related decrease in bone density among asthmatic patients treated with inhaled corticosteroidsJ Allergy Clin Immunol1995965Pt 15715797499672
- IsraelEBanerjeeTRFitzmauriceGMKotlovTVLaHiveKLeBoffMSEffects of inhaled glucocorticoids on bone density in premenopausal womenN Engl J Med20013451394194711575285
- ZollnerEWHypothalamic-pituitary-adrenal axis suppression in asthmatic children on inhaled corticosteroids (Part 2) – the risk as determined by gold standard adrenal function tests: a systematic reviewPediatr Allergy Immunol200718646947417680905
- ChrousosGPGhalyLSheddenAIezzoniDGHarrisAGEffects of mometasone furoate dry powder inhaler and beclomethasone dipropionate hydrofluoroalkane and chlorofluorocarbon on the hypothalamic-pituitary-adrenal axis in asthmatic subjectsChest20051281707716002918
- RachelefskyGSLiaoYFaruqiRImpact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysisAnn Allergy Asthma Immunol200798322523817378253
- FukushimaCMatsuseHSaekiSSalivary IgA and oral candidiasis in asthmatic patients treated with inhaled corticosteroidJ Asthma200542760160416169797
- StoeckMRiedelRHochhausGIn vitro and in vivo anti-inflammatory activity of the new glucocorticoid ciclesonideJ Pharmacol Exp Ther200430924925814718604
- ChristiePCiclesonide: A novel inhaled corticosteroid for asthmaDrugs Today (Barc)200440756957615510231
- NaveRDrollmanAMeyerWZechKIn vivo epimeric stability of ciclesonideAm J Respir Crit Care Med2003167 Abstract A1485.
- NaveRGunawardenaKAZechKBethkeTDPharmacokinetic disposition of inhaled ciclesonide and its metabolite desisobutyryl-ciclesonide in healthy subjects and patients with asthma are similarInt J Clin Pharm Ther200644117
- DrollmannANaveRSteinijansVWBaumgartnerEBethkeTDEquivalent pharmacokinetics of the active metabolite of ciclesonide with and without use of the AeroChamber Plus spacer for inhalationClin Pharmacokinet20064572973616802853
- NaveRFisherRZechKIn vitro metabolism of ciclesonide in human lung and liver precision-cut tissue slicesBiopharm Drug Dispos20062719720716566061
- NaveRFischerRMcCrackenNIn vitro metabolism of beclomethasone dipropionate, budesonide, ciclesonide, and fluticasone propionate in human lung precision-cut tissue slicesRespir Res200786517883839
- NaveRBethkeTDvan MarleSPZechKPharmacokinetics of [14C]-Ciclesonide after oral administration to healthy subjectsClin Pharmacokinet200443747948615139796
- DrollmanAHasselquistBEBoudreauRJCiclesonide shows high lung deposition in 2D and 3D-imagingAm J Respr Crit Care Med2002165 Abstract A40.
- RohtagiSAryaVZechKPopulation pharmacokinetics and pharmacodynamics of ciclesonideJ Clin Pharmacol20034336537812723457
- LeachCBethkeTBoudreauR2-D and 3-D imaging show ciclesonide has high lung deposition and peripheral distribution: a nonrandomized study in healthy volunteersJ Aerosol Med20061911712616796536
- NaveRSatoHNonakaTMochidukiTTakahamaSKondoSUptake, retention and metabolism of ciclesonide in human alveolar epithelial cellsEur Respir J200526suppl 49255s
- ReynoldsRAScottLJCiclesonideDrugs200464551151914977388
- DahlRNielsenLPChristensenMBEngelstätterRCiclesonide – an inhaled corticosteroid prodrug – inhibits allergen induced early and late phase reactionsEur Respir J199812suppl 2826s Abstract P0475.
- NonakaTSugiyamaHTanedaMEffect of a novel inhaled glucocorticoid, ciclesonide, on an allergen-induced asthmatic response in rats and its prolonged anti-inflammatory activity in vitroEur Respir J200220suppl 38 Abstract P652.
- WilsonAMDuongMPrattBDolovichMO’ByrnePMAnti-inflammatory effects of once daily low dose inhaled ciclesonide in mild to moderate asthmatic patientsAllergy20066153754216629781
- BatemanEKarpelJCasaleTWenzelSBanerjiDCiclesonide reduces the need for oral steroid use in adult patients with severe, persistent asthmaChest20061291176118716685007
- O’ConnorBJKilfeatherSCheungDTreatment of moderate to severe asthma with ciclesonide: a long-term investigation over 52 weeksEur Respir J200220suppl 38 Abstract 2579.
- LangdonCGAdlerMMehraSAlexanderMDrollmanAOnce-daily ciclesonide is safe and effective in patients with persistent asthmaRespir Med2005991275128516024244
- ChapmanKRPatelPD’UrzoADMaintenance of asthma control by once-daily ciclesonide in adults with persistent asthmaAllergy20056033033715679718
- GelfandEWGeorgitisJWNoonanMRuffMEOnce-daily ciclesonide in children: efficacy and safety in asthmaJ Pediatr200614837738316615971
- PostmaDSSevetteCMartinatYSchlösserNAumannJKaféHTreatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or eveningEur Respir J2001171083108811491148
- KnoxALanganJMartinotJBGrussCHäfnerDComparison of a step-down dose of once-daily ciclesonide with a continued dose of twice-daily fluticasone propionate in maintaining control of asthmaCurr Med Res Opin200723102387239417714607
- LeeDKHaggartKCurrieGPBatesCELipworthBJEffects of hydroflouroalkane formulations of ciclesonide 400 μg once daily vs fluticasone 250 μg twice daily on methacholine hyper-responsiveness in mild-to-moderate persistent asthmaBr J Clin Pharm20045812633
- BuhlRVinklerIMagyarPComparable efficacy of ciclesonide once daily versus fluticasone propionate twice daily in asthmaPulm Pharmacol Ther200619640441216310388
- UkenaDBibergerCSteinijansVCiclesonide is more effective than budesonide in the treatment of persistent asthmaPulm Pharmacol Ther200720556257016962345
- NiphadkarPJagannathKJoshiJMComparison of the efficacy of ciclesonide 160 μg QD and budesonide 200 μg BID in adults with persistent asthma: A phase III, randomized, double-dummy, open-label studyClin Ther200527111752176316368446
- BouletLPDrollmanAMagyarPComparative efficacy of once-daily ciclesonide and budesonide in the treatment of persistent asthmaRespir Med200610078579416427266
- HanselTTBenezetOKaféHA multinational, 12-week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthmaClin Ther200628690692016860173
- von BergAEngelstätterRMinicPComparison of the efficacy and safety of ciclesonide 160 μg once daily vs budesonide 400 μg once daily in children with asthmaPediatr Allergy Immunol20071839140017617808
- VermeulenJHGyurkovitsKRauerHEngelstätterRRandomized comparison of the efficacy and safety of ciclesonide and budesonide in adolescents with severe asthmaRespir Med2007101102182219117614270
- BouletLPBatemanEDVovesRMüllerTWolfSEngelstätterRA randomized study comparing ciclesonide and fluticasone propionate in patients with moderate persistent asthmaRespir Med200710181677168617448650
- BatemanEDLinnhofAEHomikLFreudensprungUSmauLEngelstätterRComparison of twice-daily inhaled ciclesonide and fluticasone propionate in patients with moderate-to-severe persistent asthmaPulm Pharmacol Ther200821226427517604664
- PedersenSGarcia GarciaMLManjraAITheronIEngelstätterRA comparative study of inhaled ciclesonide 160 μg/day and fluticasone propionate 176 μg/day in children with asthmaPediatr Pulmonol2006411095496116868976
- RichterKKanniessFBibergerCNaveRMagnussenHComparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthmaJ Clin Pharmacol20054514615215647406
- NaveRZechKBethkeTDLower oropharyngeal deposition of inhaled ciclesonide via hydroflouroalkane metered-dose inhaler compared with budesonide via chlorofluorocarbon metered-dose inhaler in healthy subjectsEur J Clin Pharmacol20056120320815824911
- PearlmanDSBergerWEKerwinELaForceCKunduSBanerjiDOnce-daily ciclesonide improves lung function and is well tolerated by patients with mild-to-moderate persistent asthmaJ Allergy Clin Immunol20051161206121216337447
- AgertoftLPedersenSShort-term lower-leg growth rate and urine cortisol excretion in children treated with ciclesonideJ Allergy Clin Immunol200511594094515867849
- SzeflerSRohatagiSWilliamsJLloydMKunduSBanerjiDCiclesonide, a novel inhaled steroid, does not affect hypthalamic-pituitary-adrenal axis function in patients with moderate-to-severe persistent asthmaChest20051281104111416162694
- LipworthBJKalinerMALaForceCFBakerJWKaiserHBAminDEffect of ciclesonide and fluticasone on hypothalamic-pituitary-adrenal axis function in adults with mild-to-moderate persistent asthmaAnn Allergy Asthma Immunol20059446547215875528
- DeromEVan De VeldeVMarissensSEngelstätterRVinckenWPauwelsREffects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and airway responsiveness to adenosine 5’monophosphate in asthmatic patientsPulm Pharmacol Ther200518532833615939311
- SkonerDPMasperoJBanerjiDthe Ciclesonide Pediatric Growth Study GroupAssessment of the long-term safety of inhaled ciclesonide on growth in children with asthmaPediatrics20081211e11418070931
- AgertoftLPedersenSEffect of long-term treatment with inhaled budesonide on adult height in children with asthmaN Engl J Med2000343151064106911027740
- RohatagiSLuoYShenLGuoZSchemmCHuangYProtein binding and its potential for eliciting minimal systemic side effects with a novel inhaled corticosteroid, ciclesonideAm J Ther200512320120915891262
- NaveRMeyerWFuhstRZechKFormation of fatty acid conjugates of ciclesonide active metabolite in the rat lung after 4-week inhalation of ciclesonidePulm Pharmacol Ther20051839039616179214