Abstract
The undertreatment of acute pain is common in many health care settings. Insufficient management of acute pain may lead to poor patient outcomes and potentially life-threatening complications. Opioids provide relief of moderate to severe acute pain; however, therapy with pure μ-opioid agonists is often limited by the prevalence of side effects, particularly opioid-induced nausea and vomiting. Tapentadol is a novel, centrally acting analgesic with 2 mechanisms of action, μ-opioid receptor agonism and norepinephrine reuptake inhibition. The analgesic effects of tapentadol are independent of metabolic activation and tapentadol has no active metabolites; therefore, in theory, tapentadol may be associated with a low potential for interindividual efficacy variations and drug–drug interactions. Previous phase 3 trials in patients with various types of moderate to severe acute pain have shown that tapentadol immediate release (IR; 50 to 100 mg every 4 to 6 hours) provides analgesia comparable to that provided by the pure μ-opioid agonist comparator, oxycodone HCl IR (10 or 15 mg every 4 to 6 hours), with a lower incidence of nausea, vomiting, and constipation. Findings suggest tapentadol may represent an improved treatment option for acute pain.
Introduction
Appropriate management of acute pain remains a considerable challenge for health care providers. Unrelieved acute pain may cause anxiety, sleep disturbances, and demoralization and may interfere with mental activity and social interactions.Citation1,Citation2 Acute pain can also increase heart rate and blood pressure, suppress immune functioning, and reduce pulmonary function, leading to an increased risk of dangerous complications, including myocardial ischemia, deep vein thrombosis, pulmonary embolism, hypoxia, pneumonia, and stroke.Citation2 In addition to these more severe adverse effects, uncontrolled acute pain is also associated with gastrointestinal effects, including the development of ileus, nausea, and vomiting.Citation3
The psychologic and physiologic effects of uncontrolled acute pain can result in longer hospital stays and unscheduled readmissions following surgery.Citation4,Citation5 A retrospective analysis of the medical records of 20,817 patients who had undergone same-day surgery in 1999 found that pain was the most common reason that patients were hospitalized immediately after surgery or returned to the hospital within 30 days of surgery, accounting for 38% of unscheduled postoperative hospital admissions or readmissions.Citation4 In addition, prolonged acute pain can cause sensitization of the central and peripheral nervous systems, leading to the development of chronic pain, which is often difficult and costly to treat.Citation6–Citation8
Although guidelines have been developed to improve acute pain management,Citation9,Citation10 pain relief remains suboptimal for many patients.Citation11–Citation16 Surveys conducted among patients who had undergone ambulatory surgery indicated that 30%Citation15 to 40%Citation12 of patients experience moderate to severe pain following discharge. Among hospitalized patients who were receiving treatment in various medical wardsCitation14 or who had undergone surgery,Citation11 the percentages were even higher, with 52%Citation14 to 80%Citation11 of patients experiencing acute pain; of these patients, as many as 86% experienced moderate to extreme pain.Citation11 The undertreatment of acute pain may result from several contributing factors related to patient care, such as infrequent pain assessments, underestimation of the severity of pain, concerns about side effects associated with analgesic treatment, and delays or dose reductions in the administration of analgesics by health care providers.Citation13,Citation14,Citation17–Citation20 In addition, patients may underreport acute pain because of low expectations of pain relief and concerns about the adverse effects of analgesic treatment.Citation19,Citation21 A 2004 survey of patients undergoing major abdominal surgery found that many patients were willing to sacrifice pain relief for a reduction in the severity of side effects.Citation22
Current treatment options for the management of acute pain include opioid analgesics (eg, morphine, hydromorphone, and oxycodone) and nonopioid analgesics (eg, acetaminophen, acetylsalicylic acid, and nonsteroidal anti-inflammatory drugs [NSAIDs]).Citation23 Most NSAIDs are limited by a therapeutic ceiling and are appropriate for the relief of only mild to moderate pain.Citation23 In addition, NSAIDs are contraindicated in patients with peptic ulcer disease, renal impairment, and any tendency for bleeding.Citation24 Cyclooxygenase-2 (COX-2)-specific inhibitors do not impair platelet function and have an improved gastrointestinal tolerability profile relative to other NSAIDsCitation25; however, certain COX-2–specific inhibitors have been linked to an increase in the risk of cardiovascular side effects, including myocardial infarction.Citation26
Opioids are typically used for the management of moderate to severe acute pain,Citation27 but opioid use is limited by the occurrence of a range of side effects.Citation28 Opioids exert their analgesic effects primarily through agonistic interactions with μ-opioid receptors in neurons in the pain pathway, which lead to a reduction in neurotransmitter release and associated pain.Citation29 However, the agonistic interactions responsible for opioid activity are not limited to neurons of the pain pathway.Citation23 Opioid receptors are present throughout the nervous system, and the interactions of opioids with nonanalgesic receptors are responsible for many of the side effects associated with opioid treatment.Citation23 For example, opioids may induce nausea and vomiting by direct stimulation of receptors at the chemoreceptor trigger zone and vestibular apparatus.Citation30
A systematic reviewCitation31 of randomized controlled trials of opioids in postoperative patients found that the most common side effects occurring in these patients were gastrointestinal side effects, central nervous system (CNS) side effects, pruritus, and urinary retention. Pruritus occurred in 18.3% of patients who were treated with opioids following surgery and was most common with epidural administration of opioids.Citation31 Somnolence and sedation were the most commonly reported CNS side effects; the incidence of somnolence ranged from less than 2% to more than 90%, depending on the route of administration and type of opioid used.Citation31 Gastrointestinal side effects, including nausea, vomiting, and constipation, were the most common side effects associated with opioid analgesia and were reported by 31.0% of patients.Citation31 Opioid-induced postoperative nausea and vomiting (PONV) is associated with negative effects on patient outcomes and quality of life,Citation32 which may lead to increases in recovery time, duration of hospitalization, and cost of medical care.Citation33,Citation34 The underuse of opioid analgesics by health care providers to relieve acute pain may be related to attempts to balance analgesia against concerns about opioid-induced side effects and subsequent deleterious repercussions for patient outcomes.Citation20
There is a continuing need for a potent analgesic agent that will provide adequate relief of acute pain, but with a reduction in side effects. Tapentadol is a novel, centrally acting analgesic that offers analgesic efficacy that is similar to that provided by a pure μ-opioid agonist comparator, but with an improved side effect profile, which may represent a significant advancement in the management of moderate to severe acute pain.
Pharmacology and pharmacokinetics of tapentadol
Tapentadol () is a centrally acting analgesic with 2 complementary mechanisms of action, μ-opioid receptor agonism and norepinephrine reuptake inhibition.Citation35,Citation36 In opioid receptor binding studies, tapentadol was found to have only a modest affinity (dissociation constant [Ki] = 0.096 μM [rat])Citation35 for the μ-opioid receptor relative to pure μ-opioid receptor agonists such as oxycodone (Ki = 0.018 μM [rat]) or morphine (Ki = 0.002 μM [rat]).Citation37 A similar binding affinity to that observed in native rat receptors was demonstrated for tapentadol at the human recombinant μ-opioid receptor (Ki = 0.16 μM [human]).Citation35 Despite the approximately 50-fold difference in binding affinity for the μ-opioid receptor relative to morphine, tapentadol demonstrated only a 2- to 3-fold reduction in analgesic potency in a series of acute and persistent animal pain models.Citation36 This disparity in potency and binding affinity for the μ-opioid receptor may be related to the contribution of the second mechanism of action, inhibition of norepinephrine reuptake, to the analgesic effects of tapentadol. In rat synaptosomal reuptake assays, tapentadol inhibited the norepinephrine reuptake transporter with a Ki of 0.48 ± 0.11 μM and, when administered in intraperitoneal doses of 4.64 to 10 mg/kg, produced a dose-dependent increase in extracellular levels of norepinephrine in the ventral hippocampus of freely moving rats to a maximum of 450% above baseline with 10 mg/kg, as measured by microdialysis.Citation35 In contrast to tapentadol, morphine administered to rats in intraperitoneal doses of 1 to 10 mg/kg produced a small, nonsignificant decrease in extracellular norepinephrine levels.Citation35
The contributions of both μ-opioid receptor agonism and norepinephrine reuptake inhibition to the analgesic effects of tapentadol were further elucidated by examining the extent to which analgesia was blocked by the selective μ-opioid receptor antagonist naloxone and the norepinephrine α2-receptor antagonist yohimbine.Citation35 In an animal modelCitation35 of acute (writhing) pain, it was observed that intravenous tapentadol and morphine induced dose-dependent inhibition of writhing (ED50 for tapentadol, 0.7 mg/kg; ED50 for morphine, 0.4 mg/kg). When combined with naloxone, the antinociceptive effect of morphine (0.681 mg/kg) was more potently reduced than that of tapentadol (3.16 mg/kg); the ED50 for naloxone antagonism was 0.007 mg/kg when combined with morphine and 0.099 mg/kg when combined with tapentadol (P < 0.001 for tapentadol vs morphine). In a spinal nerve ligation model of mononeuropathic pain,Citation35 coadministration of intraperitoneal naloxone (0.3 mg/kg) with equianalgesic intravenous doses of tapentadol (10 mg/kg) or morphine (6.81 mg/kg) reduced the anti-allodynic effect of tapentadol from 72% to 42% of the maximal possible effect (MPE); the anti-allodynic effect of morphine was reduced from 83% to 25% of the MPE. In contrast, when yohimbine (2.15 mg/kg) was administered intraperitoneally in combination with intravenous doses of morphine (6.81 mg/kg) or tapentadol (10 mg/kg), the anti-allodynic effect of tapentadol was reduced from 81% to 19% of the MPE, whereas the anti-allodynic effect of morphine was only minimally reduced (from 80% to 54% of the MPE).Citation35 These results indicate that both μ-opioid receptor agonist and norepinephrine reuptake inhibitor mechanisms are involved in the analgesic effect of tapentadol and that, in contrast to morphine, norepinephrine reuptake inhibition plays a prominent role in tapentadol-induced analgesia.
In addition to contributing to the analgesic activity of tapentadol, the opioid-sparing effect of norepinephrine reuptake inhibition may also contribute to a reduction of adverse effects associated with pure μ-opioid agonists.Citation36 Such an opioid-sparing effect has been achieved by combining other analgesics, such as NSAIDs or COX-2–specific inhibitors, with opioids to control acute pain.Citation38 This type of multimodal strategy achieves an additive analgesic effect by combining 2 different mechanisms of analgesic activity, but reduces the consumption of opioid analgesics and, thereby, the incidence of adverse effects associated with μ-opioid agonist activity.Citation38 For example, in a study of 200 patients undergoing outpatient anterior cruciate ligament surgery, patients who received perioperative doses of the COX-2–specific inhibitor celecoxib in addition to oxycodone experienced less pain (P < 0.01) in the recovery room, had lower postoperative opioid consumption (P < 0.001), and reported a lower incidence of PONV (P < 0.05) than patients taking oxycodone alone.Citation39 By combining a second mechanism of analgesic activity with μ-opioid receptor agonism, tapentadol may offer the benefits of multimodal analgesia within a single molecule.
The analgesic effects of tapentadol are independent of metabolic activation, and tapentadol has no active metabolites.Citation40 Orally administered tapentadol is principally cleared by hepatic glucuronidation via the uridine 5′-diphospho-glucuronosyl transferases (UGTs) UGT1A9 and UGT2B7, which are responsible for approximately 55% of tapentadol metabolism in humans.Citation41 The major metabolite of tapentadol, tapentadol-O-glucuronide, has no activity at opioid receptors, synaptosomal reuptake systems, or other binding sites.Citation35 Morphine is likewise primarily metabolized by hepatic glucuronidation via UGT2B7 to morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G).Citation42 Morphine-3-glucuronide has no analgesic activity, but M6G has an affinity for the μ-opioid receptor and contributes significantly to the analgesic effect of morphine.Citation43 In patients with renal insufficiency, M6G accumulates and may contribute to the higher incidence of morphine toxicity observed in these patients.Citation44,Citation45
Many other opioids, including oxycodone, codeine, dihydrocodeine, hydrocodone, and tramadol, are primarily metabolized by the cytochrome P450 (CYP) enzymes CYP2D6 or CYP3A4.Citation46 Mutations in the CYP2D6 gene, which occur in approximately 1% to 7% of the Caucasian population, can either decrease or increase enzyme activity, leading to alterations in opioid analgesia.Citation47 The analgesic effects of codeine are highly dependent on conversion of codeine to morphine by CYP2D6; a poor CYP2D6 metabolic phenotype can suppress codeine analgesia, and an ultra-rapid CYP2D6 metabolic phenotype can lead to increased opioid effects, such as euphoria, dizziness, and visual disturbances.Citation47
In addition to the potential for varied individual responses to cytochrome P450-metabolized opioids due to genetic mutations, opioids metabolized by the cytochrome P450 pathway are associated with an increased risk for drug–drug interactions. More than half of all drugs are metabolized by CYP3A4, and opioids metabolized by this isozyme (including fentanyl, buprenorphine, and methadone) are prone to drug–drug interactions with antiretroviral agents and antidepressants.Citation48 Opioids metabolized by CYP2D6, including codeine, dihydrocodeine, and oxycodone, are also associated with a number of drug–drug interactions. Substrates of CYP2D6 include antiarrhythmic agents, antidepressants, antipsychotics, antiparasitic agents, and tamoxifen.Citation48 The analgesic activity of codeine is particularly impaired by inhibition of CYP2D6 because the analgesic effects of codeine result from the formation of metabolites, including morphine and, possibly, codeine-6-glucuronide.Citation48
In vitro studiesCitation41 were used to evaluate the inhibitory or inducing effects of tapentadol on the 7 major CYP isoforms involved in drug metabolism (CYP2D6, CYP3A4, CYP1A2, CYP2A6, CYP2C9, CYP2C19, and CYP2E1). Tapentadol did not undergo significant metabolism by CYP enzymes and did not inhibit or induce the activity of any of the CYP isoforms tested, with the exception of CYP2D6.Citation41 Limited inhibition of CYP2D6 was observed with tapentadol, with competitive inhibition occurring with a Ki of 181 μM and noncompetitive inhibition with a Ki of 1,410 μM.Citation41 The Ki values for both competitive and noncompetitive inhibition are much higher than the expected tapentadol plasma concentrations of 0.5 to 1 μM (following therapeutic dosing), indicating that CYP2D6 inhibition by tapentadol is unlikely to be clinically relevant.Citation41
Two randomized, open-label studies were performed to evaluate the potential for drug–drug interactions between tapentadol and 3 common analgesics that, like tapentadol, are metabolized by UGT pathways (acetaminophen, naproxen, and acetylsalicylic acid).Citation49 Mean serum concentrations of tapentadol and tapentadol-O-glucuronide were similar after administration of tapentadol IR alone and after coadministration with acetaminophen and acetylsalicylic acid. A slight increase in the serum concentration of tapentadol and a slight decrease in the serum concentration of tapentadol-O-glucuronide were observed after coadministration with naproxen, but these changes were not considered clinically relevant, due to the relatively small magnitude of change. Thus, no dosing adjustments are needed for administration of tapentadol with any of these commonly coadministered analgesics.
Tapentadol IR for moderate to severe pain
The efficacy of tapentadol IR for the relief of moderate to severe pain has been evaluated in 3 randomized, double-blind, phase 3 studies in patients with postoperative (bunionectomy) painCitation50,Citation51 and pain related to end-stage degenerative joint diseaseCitation52 and as a secondary measure in a phase 3 randomized, double-blind, 90-day safety study.Citation53 In one of the postoperative pain studies,Citation51 patients received tapentadol IR (50, 75, or 100 mg), oxycodone HCl IR (15 mg), or placebo every 4 to 6 hours for 72 hours following bunionectomy; in the other postoperative pain study,Citation50 patients received tapentadol IR (50 or 75 mg), oxycodone HCl IR (10 mg), or placebo every 4 to 6 hours for 72 hours following bunionectomy. In the end-stage joint disease study,Citation52 patients received tapentadol IR (50 or 75 mg), oxycodone HCl IR (10 mg), or placebo every 4 to 6 hours for 10 days. In the 90-day safety study,Citation53 patients received flexible doses of tapentadol IR 50 or 100 mg (up to 600 mg/day) or oxycodone HCl 10 mg or 15 mg (up to 90 mg/day) every 4 to 6 hours as needed for up to 90 days.
In all 4 phase 3 studiesCitation50–Citation53 of tapentadol IR for acute pain, improvements in pain intensity were observed with tapentadol IR treatment (50, 75, or 100 mg every 4 to 6 hours) that were similar to those observed with oxycodone HCl IR treatment (10 or 15 mg every 4 to 6 hours) based on pain intensity measurements on an 11-point numerical rating scale (NRS; 0 = “no pain” to 10 = “worst pain imaginable”). For example, in the postoperative pain studyCitation50 in which patients received tapentadol IR (50 or 75 mg) for 72 hours following bunionectomy, 901 patients were randomized to treatment. In that study,Citation50 efficacy was evaluated based on the following measures: the sum of the pain intensity difference (SPID) over the first 12, 24, 48 (primary efficacy endpoint), and 72 hours of treatment; responder rates at 48 hours; and the patient global impression of change (PGIC). Based on increases in the mean (SD) SPID48, significantly greater reductions in pain intensity from baseline were observed with tapentadol IR 50 mg (122.2 [98.66]), tapentadol IR 75 mg (143.7 [96.52]), and oxycodone HCl IR 10 mg (140.3 [99.52]) than with placebo (54.1 [105.74]; P < 0.001 for all comparisons; ). Additionally, the efficacy of both doses of tapentadol IR was noninferior to the efficacy of oxycodone HCl IR 10 mg, based on the lower bounds of the 2-sided 97.5% confidence intervals for tapentadol IR 50 mg (−36.05) and 75 mg (−12.91), which were within the prespecified noninferiority margin of −48 (10% of the total possible value).
Figure 2 Differences from placeboa in mean SPID scores at 12, 24, 48, and 72 hours in patients with moderate to severe pain following bunionectomy treated with tapentadol IR 50 or 75 mg or oxycodone HCl IR 10 mg.Citation50
aP < 0.001 vs placebo for all comparisons.
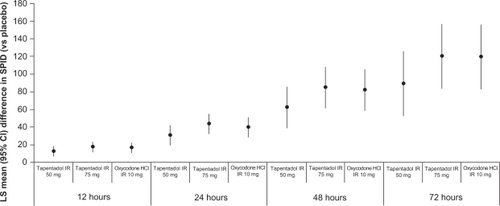
Secondary efficacy measurements supported the results of the primary efficacy endpoint in this phase 3 postoperative pain study.Citation50 Compared with placebo, significant reductions in pain intensity (based on the SPID) were observed for all active treatment groups at 12, 24, and 72 hours (P < 0.001 for all comparisons; ).
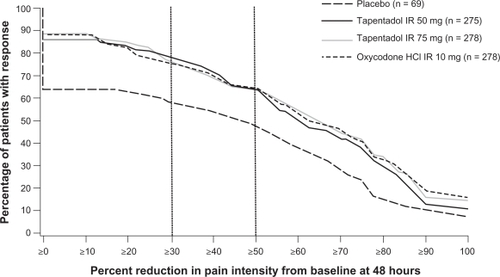
The distribution of responder rates was also significantly different between both tapentadol IR dose groups and placebo (P < 0.001) and oxycodone HCl IR 10 mg and placebo (P < 0.001; ). Compared with placebo, a significantly greater percentage of patients in all active treatment groups reported reductions in pain intensity at 48 hours of at least 30% (tapentadol IR 50 mg, 77.5%; tapentadol IR 75 mg, 76.3%; oxycodone HCl IR 10 mg, 75.2%; placebo, 58.0%; P ≤ 0.004 for all comparisons) and at least 50% (tapentadol IR 50 mg, 64.7%; tapentadol IR 75 mg, 64.0%; oxycodone HCl IR 10 mg, 64.4%; placebo, 47.8%; P ≤ 0.012 for all comparisons).
Figure 3 Distribution of responder rates based on pain intensity at 48 hours in patients with postoperative pain following bunionectomy. Reproduced from Daniels et al (2009).Citation50 Copyright © 2009 Informa Healthcare.
For the PGIC, patients rated their overall status since beginning study medication on a 7-point scale (1 = “very much improved” to 7 = “very much worse”). At the end of the study or early discontinuation, a rating of “much improved” or “very much improved” on the PGIC was reported by 83% of patients in the tapentadol IR 50-mg group, 88% of patients in the tapentadol IR 75-mg group, 86% of patients in the oxycodone HCl IR 10-mg group, and 65% of patients in the placebo group. The overall distribution of PGIC scores was significantly more favorable for all active treatment groups compared with placebo (P < 0.001 for all comparisons).
Safety and tolerability of tapentadol IR
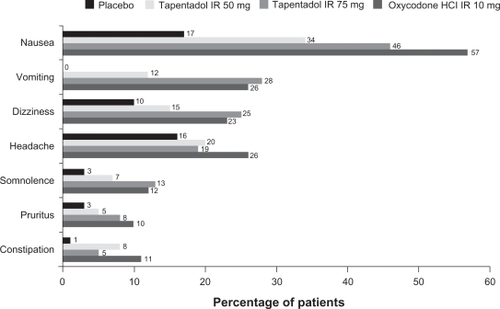
In all 4 studies,Citation50–Citation53 the most commonly reported treatment emergent adverse events (TEAEs) for patients who received any dosage of tapentadol IR were typical of drugs with μ-opioid agonist activity, and there were no major TEAEs suggestive of hyper-adrenergism. In the phase 3 studyCitation50 of tapentadol IR 50 and 75 mg in patients with postoperative pain following bunionectomy, the most common TEAEs (reported by ≥10% of patients in any treatment group) were nausea, vomiting, dizziness, headache, somnolence, pruritus, and constipation. Tapentadol IR 50 mg was associated with a lower incidence of all of these TEAEs than oxycodone HCl IR 10 mg, and tapentadol IR 75 mg was associated with a lower incidence of nausea, headache, and constipation than oxycodone HCl IR 10 mg (). A significantly lower percentage of patients reported nausea and/or vomiting in the tapentadol IR 50-mg group (35%) than in the oxycodone HCl IR 10-mg group (59%; P < 0.001). Thus, at a dose (50 mg) that provided efficacy that was noninferior to that provided by oxycodone HCl IR 10 mg, tapentadol IR was associated with a significantly lower incidence of gastrointestinal adverse events than oxycodone IR. A lower percentage of patients in the tapentadol IR 75-mg (51%) than in the oxycodone HCl IR 10-mg group (59%) reported nausea and/or vomiting, but the difference did not reach statistical significance (P = 0.057). A low percentage (<3% in any treatment group) of patients reported TEAEs that led to study discontinuation in the tapentadol IR 50-mg group (1.1%), the tapentadol IR 75-mg group (2.9%), the oxycodone HCl IR 10-mg group (1.8%), and the placebo group (1.5%).
Figure 4 Treatment-emergent adverse events occurring in ≥10% of patients in any treatment group among patients treated with placebo, tapentadol IR 50 or 75 mg, or oxycodone HCl IR 10 mg following bunionectomy.Citation50
The 90-day safety studyCitation53 permitted an evaluation of the long-term safety of flexible doses of tapentadol IR (50 or 100 mg every 4 to 6 hours) in comparison with flexible doses of oxycodone HCl IR (10 or 15 mg every 4 to 6 hours) in patients with acute osteoarthritis hip or knee pain or low back pain. Similar to the phase 3 postoperative pain study,Citation50 the most common TEAEs (occurring in ≥10% of patients in either treatment group) included gastrointestinal TEAEs (nausea, vomiting, and constipation), nervous system TEAEs (dizziness, headache, and somnolence), and pruritus. A lower percentage of patients in the tapentadol IR group than in the oxycodone IR group reported nausea (18.4% vs 29.4%), vomiting (16.9% vs 30.0%), constipation (12.8% vs 27.1%), and pruritus (4.3% vs 11.8%). Odds ratios demonstrated that patients treated with tapentadol IR were significantly less likely than patients treated with oxycodone IR to report nausea (0.542), vomiting (0.476), the composite of nausea and/or vomiting (0.458), or constipation (0.396; P < 0.001 for all comparisons). In addition, a lower percentage of patients discontinued from the study because of AEs in the tapentadol IR group (20.8%) than in the oxycodone IR group (30.6%), and patients in the oxycodone IR group discontinued significantly earlier than those in the tapentadol IR group (nominal P < 0.05; ). The percentage of patients who discontinued from the study due to gastrointestinal AEs was lower in the tapentadol IR group (8.8%) than in the oxycodone IR group (21.1%).
Figure 5 The distribution of times to discontinuation due to adverse events from the 90-day safety study among patients with osteoarthritis hip or knee pain or low back pain treated with tapentadol IR or oxycodone IR. Reproduced from Hale et al (2009).Citation53
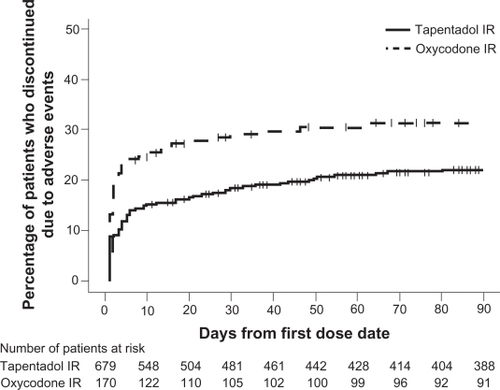
Compared with placebo, tapentadol IR (50 to 100 mg every 4 to 6 hours) and oxycodone HCl IR (10 or 15 mg every 4 to 6 hours) were associated with a higher incidence of TEAEs at all doses studied, and the majority of TEAEs increased with increasing dose.Citation50–Citation52 However, tapentadol IR was associated with a reduction in TEAEs, particularly gastrointestinal TEAEs and pruritus, relative to oxycodone IR in all 4 phase 3 studies.Citation50–Citation53 The lower incidence of gastrointestinal TEAEs observed with tapentadol IR relative to oxycodone IRCitation50–Citation53 was associated with a lower percentage of patients who discontinued treatment because of TEAEs. Nausea and vomiting are often associated with the initiation of opioid therapyCitation27 and are considered to be among the most undesirable adverse effects associated with analgesic therapy.Citation54 These opioid-induced gastrointestinal TEAEs may be dose-limiting and are often severe enough to cause patients to discontinue therapy, leading to disruption of pain relief.Citation55,Citation56
Conclusions
Tapentadol is a novel, centrally acting analgesic with 2 mechanisms of action, μ-opioid receptor agonism and norepinephrine reuptake inhibition,Citation35,Citation36 which may contribute to an improved gastrointestinal tolerability profile. In patients with moderate to severe acute pain of various etiologies, tapentadol IR has been shown to offer comparable analgesia to that provided by oxycodone IR, with lower incidences of gastrointestinal adverse effects and pruritus and lower rates of discontinuation due to adverse effects.Citation50–Citation53 This combination of potent analgesia and tolerability may represent a substantial improvement over current acute pain relief strategies.
Acknowledgements
Editorial support for the writing of this manuscript was provided by Megan Knagge, PhD, of MedErgy, and was funded by Johnson & Johnson Pharmaceutical Services, L.L.C. The authors were not compensated and retained full editorial control over the content of the manuscript.
Disclosures
D. Upmalis is an employee and shareholder of Johnson & Johnson. J.-U. Stegmann is an employee of Gr nenthal GmbH.
References
- BreivikHPostoperative pain management: why is it difficult to show that it improves outcome?Eur J Anaesthesiol19981567487519884866
- MacIntyrePon behalf of the Working Party of the Australian and New Zealand College of AnaesthetistsAcute pain management: scientific evidence2nd edMelbourne, AustraliaAustralian and New Zealand College of Anaesthetists2005 Available from: http://www.nhmrc.gov.au/publications/synopses/cp104syn.htm. Accessed October 30, 2009.
- SpacekAModern concepts of acute and chronic pain managementBiomed Pharmacother200660732933516814978
- ColeyKCWilliamsBADaPosSVChenCSmithRBRetrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costsJ Clin Anesth200214534935312208439
- GoldBSKitzDSLeckyJHNeuhausJMUnanticipated admission to the hospital following ambulatory surgeryJAMA198926221300830102810644
- CrombieIKDaviesHTMacraeWACut and thrust: antecedent surgery and trauma among patients attending a chronic pain clinicPain1998761–21671719696470
- MacraeWAChronic pain after surgeryBr J Anaesth2001871889811460816
- TailleferMCCarrierMBelisleSPrevalence, characteristics, and predictors of chronic nonanginal postoperative pain after a cardiac operation: a cross-sectional studyJ Thorac Cardiovasc Surg200613161274128016733157
- American Society of Anesthesiologists Task Force on Acute Pain ManagementPractice guidelines for acute pain management in the perioperative setting. A report by the American Society of Anesthesiologists Task Force on Pain Management, Acute Pain SectionAnesthesiology1995824107110817717542
- American Society of Anesthesiologists Task Force on Acute Pain ManagementPractice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain ManagementAnesthesiology200410061573158115166580
- ApfelbaumJLChenCMehtaSSGanTJPostoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanagedAnesth Analg200397253454012873949
- BeauregardLPompAChoiniereMSeverity and impact of pain after day-surgeryCan J Anaesth19984543043119597202
- CarrollKCAtkinsPJHeroldGRPain assessment and management in critically ill postoperative and trauma patients: a multisite studyAm J Crit Care19998210511710071701
- DixPSandharBMurdochJMacIntyrePAPain on medical wards in a district general hospitalBr J Anaesth200492223523714722175
- McGrathBElgendyHChungFKammingDCurtiBKingSThirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: a survey of 5,703 patientsCan J Anaesth200451988689115525613
- McNeillJASherwoodGDStarckPLThompsonCJAssessing clinical outcomes: patient satisfaction with pain managementJ Pain Symptom Manage199816129409707655
- MannERedwoodSImproving pain management: breaking down the invisible barrierBr J Nurs20009192067207211868183
- SummersSEvidence-based practice part 3: acute pain management of the perianesthesia patientJ Perianesth Nurs200116211212011290994
- WarfieldCAKahnCHAcute pain management. Programs in US hospitals and experiences and attitudes among US adultsAnesthesiology1995835109010947486160
- McHughGALukerKACampbellMKayPRSilmanAJA longitudinal study exploring pain control, treatment and service provision for individuals with end-stage lower limb osteoarthritisRheumatology (Oxford)200746463163717043045
- DonovanBDPatient attitudes to postoperative pain reliefAnaesth Intensive Care19831121251296869774
- GanTJLubarskyDAFloodEMPatient preferences for acute pain treatmentBr J Anaesth200492568168815003986
- BrownAKChristoPJWuCLStrategies for postoperative pain managementBest Pract Res Clin Anaesthesiol200418470371715460554
- PowerIBarrattSAnalgesic agents for the postoperative period: nonopioidsSurg Clin North Am199979227529510352655
- StephensJLaskinBPashosCPenaBWongJThe burden of acute postoperative pain and the potential role of the COX-2-specific inhibitorsRheumatology (Oxford)200342Suppl 3iii40iii5214585917
- McGettiganPHenryDCardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and non-selective inhibitors of cyclooxygenase 2JAMA2006296131633164416968831
- NicholsonBResponsible prescribing of opioids for the management of chronic painDrugs2003631173212487620
- ShangABGanTJOptimising postoperative pain management in the ambulatory patientDrugs200363985586712678572
- ChevlenEOpioids: a reviewCurr Pain Headache Rep200371152312525266
- HerndonCMJacksonKCIIHallinPAManagement of opioid-induced gastrointestinal effects in patients receiving palliative carePharmacotherapy200222224025011837561
- WheelerMOderdaGMAshburnMALipmanAGAdverse events associated with postoperative opioid analgesia: a systematic reviewJ Pain20023315918014622770
- AparasuRMcCoyRAWeberCMairDParasuramanTVOpioid-induced emesis among hospitalized nonsurgical patients: effect on pain and quality of lifeJ Pain Symptom Manage199918428028810534968
- OderdaGMEvansRSLloydJCost of opioid-related adverse drug events in surgical patientsJ Pain Symptom Manage200325327628312614962
- OderdaGMSaidQEvansRSOpioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stayAnn Pharmacother200741340040617341537
- TzschentkeTMChristophTKögelB(-)-(1R,2R)-3-(3-Dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel μ opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic propertiesJ Pharmacol Exp Ther2007323126527617656655
- TzschentkeTMDe VryJTerlindenRTapentadol HClDrugs Future2006311210531061
- MonoryKGreinerESartaniaNOpioid binding profiles of new hydrazone, oxime, carbazone and semicarbazone derivatives of 14-alkoxymorphinansLife Sci199964222011202010374926
- HartrickCTMultimodal postoperative pain managementAm J Health Syst Pharm200461Suppl 1S4S1015119755
- ReubenSSEkmanEFCharronDEvaluating the analgesic efficacy of administering celecoxib as a component of multimodal analgesia for outpatient anterior cruciate ligament reconstruction surgeryAnesth Analg2007105122222717578978
- TerlindenRKögelBEnglbergerWTzschentkeTIn vitro and in vivo characterization of tapentadol metabolitesMethods Find Exp Clin Pharmacol2009 Submitted
- KneipCTerlindenRBeierHChenGInvestigations into the drug-drug interaction potential of tapentadol in human liver microsomes and fresh human hepatocytesDrug Metab Letters2008216775
- WittwerEKernSERole of morphine’s metabolites in analgesia: concepts and controversiesAAPS J200682E348E35216796385
- PensonRTJoelSPGloyneAClarkSSlevinMLMorphine analgesia in cancer pain: role of the glucuronidesJ Opioid Manag200512839017319252
- AngstMSBuhrerMLotschJInsidious intoxication after morphine treatment in renal failure: delayed onset of morphine-6-glucuronide actionAnesthesiology20009251473147610781294
- OsborneRJoelSGrebenikKTrewDSlevinMThe pharmacokinetics of morphine and morphine glucuronides in kidney failureClin Pharmacol Ther19935421581678354025
- ArmstrongSCCozzaKLPharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, Part IIPsychosomatics200344651552014597688
- LotschJSkarkeCLiefholdJGeisslingerGGenetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectivesClin Pharmacokinet20044314983101315530129
- LotschJSkarkeCTegederIGeisslingerGDrug interactions with patient-controlled analgesiaClin Pharmacokinet2002411315711825096
- SmitJWOhCRengelshausenJEffects of acetaminophen, naproxen, and acetylsalicylic acid on tapentadol pharmacokinetics: results of two randomized open-label, crossover drug-drug interaction studiesPharmacotherapy2010301253420030470
- DanielsSCassonEStegmannJUA randomized, double-blind, placebo-controlled phase 3 study of the relative efficacy and tolerability of tapentadol IR and oxycodone IR for acute painCurr Med Res Opin20092561551156119445652
- DanielsSEUpmalisDOkamotoALangeCHaeusslerJA randomized, double-blind, phase III study comparing multiple doses of tapentadol IR, oxycodone IR, and placebo for postoperative (bunionectomy) painCurr Med Res Opin200925376577619203298
- HartrickCVan HoveIStegmannJUOhCUpmalisDEfficacy and tolerability of tapentadol immediate release and oxycodone HCl immediate release in patients awaiting primary joint replacement surgery for end-stage joint disease: a 10-day, phase III, randomized, double-blind, active- and placebo-controlled studyClin Ther200931226027119302899
- HaleMUpmalisDOkamotoALangeCRauschkolbCTolerability of tapentadol immediate release in patients with lower back pain or osteoarthritis of the hip or knee over 90 days: a randomized, double-blind studyCurr Med Res Opin20092551095110419301989
- McNicolEHorowicz-MehlerNFiskRAManagement of opioid side effects in cancer-related and chronic noncancer pain: a systematic reviewJ Pain20034523125614622694
- BellTJPanchalSJMiaskowskiCBolgeSCMilanovaTWilliamsonRThe prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European Patient Survey (PROBE 1)Pain Med2009101354218721170
- BenyaminRTrescotAMDattaSOpioid complications and side effectsPain Physician2008112 supplS105S12018443635