Abstract
Cancer pain is often difficult to treat. Growing evidence indicates that chemical mediators secreted by the tumor itself play an important role in the development of cancer pain. One such mediator, endothelin-1 (ET-1) is secreted by different tumor types. Studies have indicated that ET-1 induces spontaneous and evoked nociception in rodents and in humans. The focus of all these studies has always been on a single administration of ET-1. Such an acute exposure to ET-1 however bears little resemblance to the clinical condition in which cancer patients are exposed continuously for many months to increased levels of ET-1. To improve the knowledge of the pathological role of ET-1 in cancer, we developed an animal model of prolonged exposure to ET-1. Rats were exposed to subcutaneous administration of ET-1 for seven consecutive days, with a total amount of 67.4 nmol. On days +2, +3, +5, +7, and +10 sensitivity to von Frey hairs and to pin-prick stimulation were evaluated. Prolonged administration of ET-1 induced signs of mechanical allodynia on several time points. Although the administered doses were very small, prolonged administration of ET-1 seems to lead to a state of mechanical allodynia.
Introduction
Pain associated with metastatic cancer is often debilitating and difficult to treat.Citation1 Among all cancer patients, the prevalence of metastatic cancer pain may exceed 75%.Citation2 These patients often require large systemic doses of analgesics (eg, opioids) that do not always provide complete pain relief and that frequently produce undesirable side effects. There is growing preclinical evidence that peripheral sensitization of nociceptors is involved in the generation and maintenance of cancer pain.Citation3–Citation5 Chemical mediators that are synthesized by either cancer cells or other cell types present in the tumor stroma, such as immune cells, may contribute to the lowering of nociceptor threshold of activation.Citation6–Citation8 One candidate mediator for lowering this threshold of activation is the potent vasoconstrictor peptide, endothelin-1 (ET-1), whose expression levels are high in several types of tumors.Citation9–Citation12
Most efforts to establish a pathological role of ET-1 have focused on its role in cardiovascular disease (for reviews see Sugden and Clerk,Citation13 ShiffrinCitation14). Recently, ET-1 has been recognized as a proalgesic mediator that is involved in the pathophysiology of many different pain syndromes which range from inflammatory states, complex regional pain syndromes (CRPS), sickle cell disease to cancer.Citation9,Citation15–Citation17 Furthermore, its nociceptive effects are independent from its vasoconstrictive effects.Citation18 Application of ET-1 has been shown to induce nociceptive behavior in rodents, and pain in humans.Citation15,Citation18–Citation21 The results of clinical studies have shown the existence of a correlation between the severity of the pain and plasma levels of endothelins in patients with prostate cancer.Citation22
Despite the abundance of preclinical reports, the experimental conditions of these studies often bear little resemblance with clinical pathological states. These experiments usually use a single administration of ET-1, whereas in pathological conditions, such as cancer, patients have chronically (uninterrupted) elevated ET-1 levels in their plasma. The results of human studies have reported that mean plasma ET-1 levels in patients with different cancerous conditions are between 4–5 pg/mL.Citation12,Citation23–Citation25 Although preclinical studies have applied very diverse concentrations of ET-1, these are probably consistently higher than the observed levels in human pathological conditions. Picomolar doses of injected ET-1 that yield very low local ET-1 concentrations are probably closer to the plasma levels of endogenous ET-1 that are found in human pathological conditions. It should be noted that tissue levels of ET-1 could well be extremely important for pain induction in cancer syndromes. ET-1 is indeed released from endothelial cells in a polarized fashion into the direction of surrounding vascular tissue and thus, plasma levels of ET-1 are likely to be only a fraction of the levels in surrounding tissues and should perhaps be considered as a merely “spill over” from the tissue compartment.
Until now, only one study has investigated the nociceptive effects of repeated or prolonged administration of ET-1.Citation26 These investigators reported that repeated administration of low doses of ET-1 in rats led to the development of an acute desensitization period that persist for up to 24 hours. In the absence of confirming data, we decided to further investigate the effects of chronic delivery of exogenous ET-1 on spontaneous and evoked pain sensations in rats. The results of our preliminary experiments (unpublished data) revealed that prolonged perineural administration of ET-1 quickly (within 24 hours) results in the development of an intense fibrotic reaction around the subcutaneous tip of the cannula. This fibrotic reaction can rapidly result in complete blockage of the cannula, thereby reducing its performance and preventing further delivery of ET-1. Based on these preliminary findings, we opted to use very low doses of ET-1 to avoid any confounding inflammatory response. The final dose of ET-1 was chosen in accordance with the results of previous ET-1-based behavioral studies.Citation18,Citation20,Citation27,Citation28
The present project was undertaken to further characterize the exact role of ET-1 in nociception. To this end, we assessed the effect of prolonged administration of minute doses of ET-1 on the perception of mechanically evoked nociception.
Material and methods
Experimental animals
Twenty male Sprague–Dawley rats (180–200 g at arrival, Charles River®) were used in this protocol. They were housed in pairs in conventional plastic cages (24 × 40 × 15 cm) in a rodent colony room whose room temperature was 21 ± 1 °C and a relative humidity of 45 ± 5%. Water and food were available ad libitum. Rats were kept under a reversed 12:12 hour dark/light cycle (lights on at 20:00 hours) and were acclimated for at least three weeks to the housing conditions before testing. The rats were habituated to the test procedure (see later for detailed description) three times before baseline values were obtained. The experimental protocol was approved by the Animal Ethical Committee of the University of Antwerp, and the experiments were performed in accordance with the Directives of European Community Council on the use and care of laboratory animals.
Experimental groups and drug administration
Rats were randomly assigned into two groups. Group 1 received ET-1 and group 2 received saline. Rats were implanted with an osmotic pump delivering either ET-1 (24 μg/day) or saline. The total amount of ET-1 administered to the rats over seven days was 67.4 nmol. ET-1 solution was prepared by dissolving the dry compound in saline. Preliminary testing has confirmed the stability of the ET-1 solution in these pumps. Additionally, samples of the ET-1 solution, after being contained in the osmotic pumps during seven consecutive days, were afterwards injected into the rat’s paw to check any remaining biological activity. Indeed, such injections induced the previously described paw-flinching syndrome, evidencing a remaining biological (nociceptive) activity of this ET-1 solution. Pumps (Alzet 2ML1) were filled by injecting the solution into the pumps using a 1 ml sterile syringe and a specialized blunt needle. These pumps have a nominal pumping rate of 10.0 μl/hr and a nominal duration of seven days. In order to obtain immediate pumping after implantation, the pumps were primed before implantation, according to the guidelines provided by the manufacturer. The pumps were prefilled in the usual manner and placed in sterile 0.9% saline at 37 °C overnight. During this priming period the catheter was draped outside the beaker to avoid any mixing of solutions. The next morning the pump was removed from the saline and implanted immediately.
The pumps were implanted into the rats under anesthesia. To this end, the rats were placed in a chamber containing 4% isoflurane. When the rats lost consciousness, they were removed from the chamber, and anesthesia was maintained by 2.5% isoflurane delivered by a face mask. The dorsal area was shaved and then disinfected with Hibitane® (chlorhexidine 0.5% dissolved in 70% alcohol). A skin incision of approximately 2 cm made at the distal end of the scapula and a small subcutaneous pocket was created using hemostatic forceps. Ampicillin (0.1 ml of Pentrexyl®, Na ampicillin 1 g) was then injected into the pocket and the pump was inserted with its opening facing towards the back of the rat and away from the incision site (tip of the catheter pointing towards the lumbar region of the rat). Finally, the incision was closed with stainless steel staples (Disposable skin stapler with auto-release Appose 35 Regular; Sherwood– Davis and Geck, St. Louis, MO, USA). After seven days the pumps were removed under general anesthesia.
In order to observe possible hemodynamic effects of the injected ET-1, systolic blood pressure was measured non-invasively in all animals daily by tail cuff plethysmography (Letica 5100; PanLab, Barcelona, Spain). Blood pressure monitoring was performed according to standard procedures, described previously.Citation29 Additionally, body weight was measured daily. Other clinical signs of distress were also followed, such as the appearance of red tears.
Behavioral testing
Rats were individually transported from the colony room to the test room (15 sec trip) in a covered plastic cage without bedding (24 (l) × 14 (w) × 17 (h) cm). von Frey and pinprick testing were conducted in a darkened room in which light was provided by a 60W red light bulb suspended 1 m above the test area and with a 45 dB background noise that was sufficient to decrease the interference of sudden external auditory stimuli. Testing of evoked behavior was always performed at the same moment, more precisely during late morning. An extensive training phase for the rats was performed, in order to obtain highly reproducible results. All rats were as such acclimated to the testing conditions (ie, manipulation and transportation of the rats, stay in the darkened room and in the test box, performance of mechanical testing, procedure of blood pressure measurement) during 10 days before the start of the actual experiment. Baseline data were obtained one day before pump implantation. Further data were obtained on post-implantation days +2, +3, +5, +7, and +10. Data that were collected on the tenth day were at approximately three days after the end of pump infusion and the removal of the pump. Behavioral testing was performed by the same examiner, who was blinded with respect to administration of ET-1 or saline. The code was broken after data entry and analysis.
Local cutaneous sensitivity
Responsiveness to mechanical stimulation of a dorsal area, approximately 25 mm caudal to the opening of the subcutaneous pump, was measured using a series of three von Frey filaments (Stoelting Co, Wood Dale, IL, USA): 2.150 g, 7.370 g, and 46.540 g and a pin-prick (21 gauge needle bent at a 45° angle). The cages of the rats were placed next to each other on a table and the experimenter sat in front of them. The scoring system used was adapted from Vos and colleaguesCitation30,Citation31 to evaluate the reaction of the rats to the stimulation, and we have used this modified method previously in different behavioral and pharmacological studies.Citation32–Citation35 The response of an animal is analyzed according to different response categories: 0: no response, 1: detection, 2: withdrawal reaction, 3: escape/attack. Lower scores indicate a weak responsiveness to stimulation while higher scores indicate a strong responsiveness. Under normal conditions (nonoperated rats and in absence of sensitivity modifying drugs) rats will display mechanical sensory thresholds ranging from 0 (no response at all to stimulation) to 1 (detective response) to the above mentioned mechanical stimulations in this particular skin region. These mechanical stimulations should therefore be considered as absolutely nonpainful in nonpathological conditions. In order not to sensitize the animals too much by performing too many manipulations and (mechanical) stimulations we decided not to include stimulation of distal dermatomes into the current study protocol.
Statistical analysis
All statistical analyses were performed using SPSS for Mac (version 13.0.0; SPSS Inc., Chicago, IL, USA). In order to analyze data from mechanical stimulation, preliminary Kruskal–Wallis testing was performed in order to detect any time-or treatment-dependent differences between the different experimental groups. Comparisons between responses to mechanical stimulation (von Frey filaments and pin-prick) between experimental groups on a single time point were performed using the Mann–Whitney test. Exact significance [2*(1-tailed sig.)] was hereby calculated. The parameters of systolic blood pressure in both experimental groups at the individual time points were compared using an unpaired t-test. The significance level for this study was set at 5%.
Results
Hemodynamic and distress monitoring
No rats displayed significant alterations in systolic blood pressure (SBP) during the course of the administration of ET-1 or saline. At the starting point of the experiment, SBPs of both experimental groups were comparable (108 ± 5 mmHg). Mean SBPs over seven days (during ET-1 and placebo administration) were not significantly different in ET-1-treated rats compared with those in placebo-infused rats (114 ± 3 vs 111 ± 2 mmHg). ET-1 and placebo-treated animals displayed identical increases in body weight. No signs of distress were observed in any rats.
Cutaneous sensitivity to mechanical stimulation
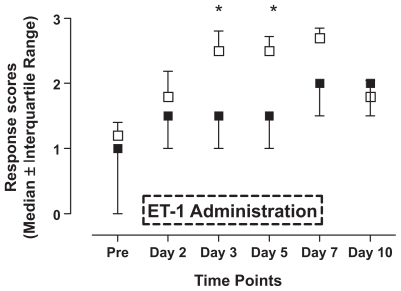
Chronic administration of low doses ET-1 led to changes in sensitivity to different types of mechanical stimulation. Stimulation with the smallest von Frey hair (2.150 g) did not induce significant changes in response scores compared to saline-treated rats (). However, stimulation with 7.370 g von Frey filament () led to significantly increased response scores on day 3 (p < 0.05). Stimulation with the largest of the three von Frey hairs (46.540 g; ) resulted in significantly higher response scores in ET-1-treated animals on days 3, 5, and 7 compared to the control animals stimulated with the identical hair (p < 0.05). Finally, behavioral responses to pin-prick stimulation () displayed a somewhat similar time course with significantly increased responsiveness in ET-1-treated rats on days 3 and 5 (p < 0.05). Saline-treated animals displayed some changes in responsiveness to mechanical stimulation following the implantation of the pumps, but these changes always failed to reach statistical significance when compared to pre-implantation values (p > 0.05).
Figure 1 Time course of changes in response scores to (A) stimulation with 2.150 g von Frey filament, (B) 7.370 g von Frey filament, and (C) 46.540 g von Frey filament. Saline (■) or endothelin-1 (ET-1) (□) was administered for seven consecutive days (see box in graph C). The following response codes were applied: ‘no response’ (indicated by 0 in the X-axis of the graph), ‘detection’ (1), ‘withdrawal reaction’ (2), ‘escape/attack’ (3). Data are expressed as median values ± interquartile range. Asterisks indicate a significant difference in response scores between ET-1 administration and saline (Mann–Whitney U test; p < 0.05).
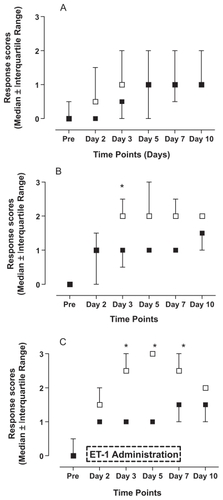
Figure 2 Effects of endothelin-1 (ET-1) administration on the reaction to a pin-prick (21 gauge needle). Saline (■) or ET-1 (□) was administered for seven consecutive days (see box). The following response scores were applied: ‘no response’ (indicated by 0 in the X-axis of the graph), ‘detection’ (1), ‘withdrawal reaction’ (2), ‘escape/attack’ (3). Data are expressed as median values ± interquartile range. Asterisks indicate a significant difference between ET-1 and saline administration (Mann–Whitney U test; p < 0.05).
Discussion
To the best of our knowledge, the results of the present study constitute the first report on the effects on evoked nociception of a prolonged local administration of endothelin-1 (ET-1) in rats. ET-1-treated animals displayed significant changes in sensitivity to mechanical stimuli. Although the temporal changes in evoked behavior were somewhat different for the various intensities of mechanical stimulation, significant alterations occurred particularly from the third day of ET-1 administration. The observed mechanical allodynia (increased sensitivity to nonpainful mechanical stimulations) disappeared after discontinuation of ET-1 administration.
Rats receiving continuous administration of saline did display some changes in sensitivity to mechanical stimulation during the treatment period. However, none of these changes ever reached statistical significance and this result indicates that the administration of saline does not lead to significant changes in mechanical sensitivity. In addition, this result also means that the subcutaneous infusion of a fluid without pharmacological activity over seven days did not lead to the development of hypersensitivity in these animals. However, the subcutaneous implantation of a pump induces some changes in sensitivity in the animals, without ever reaching any significancy. Additionally, it should be noted that these changes occurred immediately after implantation of the pump, whereas the ET-1-induced changes occurred at a somewhat later stage. This time difference in the onset of changes in cutaneous sensitivity points to a clear difference between pump-induced and ET-1-induced changes.
The rather limited extent of changes in mechanical sensitivity that was observed in the ET-1-treated animals is probably due to the low doses of ET-1 used in this investigation. The administration of 24 μg ET-1 per day corresponds to 9.6 nmol per day (or a total of 67.4 nmol over seven days). This means that only about 6 pmol of ET-1 was released subcutaneously each minute. Previous studies investigating the algesic effects of ET-1 have involved different methods of administering ET-1. These methods range from injecting 120 pmol ET-1 into the knee joint of rats,Citation36 to application of 16 nmol ET-1 onto the epineurial surface of the sciatic nerve of a rat.Citation18 The results of a recent study have shown that single injection of 0.1 nmol of ET-1 into the rat hind paw can induce sensitization of the ipsilateral paw to tactile stimulation by von Frey hairs.Citation37 In our study, approximately 50% of this dose was delivered each minute by the subcutaneously implanted osmotic pump. Considering the results obtained from previously published dosing regimens of ET-1, it is quite remarkable that such a low dose of ET-1 used in our study indeed induces a prolonged state of mechanical hyperalgesia. This result implies that a continuous administration of ET-1 slowly leads to hypersensitivity, hereby providing an explanation for the onset of mechanical hyperalgesia after the third day of low dose ET-1 administration.
The experimental model used in our investigation reflects more accurately those clinical conditions in which patients are continuously exposed during weeks to months to elevated levels of ET-1. Nevertheless, some methodological limitations should certainly be considered. Animals were subjected to repeated mechanical stimulations that by itself could lead to some kind of hypersensitivity phenomenon. However, similar testing protocols have been used in our laboratory using other compounds and we found that the experimental protocols do not result in decreased thresholds.Citation33,Citation35 In addition, it would have been desirable to include additional testing for thermal sensitivity into the study protocol. However, this would have subjected the rats to even more testing manipulations on the same day, possible leading to a general state of sensitization and seriously jeopardizing the validity of the behavioral results. Because few data are currently available regarding the ET-1 concentrations that are present in cancerous tissues, the exact physiological relevance of the amounts of ET-1 that were applied in this protocol can not be correctly evaluated. It would have been interesting to combine the parameters of evoked sensation with measurements of (plasma/tissue) ET-1-concentration. This was not done in this protocol due to the technical difficulties linked to measurements of ET-1 levels. Additionally, repetitive blood sampling would have greatly increased the stress for the animals. In future experiments we plan to use enzyme-linked immunosorbent assays to obtain ET-1 measurements in plasma and tissue. Finally, we opted not to use a cancer pain model, in order to exclude any possible confounding factors due to the presence of a growing tumor. Studies using different cancer pain models have already shown the development of reduced thresholds to mechanical stimulation, as well as pain reduction after administration of ET-1 antagonists.Citation38–Citation40 However, a growing tumor mass will result in many pathophysiological changes in the rodents, hereby possibly confounding the nociceptive effects of ET-1.
Conclusion
This report is the first description of the neurosensory changes that develop following prolonged administration of ET-1. Although the administered doses were very small, prolonged administration of ET-1 seems to lead to a state of mechanical and heat hyperalgesia. This ET-1-based animal model could prove to be of great value for future investigations into the pathophysiology of ET-1-induced pain syndromes because the model, more closely mimics human clinicopathological conditions. Further studies are warranted to establish a dose-response relationship of chronic delivery of ET-1 and to evaluate the effects of longer administration periods as well as different routes of administration. Finally, the differential effects of chronic administration of ET-1 on spontaneous behavior and evoked nociception should be carefully investigated in future experiments.
Acknowledgments
The authors gratefully acknowledge the financial support by the ‘Benoît Foundation’. The authors report no competing or conflicts of interest in this work.
References
- MantyhPWCancer pain and its impact on diagnosis, survival and quality of lifeNat Rev Neurosci2006779780916988655
- PortenoyRKPayneDJacobsenPBreakthrough pain: characteristics and impact in patients with cancer painPain19998112913410353500
- HonorePLugerNMSabinoMAOsteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cordNat Med2000652152810802707
- LugerNMHonorePSabinoMAOsteoprotegerin diminishes advanced bone cancer painCancer Res2001614038404711358823
- SchweiMJHonorePRogersSDNeurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer painJ Neurosci199919108861089710594070
- PetersCMLindsayTHPomonisJDEndothelin and the tumorigenic component of bone cancer painNeuroscience20041261043105215207337
- SabinoMAMantyhPWPathophysiology of bone cancer painJ Support Oncol20053152415724942
- SabinoMCGhilardiJRFeiaKJThe involvement of prostaglandins in tumorigenesis, tumor-induced osteolysis and bone cancer painJ Musculoskelet Neuronal Interact2002256156215758394
- DavarGEndothelin-1 and metastatic cancer painPain Med20012242715102314
- KurbelSKurbelBKovacicDEndothelin-secreting tumors and the idea of the pseudoectopic hormone secretion in tumorsMed Hypotheses19995232933310465672
- NelsonJBCarducciMAThe role of endothelin-1 and endothelin receptor antagonists in prostate cancerBJU Int200085Suppl 2454810781185
- ShankarALoizidouMAlievGRaised endothelin 1 levels in patients with colorectal liver metastasesBr J Surg1998855025069607532
- SugdenPHClerkAEndothelin signalling in the cardiac myocyte and its pathophysiological relevanceCurr Vasc Pharmacol2005334335116248777
- SchiffrinELState-of-the-Art lecture. Role of endothelin-1 in hypertensionHypertension19993487688110523377
- FerreiraSHRomitelliMde NucciGEndothelin-1 participation in overt and inflammatory painJ Cardiovasc Pharmacol198913Suppl 5S220S2222473319
- GroenewegJGHuygenFJHeijmans-AntonissenCNiehofSZijlstraFJIncreased endothelin-1 and diminished nitric oxide levels in blister fluids of patients with intermediate cold type complex regional pain syndrome type 1BMC Musculoskelet Disord200679117137491
- HammermanSIKourembanasSConcaTJTucciMBrauerMFarberHWEndothelin-1 production during the acute chest syndrome in sickle cell diseaseAm J Respir Crit Care Med19971562802859230761
- DavarGHansGFareedMUSinnottCStrichartzGBehavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerveNeuroreport19989227922839694215
- ChichorroJGZampronioARRaeGAEndothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic painExp Biol Med (Maywood)20062311136114016741064
- GokinAPFareedMUPanHLHansGStrichartzGRDavarGLocal injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in ratsJ Neurosci2001215358536611438612
- PiovezanAPD’Orleans-JustePFrighettoMSouzaGEHenriquesMGRaeGAEndothelins contribute towards nociception induced by antigen in ovalbumin-sensitised miceBr J Pharmacol200414175576314744803
- NelsonJBHedicanSPGeorgeDJIdentification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostateNat Med199519449497585222
- ArunCDeCatrisMHemingwayDMLondonNJO’ByrneKJEndothelin-1 is a novel prognostic factor in non-small cell lung cancerInt J Biol Markers20041926226715646831
- ArunCLondonNJHemingwayDMPrognostic significance of elevated endothelin-1 levels in patients with colorectal cancerInt J Biol Markers200419323715077924
- MaiHQZengZYZhangCQElevated plasma big ET-1 is associated with distant failure in patients with advanced-stage nasopharyngeal carcinomaCancer20061061548155316518816
- FareedMUHansGHAtandaAPharmacological characterization of acute pain behaviour produced by application of Endothelin-1 to rat sciatic nerveJ Pain200014653
- KhodorovaAFareedMUGokinAStrichartzGRDavarGLocal injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive mannerJ Neurosci2002227788779612196602
- KhodorovaANavarroBJouavilleLSEndothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injuryNat Med200391055106112847519
- BunagRDValidation in awake rats of a tail-cuff method for measuring systolic pressureJ Appl Physiol1973342792824686367
- VosBPStrassmanAMMaciewiczRJBehavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerveJ Neurosci199414270827238182437
- VosBPHansGAdriaensenHBehavioral assessment of facial pain in rats: face grooming patterns after painful and non-painful sensory disturbances in the territory of the rat’s infraorbital nervePain1998761731789696471
- DeseureKBreandSColpaertFCCurative-like analgesia in a neuropathic pain model: parametric analysis of the dose and the duration of treatment with a high-efficacy 5-HT(1A) receptor agonistEur J Pharmacol200756813414117512927
- DeseureKKoekWAdriaensenHColpaertFCContinuous administration of the 5-hydroxytryptamine1A agonist (3-Chloro-4-fluoro-phenyl)-[4-fluoro-4-[[(5-methyl-pyridin-2-ylmethyl) -amino]-methyl]piperidin-1-yl]-methadone (F 13640) attenuates allodynia-like behavior in a rat model of trigeminal neuropathic painJ Pharmacol Exp Ther200330650551412730352
- DeseureKKoekWColpaertFCAdriaensenHThe 5-HT(1A) receptor agonist F 13640 attenuates mechanical allodynia in a rat model of trigeminal neuropathic painEur J Pharmacol2002456515712450569
- DeseureKRAdriaensenHFColpaertFCEffects of the combined continuous administration of morphine and the high-efficacy 5-HT1A agonist, F 13640 in a rat model of trigeminal neuropathic painEur J Pain2004854755415531223
- De-MeloJDTonussiCRD’Orleans-JustePRaeGAArticular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonistsPain1998772612699808351
- BalonovKKhodorovaAStrichartzGRTactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptorsExp Biol Med (Maywood)20062311165117016741070
- PickeringVJay GuptaRQuangPJordanRCSchmidtBLEffect of peripheral endothelin-1 concentration on carcinoma-induced pain in miceEur J Pain20081229330017664075
- SchmidtBLPickeringVLiuSPeripheral endothelin A receptor antagonism attenuates carcinoma-induced painEur J Pain20071140641416807013
- YuyamaHKoakutsuAFujiyasuNInhibitory effects of a selective endothelin-A receptor antagonist YM598 on endothelin-1-induced potentiation of nociception in formalin-induced and prostate cancer-induced pain models in miceJ Cardiovasc Pharmacol200444Suppl 1S479S48215838353