Abstract
Epilepsy is a common pediatric neurologic disorder that is difficult to manage in a substantial portion of children. Levetiracetam (LEV) is a novel antiepileptic drug (AED) that has recently been approved as add-on treatment for various seizure types in epilepsy populations that include children: for refractory partial seizures in epilepsy patients ≥4 years old, for myoclonic seizures in juvenile myoclonic epilepsy patients ≥12 years old, and for primary generalized tonic-clonic seizures in idiopathic generalized epilepsy patients (≥6 years old with FDA approval; ≥12 years old with EMEA approval). A review of published pediatric studies indicates that the efficacy of LEV is best established for partial seizures; however, results from recent double-blind and open-label trials indicate that adjunctive LEV also controls generalized seizures – particularly myoclonic and generalized tonic-clonic – in children and adolescents with primary generalized epilepsy. LEV was well-tolerated in pediatric studies. The most common adverse events (AEs) reported were sedation related. Behavioral AEs were among the most commonly reported events in some trials; conversely, improvements in behavior and cognition were also frequently reported. LEV appears to be a safe and effective AED with unique characteristics that benefit the treatment of children with epilepsy.
Keywords:
Introduction
Epilepsy is a common neurologic disorder in children and frequently presents a significant challenge for treatment (CitationShinnar and Pellock 2002). Seizures are refractory in about a quarter of children (CitationPellock 1999) and pediatric epilepsy often occurs in the context of mental retardation (CitationShinnar and Pellock 2002) or significant behavioral problems (CitationOtt et al 2003). Children with refractory epilepsy may receive trials of several antiepileptic drugs (AEDs) without achieving adequate seizure control or acceptable tolerability (CitationHerranz 2003; CitationGrosso et al 2005; CitationMandelbaum et al 2005; CitationOpp et al 2005). Despite the availability of a number of AEDs that are approved for pediatric use, additional AEDs that are effective and well-tolerated in children are still needed. Levetiracetam (LEV) was recently approved for use as add-on therapy in children with partial seizures and now provides another alternative in the treatment of pediatric epilepsy.
LEV is a broad-spectrum AED with a unique preclinical and pharmacological profile. In rodent studies, it demonstrated no activity in traditional acute seizure models (maximal electroshock and pentylenetetrazol seizure tests) but exhibited potent seizure protection in chronic epilepsy models, ie, in kindled animals and in genetic models of generalized epilepsy. It was also protective against seizures in rodent models of chemoconvulsant-induced partial seizures. LEV exhibited antiepileptogenic properties through its ability to inhibit the development of kindling in mice and rats and demonstrated a high safety margin compared with other AEDs in genetic models and kindled animals (CitationKlitgaard 2001).
The molecular mechanisms through which LEV exerts its antiepileptic effects are not fully known but are unlike those of any other AED. LEV binds to a unique binding site in the brain, the synaptic vesicle protein SV2A. The function of this protein is still under investigation, but current evidence suggests that it modulates synaptic vesicle fusion and the consequent release of neurotransmitter into the synapse. Because LEV does not appear to affect normal brain physiology, it is believed to modulate SV2A function only under pathophysiologic conditions (CitationLynch et al 2004). LEV is also known to selectively inhibit N-type calcium channels (CitationLukyanetz et al 2002) and to block the inhibition of GABA- and glycine-gated currents by negative allosteric modulators (CitationRigo et al 2002). Whether LEV’s binding at SV2A proteins mediates these mechanisms is unknown (CitationLynch et al 2004).
LEV has been FDA approved since 1999 for adjunctive treatment of refractory partial seizures in adults (CitationLeppik 2001) and in 2005, that approval was extended to include children ≥4 years old (CitationMedical News Today 2005). Most recently, the approval was further broadened to include adjunctive treatment of myoclonic seizures in adults and adolescents ≥12 years of age with juvenile myoclonic epilepsy and adjunctive treatment of primary generalized tonic-clonic (PGTC) seizures in adults and children ≥6 years of age with idiopathic generalized epilepsy. EMEA indications are similar, except that the age range approved for PGTC seizures is ≥12 years, and LEV is also approved as monotherapy in adults with newly diagnosed partial seizures (CitationBioSpace Beat 2007). LEV, as add-on therapy, has also demonstrated efficacy for refractory idiopathic generalized epilepsies in open-label trials that included adult patients with myoclonic, tonic-clonic, and absence seizures (CitationKrauss et al 2003; CitationCoppola et al 2004; CitationKumar and Smith 2004).
Although the efficacy, safety, and tolerability profile of LEV is well established in the treatment of adult epilepsy patients, data in children are somewhat limited. This article reviews available published data on LEV in the treatment of pediatric epilepsy, including pertinent information on LEV’s pharmacokinetics, formulations, efficacy, and safety.
Pharmacokinetics of LEV in adults versus children
The pharmacokinetic profile of LEV as demonstrated in adults is very favorable and allows for easy titration and use of the drug, even in the presence of comedications. LEV is rapidly absorbed after oral dosing and exhibits linear kinetics with minimal protein binding (<10%). Oral bioavailability is almost 100% with peak plasma concentrations achieved after 1 hour and LEV has a half-life of 6–8 hours in adults (CitationPatsalos, 2000). In a rat model, the half-life of LEV in brain extracellular fluid was ~50% longer than in serum and may explain its long duration of effect and need for only twice-a-day dosing (CitationTong and Patsalos 2001). LEV is not metabolized hepatically but is eliminated renally, primarily as unchanged drug. The half-life is extended to 10–11 hours in the elderly because of age-associated reduction in renal function. (CitationPatsalos 2000) LEV is not known to participate in clinically relevant pharmacokinetic drug-drug interactions (CitationPerucca 2006).
The pharmacokinetic profile of LEV appears similar in pediatric patients compared with adults, except that the drug is cleared more quickly. Body clearance is 30% to 40% higher and the half-life is decreased to about 6 hours. Higher doses of LEV (on a mg/kg basis) may be required in children to accommodate their increased clearance (CitationPellock et al 2001). The product labeling recommends a starting dose in children of 20 mg/kg/day, and the daily dose can be increased every 2 weeks to a maximum of 60 mg/kg/day. Similar to adults, the pharmacodynamic action of LEV in children appears prolonged relative to its plasma half-life and twice-daily dosing is recommended (CitationKeppra 2004).
LEV is currently available as an oral tablet (250, 500, and 750 mg in the US and EU; 1000 mg in the EU only), a 10% oral solution (100 mg/mL), and an i.v. formulation (100 mg/mL). The solution is grape flavored and contains no alcohol or sugar (CitationKeppra 2004). The oral formulations are bioequivalent and can be used interchangeably (CitationCoupez et al 2003). Children weighing less than 20 kg will need to initiate treatment with the oral solution to achieve their lower dose requirements. Oral LEV can be administered with or without food (CitationKeppra 2004). The i.v. formulation is also bioequivalent and can be used interchangeably with the oral formulations; however, the FDA approval was applicable only for use in adult patients (CitationMedical News Today 2006; CitationRamael et al 2006).
Efficacy of LEV as adjunctive therapy in pediatric epilepsy
Prospective studies in partial seizures
Two prospective trials have evaluated the efficacy of LEV for refractory partial seizures in children. The first trial was a multicenter, prospectively designed, open-label trial that enrolled 24 children aged 6–12 years (CitationGlauser et al 2002). During a 4-week baseline period, patients received a stable dose of one standard AED and were required to have at least 2 seizures. During the titration period, LEV was added to the baseline regimen at 10 mg/kg/day (bid dosing) and was increased to a maximum of 40 mg/kg/day over 6 weeks. After titration, patients entered an 8-week evaluation phase where LEV dose remained constant. Twenty-three patients entered the evaluation phase and were included in the efficacy analysis.
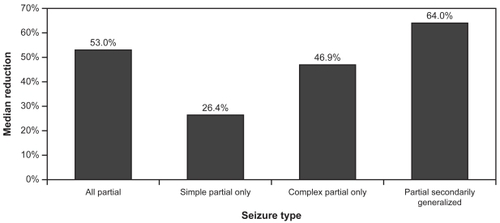
The mean age of patients was 9.4 ± 2.2 years and the most commonly reported partial seizure subtype was complex partial with 83.3% of patients reporting them at baseline. Over half (52.2%) of the patients showed a ≥50% reduction in partial seizures from baseline, and a ≥75% reduction was documented in 21.7%. Two patients (8.7%) were seizure free throughout the 8-week evaluation period. depicts the median percentage reduction in seizures by seizure subtype. Based on this endpoint, LEV appeared to be most effective for secondarily generalized seizures (CitationGlauser et al 2002).
Figure 1 Mean percentage reduction in seizure frequency per week: open-label pediatric trial in partial seizures.
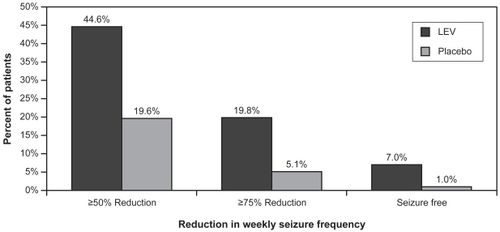
After these positive findings were reported in the preliminary open-label study, a double-blind, placebo-controlled study was implemented. Children (4–16 years) with at least 8 partial seizures reported during an 8-week baseline were randomized to receive placebo or LEV 60 mg/kg/day bid (CitationGlauser et al 2006). Patients had uncontrolled seizures on 1 or 2 standard AEDs during baseline, and LEV or placebo was added to the baseline regimen. Drug was titrated over a 4-week period (in 20 mg/kg/day increments every 2 weeks to a target dose of 60 mg/kg/day) after which patients entered a 10-week evaluation phase. The titration and evaluation periods were considered the treatment period, and efficacy endpoints were calculated over this entire 14-week period. One hundred ninety-eight patients were evaluable for efficacy (LEV = 101; placebo = 97). The mean age was 10.2 years in the LEV group and 9.8 years in the placebo group. LEV significantly reduced weekly partial seizure frequency (the primary endpoint) by 26.8% over placebo (p = 0.0002). shows response rates based on weekly percent reduction in partial seizure frequency. A 50% or greater reduction in partial seizures was attained in 44.6% of the LEV group and 19.6% of the placebo group (p = 0.0002). Seizure freedom rates were also higher in the LEV group (7% vs 1% for placebo). This study was the basis for the FDA’s approval of LEV’s use in children.
Prospective studies in partial and/or generalized seizures
Several prospectively designed, open-label studies have tested the effects of LEV in children with various epilepsy syndromes or epilepsy types. These studies report efficacy for both partial and generalized seizures. In the first published study, 39 patients (mean age 8.6 years) were followed for up to 9 months (CitationWheless and Ng 2002). LEV was titrated over 8 weeks (on average) to a mean maintenance dose of 53.3 mg/kg/day (given bid or tid). The mean number of concomitant AEDs was 1.6. Seizures were reduced by >50% in 33.3% of patients, by >90% in 23.1% of patients, and 7.7% of patients were seizure free. An analysis by seizure type indicated that efficacy was better in partial seizures than in generalized seizure subtypes. In another small trial (n = 21) in refractory epilepsy, Lagae and colleagues enrolled children from the age of 11 months to 14 years (CitationLagae et al 2003). This population included children with identifiable lesions on MRI, children with Lennox Gastaut or West syndrome, and children with idiopathic partial or generalized epilepsy. Most patients (17/21) were receiving at least 2 AEDs at inclusion. LEV was titrated from 10 mg/kg/day to a maximum of 60 mg/kg/day with dose increases occurring every 4th day. There was a significant reduction in total seizure frequency (from baseline) overall (p <0.01) and 10 patients (47%) had at least a 50% reduction in all seizures. One child was seizure free (4.8%). Efficacy did not appear to differ based on syndrome or epilepsy type. Sixty percent (3/5) of children with Lennox Gastaut and the 1 child with West syndrome responded well. Similarly, seizure type did not predict efficacy. The response rate was roughly 50% whether patients had partial seizures only, generalized seizures only, or experienced both seizure types.
Two larger studies found generally similar results with LEV as adjunctive therapy. Grosso et al studied 110 children (6 months to 16 years old) with refractory epilepsy (CitationGrosso et al 2005). Patients were followed for 2–20 months and the median LEV dose was 38 mg/kg/day. The overall response rate (≥50% reduction in seizures from baseline) was 39%, and 9% of children were seizure free. Response rate in patients with partial seizures was somewhat higher than in those with generalized seizures (58% vs 37%). Response rates in patients with Lennox Gastaut or West syndrome was lower than in the Lagae et al study noted above (2/8 with Lennox Gastaut and 1/5 with West Syndrome responded). A later study performed by Lagae and others evaluated adjunctive LEV (12–62 mg/kg/day) in 67 children with a median age of 8 years (CitationLagae et al 2005). Most (76%) of these children were receiving 2 or more concomitant AEDs. Overall, there was a median percent reduction in seizures of 50% and 49% of patients were responders (>50% seizure reduction). Five percent were seizure free. Response rate was similar in patients with either generalized or partial seizures (14 of 32 patients with generalized seizures responded vs 16 of 31 patients with partial seizures). The response rate was 75% in patients with mixed seizure types (n = 4). As in the earlier Lagae et al study, the etiology of epilepsy did not predict efficacy.
Retrospective studies in partial and/or generalized seizures
A number of centers have retrospectively reviewed charts or databases to extract data on the use of LEV as add-on therapy in children with epilepsy. The largest of these evaluated efficacy in 209 patients (under the age of 18) for a minimum of 12 weeks (CitationOpp et al 2005). These patients had highly refractory seizures and had been treated with as many as 20 different AEDs before receiving LEV as add-on therapy. A response (>50% reduction in seizure frequency) was achieved in 25% of patients and 6% were rendered seizure free. There were no significant differences in outcome based on the type of epilepsy syndrome (generalized versus focal epilepsy) or its etiology (symptomatic, idiopathic, or cryptogenic). However an analysis of seizure type determined that secondarily generalized tonic-clonic and simple partial seizures were best controlled by LEV, followed by generalized tonic-clonic, complex partial, myoclonic, tonic, absence, and atonic seizures.
Three smaller retrospective studies reported better response rates than those reported by CitationOpp et al (2005). Herranz and colleagues studied 43 patients under the age of 18 (mean age = 5.2 ± 4.4 years) who had been treated with LEV as add-on therapy (to 1–3 AEDs) for over 6 months (CitationHerranz et al 2003). The predominant seizure type reported at baseline was partial onset (74% of seizures), and the mean LEV dose was 45 ± 33 mg/kg/day. Sixty-five percent of patients had seizure frequency reduced by >50%, and 14% of patients were seizure free. The authors noted that LEV was especially effective for partial seizures, but also exhibited efficacy in generalized seizures (tonic-clonic, absence, and myoclonic). In another study, Mandelbaum et al studied 59 patients aged 9 months to 23 years for up to 12 months (CitationMandelbaum et al 2005). Most patients received from 1 to 5 AEDs in addition to LEV, but 8 children received LEV as monotherapy. After 12 months, 53% of patients had seizure reductions of at least 50%, and 20% were seizure free. In this study, response rates were a bit higher in patients with predominantly generalized seizures or with mixed seizure types, than in those with focal seizures (55% [generalized] and 62% [mixed] versus 40% [focal]).
Another small retrospective study focused on younger children only (CitationTan and Appleton 2004). A computerized hospital pharmacy database (from a tertiary pediatric epilepsy center) was searched to obtain records on young children who were treated with LEV for epilepsy. Twenty-six children under the age of 11 years (median age = 7 years) were identified and included in the study. Patients received between 1 and 3 AEDs in addition to LEV, which was administered at a mean maintenance dose of 36.9 mg/kg/day. Sixty-one percent of children were responders (≥50% seizure reduction), and 2 (8%) were seizure free. Response was high across most partial and generalized seizure types, but was highest in patients with partial seizures, epileptic spasms, tonic, or atonic seizures. Response was lowest for myoclonic seizures.
Retrospective studies in rare epilepsy syndromes
Two studies were identified in the literature that retrospectively evaluated LEV in children with rare epilepsy syndromes. Aeby and colleagues reviewed charts on 12 children (4–14 years old) who had epilepsies with continuous spikes and waves during slow-wave sleep (CSWS) (CitationAeby et al 2005). All patients had neuropsychological problems, and most were moderately or severely mentally retarded. These patients received LEV (50 mg/kg/day) in addition to their usual AED therapy. After 2 months, significant to dramatic improvements on EEG were noted for 7 patients (58%). Nine patients (75%) showed improvements in behavior and/or cognitive function. However, of the eight patients who continued on LEV for 1 year, 4 relapsed between 9 and 11 months after LEV initiation.
Another study evaluated LEV in patients with tuberous sclerosis complex (CitationCollins et al 2006). Patients with this inherited disorder have pathologic features of brain dysgenesis such as cortical and subependymal nodules, and about 80% have epilepsy (CitationShorvon 2000). In this study, 20 children and adolescents (2–19 years old) with tuberous sclerosis received LEV as adjunctive therapy for up to 6 months. The average seizure frequency at baseline was 120 per month, and the most common seizure type was complex partial seizures. LEV was dosed in the range of 8 to 135 mg/kg/day (given bid or tid). A positive response (>50% reduction in seizures) was attained in 40% of patients, and 20% became seizure free (CitationCollins et al 2006).
Efficacy of LEV as add-on treatment in primary generalized epilepsy
Results of the placebo-controlled, double-blind study of adjunctive LEV in juvenile myoclonic and absence epilepsies that was the basis for regulatory approval have been reported in abstract form (CitationAndermann et al 2005). Although this study enrolled adults as well as adolescents (age range = 12–65 years), the results are relevant to the treatment of pediatric epilepsy given the high prevalence of these epilepsies and their typical onset age (early adolescence). In this study, patients who were poorly controlled on 1 AED at baseline were titrated to LEV 1500 mg bid or placebo over 4 weeks and then entered a 12-week stable dose period. Responder rate (percentage of patients with ≥50% reduction in days with myoclonic seizures versus baseline) was calculated over the entire 16-week treatment period. The responder rate was 58.3% in the LEV group (n = 60) vs 23.3% in the placebo group (n = 60) (p = 0.0002). The median difference between groups in percent reduction in seizure days per week for all seizure types was 38.08% (p < 0.0001) and the median difference in percent reduction in weekly seizure frequency for PGTC seizures was 30.35%. Only 33 patients experienced PGTC seizures (n = 15 for LEV, n = 18 for placebo), and the difference did not reach statistical significance. There was no difference between groups in median percent reduction of absence seizures (CitationAndermann et al 2005).
In another double-blind study reported in a recent conference abstract, children and adults (age range = 4–65 years) with idiopathic generalized epilepsy received add-on LEV or placebo for PGTC seizures (CitationRosenfeld et al 2006). LEV was titrated over 4 weeks to a target dose of 3000 mg/day in adults and 60 mg/kg/day in children. Patients remained on a stable dose for 20 weeks. Among the 163 patients who were evaluable for efficacy, 72.2% of the LEV group experienced at least a 50% reduction in PGTC seizures compared with 45.2% of the placebo group (p = 0.0005). Complete seizure freedom was reported by 24.1% of patients on LEV therapy and 8.3% of patients on placebo (p = 0.009). Regulatory approvals in PGTC seizures were based on this study.
Summary of LEV efficacy in pediatric epilepsy and comparison with other AEDs
provides a summary of LEV’s efficacy from the pediatric studies reviewed above that included responder rates. Based on these studies, the median responder rate was 49% with most studies reporting a rate in the range of 40%–60%. The median seizure-free rate was 8.4%. There was no clear difference in response based on seizure type. Among the 7 studies that evaluated differences based on seizure type, 3 found no difference, 1 found better efficacy in generalized seizures, and 4 found better efficacy for partial seizures. A recently published consensus document that was based on the opinions of 39 physicians considered as experts in the treatment of pediatric epilepsy rated LEV’s usefulness (among other AEDs) in the treatment of various epilepsy types or syndromes and in particular clinical situations. LEV was rated as “usually appropriate” (ie, often used or first-line choice) for cryptogenic complex partial seizures, and “sometimes appropriate” (ie, second-line choice) in Lennox-Gastaut syndrome, in juvenile myoclonic epilepsy, in myoclonic and generalized tonic-clonic seizures, and in newly diagnosed epilepsy in the emergency department (CitationWheless et al 2005).
Table 1 Efficacy of adjunctive LEV in pediatric studies of refractory epilepsy
It is not currently possible to make truly valid efficacy comparisons between LEV and other AEDs since no head-to-head studies in children have been completed. However, it may be instructive to review results across prospective, well-controlled, well-designed, appropriately-analyzed, double-blind trials (defined as class I evidence). A committee from the American Academy of Neurology (AAN) and the American Epilepsy Society (AES) has recently done just that. Based on their review, guidelines were published on the use of the newer AEDs in children with refractory partial epilepsy and 4 of the newer AEDs were recommended as adjunctive therapy based on data from class I trials (CitationFrench et al 2004). A single class I trial was available for each of these 4 AEDs. Class I evidence for LEV did not exist at the time of their review, but since that time it has become available and has been compared with the 4 newer AEDs recommended by AAN/AES () (CitationVerdru 2005). Review of the data in suggests that the efficacy of adjunctive LEV in children with refractory partial epilepsy may be comparable to that of three of the four recommended AEDs (topiramate [TPM], lamotrigine [LTG], and oxcarbazepine [OXC]) and is potentially better than the efficacy of gabapentin (GBP).
Table 2 Results of class I trials of newer AEDs for refractory pediatric partial epilepsyTable Footnotea
Pediatric trials comparing AEDs directly are needed to determine which AEDs may be “first-line” as add-on therapy or monotherapy for partial and generalized seizures in children. In an adult population, LEV has demonstrated similar efficacy to CBZ-CR in a double-blind monotherapy trial in partial and generalized tonic-clonic seizures (CitationBrodie et al 2007).
Safety and tolerability
Review of pediatric trials
LEV was generally well-tolerated in all of the pediatric studies reviewed above. lists the overall AE rates, most common AEs, and any positive psychotropic effects that were reported in these trials. The overall incidence of AEs ranged from 4% to 88%. Interestingly, the highest rate (88%) was reported in the only double-blind trial for which AE rates were available. However, the placebo rate for AEs was even higher in this trial (92%). Somnolence (or other sedation-related AEs) was the most frequently reported AE across trials. Behavior-related problems were among the most commonly reported AEs in 7 of 12 trials. All 7 of these trials were open-label. A post-hoc analysis of the risk of neuropsychiatric AEs was undertaken in the double-blind pediatric study and determined that the risk was modestly increased in the LEV group relative to the placebo group (RR = 1.39; 95% CI: 0.93–2.08). Most of the events were categorized as mood/anxiety/behavioral problems. The relative risk was not increased in pediatric patients with psychiatric histories or with cognitive impairment as compared to those without these comorbidities. Similarly, the relative risk for neuropsychiatric events in children was comparable to the relative risk in adults that was calculated in a separate, double-blind, placebo-controlled study (CitationShoaf et al 2005).
Table 3 Tolerability of LEV in pediatric trials
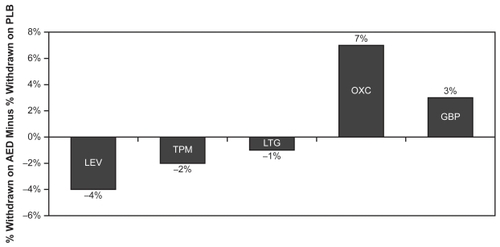
Because of the lack of head-to-head trials, direct tolerability comparisons between AEDs, like efficacy comparisons, are unavailable. However, the tolerability of LEV as reflected by the withdrawal rate (due to AEs) from the placebo-controlled, double-blind trial (class I evidence) in patients 4–16 years old has been compared with those of the four other newer AEDs that were recommended by the AAN/AES committee (CitationVerdru 2005). depicts AE-related withdrawal rates (minus the rate in the placebo group) for class I trials of LEV, TPM, LMT, OXC, and GBP. Four percent fewer patients withdrew from the LEV group than from the placebo group. For TPM and LMT, withdrawals for AEs were also higher in the placebo group (by 2% and 1% respectively). In the OXC and GBP groups, the withdrawal rate was higher for patients receiving active drug (7% higher for OXC patients and 3% higher for GBP patients).
Figure 3 AE-related withdrawal rates in class 1 pediatric trials of newer AEDs (active minus placebo rates).
Seven of the 12 pediatric trials identified for this review noted improvements in behavior or cognitive function during LEV treatment (). Improvements occurred in 9%–75% of patients, depending on the trial. In 2 of these trials, neuropsychiatric status was monitored prospectively and systematically. Lagae and colleagues (CitationLagae et al 2005) used a structured questionnaire completed by caregivers because many of the children in their study were severely retarded and could not be tested quantitatively. Approximately one quarter of the patients were reported to show improvements in alertness or behavior. Improved alertness was usually observed as improved communication (verbal or non-verbal), and behavior was improved allowing better handling and structuring of the children. Improvement in behavior and cognition did not always correlate with improved seizure control. Over half the children with improvements had a seizure reduction of less than 50%.
In the Aeby et al study, children with CSWS were evaluated using quantitative neuropsychological testing if they were capable of completing testing. Five of 7 patients tested showed improvements in at least 2 domains of cognitive function (eg, visual attention, visual or auditive memory, or graphic organization), and 6 of 7 demonstrated improved behavior during testing (CitationAeby et al 2005). In addition, all patients had behavior assessed by parents or teachers. In 7 of 12 patients, better behavior was reported. Of the remaining 5 patients, 3 were unchanged and 2 worsened. As in the Lagae et al study (CitationLagae et al 2005), improvements in cognitive function or behavior were not always associated with efficacy improvements. Two of the nine patients who showed improvement in neuropsychologic testing or behavior as assessed by parents or teachers, did not show any improvement in EEG evaluation.
AEDs and cognitive development in children
Children may be more vulnerable to neurotoxic effects of drugs because of their developing nervous systems. In children of normal intellect, it is critical to control seizures without affecting cognitive development or learning ability. In children who are already cognitively impaired, further impairment must be avoided in order to maintain maximum function. Two clinical studies were designed specifically to evaluate the cognitive effects of LEV, although neither of these studies included children. In a double-blind crossover study of 10 healthy volunteers (mean age = 29.2 years), placebo, LEV, carbamazepine (CBZ), or OXC were administered for 8 days with a 2-week washout period between treatments (CitationMecarelli et al 2004). Doses were titrated over 7 days to the final dose of 750 mg bid for LEV, 400 mg bid for CBZ, and 600 mg bid for OXC. At baseline and 12 hours after the last dose of drug on day 8, neuropsychological testing was performed. LEV did not have a negative impact on any on the tests employed, including measures of reaction time (motor task), selective attention (Stroop task), quantitative EEG, or color visually evoked potentials (VEP). For CBZ, reaction time (motor speed) was significantly slowed and delta and theta power (slowest frequency bands in the EEG) were increased, the frequency of the alpha rhythm was decreased, and P1 latency on VEP tests was slowed. These changes are indicative of CNS dysfunction and cognitive slowing. OXC also demonstrated some significant negative effects on EEG and VEP measures, but they were generally smaller and not as pervasive (ie, fewer measures were affected). A slower titration rate for CBZ may have produced fewer negative effects (CitationMecarelli et al 2004).
In another double-blind, crossover comparison in adult volunteers, CBZ and LEV were titrated more slowly (over 4 weeks) and were then continued at a maintenance dose for 4 weeks. The maintenance dose during the LEV arm was 2000 mg/day, while the CBZ dose was adjusted to achieve midrange therapeutic level. Thirty-three cognitive and behavioral variables were measured in 28 subjects after completion of the maintenance phase. Mean scores favored LEV over CBZ on 29 of 33 variables (88%), and the differences were statistically significant for 14 of 33 variables (42%). CBZ was not significantly better than LEV for any variable. Significance was achieved for LEV on various cognitive and mood measures, including the profile of mood states (POMS) and all 3 cognitive subscales (attention, language, and memory) of the QOLIE-89 (Quality of Life in Epilepsy, Long Form) (CitationMeador et al 2006).
A recently published open-label study evaluated LEV’s effects on cognitive function in patients with refractory partial epilepsy (CitationPiazzini et al 2006). A group of 35 adult patients who were receiving LEV in addition to a stable regimen of AEDs were compared with a control group (n = 35) on stable AED therapy. There was no difference between the groups as far as age, education or intelligence level, seizure frequency at baseline, duration of epilepsy, or number of AEDs at baseline (prior to addition of LEV). An extensive neuropsychological battery was administered to both groups of patients at baseline and 7 weeks later, after the LEV add-on group had been titrated to a mean dose of 1834.3 mg/day (range = 1000–3500 mg/day). Changes in cognitive performance from baseline were significantly superior for 4 of 11 tests for the LEV add-on group compared with controls. Significant improvements were noted in 3 tests of attention and a test of oral fluency. The control group was not superior to the LEV group on any cognitive measure. An analysis comparing LEV responders (n = 10) with LEV nonresponders (n = 25) detected no significant difference in any test results, suggesting that the positive cognitive effects of LEV in this study were not just a result of seizure reduction (CitationPiazzini et al 2006). A previous case study documented by some of the same researchers found improvements in verbal fluency and complete abolishment of stuttering after 12 weeks of LEV therapy in a patient with baseline deficits in verbal memory, oral comprehension, and verbal fluency (CitationPaola Canevini et al 2002).
Results of these clinical studies suggest that LEV does not negatively impact cognitive function in adults (on average) and has a better cognitive profile than CBZ. The Piazzini et al study suggests that LEV has the potential to impart positive cognitive effects in some domains of cognitive function. Preclinical studies in animal models are in agreement with these clinical findings. LEV demonstrated no effect on cognitive function in normal or amygdala-kindled rats in the Morris water maze test (CitationLamberty et al 2000), no effect on attention in rats as measured by the 5-choice serial reaction time task (CitationShannon and Love 2005), and no effect on working memory in rats as measured by spatial alternation performance (CitationShannon and Love 2004). LEV showed positive effects on cognitive function by antagonizing the amnestic effects of scopolamine in passive avoidance learning in mice (CitationVerloes et al 1988). Additionally, LEV has shown no evidence of neurotoxic effects at doses of 100 mg/kg in the developing rat brain (rat pups aged 0–7 days) (CitationManthey et al 2005). Formal, well-controlled cognitive studies are needed to assess LEV’s cognitive profile in children with epilepsy. A double-blind clinical trial in pediatric epilepsy is currently underway; however, no results are yet available.
AEDs and change in body weight
Several AEDs are known to either increase or decrease body weight, either of which may be undesirable in a developing child. Significant weight changes may lead to health problems, poor self-esteem, and eventually, noncompliance (CitationBiton 2003). Valproate, CBZ, and GBP have been associated with weight gain, while TPM and zonisamide are associated with weight loss (CitationNess-Abramof and Apovian 2005). Because body weight changes are common with some AED therapies, the effect of LEV therapy on weight was evaluated in a meta-analysis of controlled clinical trials (CitationGidal et al 2003). There was no significant difference in mean weight at baseline compared with mean weight at follow-up in 631 adult patients (after an average duration of 125 days of add-on LEV treatment). There was also no difference between LEV-treated patients and placebo-treated patients in the incidence of clinically significant weight changes (defined as >7% change from baseline). In the LEV group, 4.5% of patients had a weight increase and 4.5% had a decrease. For placebo, 5.9% of patients had an increase vs 3.5% with a decrease. Similarly, LEV did not appear to affect body weight in a prospective, open-label trial of LEV in children with partial epilepsy. In this study, 24 children aged 6–12 years were treated for up to 14 weeks (only 2 patients did not complete 14 weeks). Three children (12.5%) had clinically significant weight increases and 2 (8.3%) had clinically significant weight loss (CitationGlauser et al 2002). Based on these studies, LEV appears to be a weight-neutral AED.
AEDs and drug interactions
Children with refractory epilepsy often receive polytherapy with AEDs in order to adequately control seizures. Additionally, children with epilepsy syndromes that include other medical manifestations as a part of the syndrome are also likely to receive polytherapy in order to control all aspects of the disorder. For these reasons, it is important to be aware of any drug-drug interactions between AEDs and cotherapy. A number of studies have evaluated the potential of LEV to interact with other AEDs or with other drugs that commonly participate in interactions. Based on these studies, it was determined that LEV does not participate in clinically significant pharmacokinetic interactions with other commonly prescribed AEDs (CitationPerucca et al 2003), with low-dose oral contraceptives (CitationRagueneau-Majlessi et al 2002), with warfarin (CitationRagueneau-Majlessi et al 2001), with digoxin (CitationLevy et al 2001), or with probenecid (CitationPatsalos 2000). Since LEV is not metabolized in the liver and is not appreciably protein bound it is unlikely to interact pharmacokinetically with any drugs (CitationPatsalos 2000) and no significant interactions have been reported (CitationPerucca 2006). A pharmacodynamic interaction has been reported between LEV and CBZ and between LEV and TPM (CitationPatsalos 2003).
Other safety issues
Changes in laboratory parameters have been reported with AED treatment; therefore, these values are routinely tracked in clinical trials. Statistically significant (compared with placebo) but clinically irrelevant changes in mean values for red blood cell (RBC) count, hematocrit, and hemoglobin have been reported for adult patients treated in clinical trials (CitationBriggs and French 2004). Additionally, the incidence of possibly clinically significant decreases in white blood cell (WBC) count and in neutrophil count was slightly higher in the LEV group (WBCs: LEV = 3.2%, placebo = 1.8%; neutrophils: LEV = 2.4%, placebo = 1.4%). (2004) In the double-blind pediatric trial in partial seizures, 3.0% of LEV patients vs 0% of placebo patients reported decreases in WBC count, but there was no apparent difference in the incidence of low neutrophil counts. No patients were discontinued for low WBCs or neutrophils. Meaningful changes in liver function tests or other blood chemistries have not been detected in LEV patients compared with placebo patients in adult or pediatric trials (CitationBriggs and French 2004). Monitoring of laboratory values is not required.
Potential hypersensitivity reactions to LEV have been infrequently reported in clinical trials with an incidence similar to placebo (CitationFrench et al 2001). Similarly, LEV use has not been associated with any idiosyncratic AEs in the currently published literature (CitationBriggs and French 2004).
Summary and conclusions
LEV is a novel AED that binds to SV2A proteins and acts through a mechanism that is distinct from any other currently available AED. Its pharmacokinetic profile allows easy titration with little risk for drug interactions. As adjunctive therapy, LEV appears to be safe and effective for the treatment of pediatric epilepsy. Its efficacy is best demonstrated in the treatment of partial seizures, but several studies show that LEV is efficacious for a broad range of seizure types, including various subtypes of primary generalized seizures, and LEV is now approved for myoclonic and PGTC seizures. It has been well tolerated in pediatric studies with an AE profile similar to that demonstrated in adults. Somnolence is the most commonly reported AE across all pediatric studies, and behavioral events were among the most common types of AEs in open-label studies. Improvements in behavior and cognition were also frequently reported. The relative risk of neuropsychiatric AEs appears similar in children compared with adults. The cognitive profile of LEV as demonstrated in adults and in animal models is encouraging, but pediatric studies are needed.
In summary, LEV provides another much-needed option in the treatment of pediatric epilepsy. Further well-controlled studies are needed to fully define its potential in generalized seizures and in children younger than 4 years old.
References
- AebyAPoznanskiNVerheulpenD2005Levetiracetam efficacy in epileptic syndromes with continuous spikes and waves during slow sleep: experience in 12 casesEpilepsia4619374216393159
- AndermannEAndermannFMeyvischP2005Seizure control with levetiracetam in juvenile myoclonic epilepsiesEpilepsia46Suppl 8205
- BioSpace Beat2007Keppra approved for US epilepsy patients with one of the most debilitating seizure types [online]Accessed 25 April 2007 URL: http://www.biospace.com/news_story.aspx?StoryID=49789&full=1
- BitonV2003Effect of antiepileptic drugs on bodyweight: overview and clinical implications for the treatment of epilepsyCNS Drugs177819112921491
- BriggsDEFrenchJA2004Levetiracetam safety profiles and tolerability in epilepsy patientsExpert Opin Drug Saf34152415335297
- BrodieMJPeruccaERyvlinP2007Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsyNeurology68402817283312
- CollinsJJTudorCLeonardJM2006Levetiracetam as adjunctive antiepileptic therapy for patients with tuberous sclerosis complex: a retrospective open-label trialJ Child Neurol2153716551454
- CoppolaGManganoSTortorellaG2004Levetiracetam during 1-year follow-up in children, adolescents, and young adults with refractory epilepsyEpilepsy Res59354215135165
- CoupezRStraetemansRSehgalG2003Levetiracetam: relative bioavailability and bioequivalence of a 10% oral solution (750 mg) and 750-mg tabletsJ Clin Pharmacol431370614615473
- FrenchJEdrichPCramerJA2001A systematic review of the safety profile of levetiracetam: a new antiepileptic drugEpilepsy Res47779011673023
- FrenchJAKannerAMBautistaJ2004Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy SocietyNeurology6212617315111660
- GidalBEShethRDMagnusL2003Levetiracetam does not alter body weight: analysis of randomized, controlled clinical trialsEpilepsy Res56121614642996
- GlauserTAAyalaREltermanRD2006Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizuresNeurology6616546016641323
- GlauserTAPellockJMBebinEM2002Efficacy and safety of levetiracetam in children with partial seizures: an open-label trialEpilepsia435182412027913
- GrossoSFranzoniECoppolaG2005Efficacy and safety of levetiracetam: an add-on trial in children with refractory epilepsySeizure142485315911359
- HerranzJLRufo-CamposMArteagaR2003[Effectiveness and tolerability of levetiracetam in 43 children and adolescents with epilepsy]. SpanishRev Neurol371005814669138
- Keppra [package insert]Smyrna, GaUCB Pharma, Inc2004
- KlitgaardH2001Levetiracetam: the preclinical profile of a new class of antiepileptic drugs?Epilepsia42Suppl 413811564119
- KraussGLBettsTAbou-KhalilB2003Levetiracetam treatment of idiopathic generalised epilepsySeizure126172014630506
- KumarSPSmithPE2004Levetiracetam as add-on therapy in generalised epilepsiesSeizure13475715324824
- LagaeLBuyseGCeulemansB2005Clinical experience with levetiracetam in childhood epilepsy: an add-on and mono-therapy trialSeizure14667115642504
- LagaeLBuyseGDeconinckA2003Effect of levetiracetam in refractory childhood epilepsy syndromesEur J Paediatr Neurol7123812788038
- LambertyYMargineanuDGKlitgaardH2000Absence of negative impact of levetiracetam on cognitive function and memory in normal and amygdala-kindled ratsEpilepsy Behav13334212609164
- LeppikIE2001Issues in the treatment of epilepsyEpilepsia42Suppl 41611564117
- LevyRHRagueneau-MajlessiIBaltesE2001Repeated administration of the novel antiepileptic agent levetiracetam does not alter digoxin pharmacokinetics and pharmacodynamics in healthy volunteersEpilepsy Res4693911463510
- LukyanetzEAShkrylVMKostyukPG2002Selective blockade of N-type calcium channels by levetiracetamEpilepsia4391811879381
- LynchBALambengNNockaK2004The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetamProc Natl Acad Sci USA1019861615210974
- MandelbaumDEBunchMKuglerSL2005Efficacy of levetiracetam at 12 months in children classified by seizure type, cognitive status, and previous anticonvulsant drug useJ Child Neurol20590416159526
- MantheyDAsimiadouSStefovskaV2005Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brainExp Neurol19349750315869952
- MeadorKJLoringDWRayPG2006Cognitive and behavioral effects of carbamazepine and levetiracetam in healthy volunteersNeurology66Suppl 2A.73
- MecarelliOVicenziniEPulitanoP2004Clinical, cognitive, and neurophysiologic correlates of short-term treatment with carbamazepine, oxcarbazepine, and levetiracetam in healthy volunteersAnn Pharmacother3818162215367726
- Medical News Today2005FDA approves UCB’s Keppra® for use in childhood epilepsy [online]Accessed 30 March 2006 URL: http://www.medicalnewstoday.com/medicalnews.php?newsid=26559
- Medical News Today2006UCB receives EMEA positive opinion and FDA approvable letter for Keppra® (levetiracetam) intravenous administration [online]Accessed 30 March 2006 URL: http://www.medicalnewstoday.com/printerfriendlynews.php?newsid=37150
- Ness-AbramofRApovianCM2005Drug-induced weight gainDrugs Today (Barc)415475516234878
- OppJTuxhornIMayT2005Levetiracetam in children with refractory epilepsy: a multicenter open label study in GermanySeizure144768416182573
- OttDSiddarthPGurbaniS2003Behavioral disorders in pediatric epilepsy: unmet psychiatric needEpilepsia44591712681010
- Paola CaneviniMChifariRPiazziniA2002Improvement of a patient with stuttering on levetiracetamNeurology59128812391373
- PatsalosPN2000Pharmacokinetic profile of levetiracetam: toward ideal characteristicsPharmacol Ther85778510722121
- PatsalosPN2003The pharmacokinetic characteristics of levetiracetamMethods Find Exp Clin Pharmacol25123912731458
- PellockJM1999Managing pediatric epilepsy syndromes with new anti-epileptic drugsPediatrics10411061610545555
- PellockJMGlauserTABebinEM2001Pharmacokinetic study of levetiracetam in childrenEpilepsia421574911879369
- PeruccaE2006Clinically relevant drug interactions with antiepileptic drugsBr J Clin Pharmacol612465516487217
- PeruccaEGidalBEBaltesE2003Effects of antiepileptic comedication on levetiracetam pharmacokinetics: a pooled analysis of data from randomized adjunctive therapy trialsEpilepsy Res53475612576167
- PiazziniAChifariRCaneviniMP2006Levetiracetam: an improvement of attention and of oral fluency in patients with partial epilepsyEpilepsy Res68181816332430
- Ragueneau-MajlessiILevyRHJanikF2002Levetiracetam does not alter the pharmacokinetics of an oral contraceptive in healthy womenEpilepsia4369770212102671
- Ragueneau-MajlessiILevyRHMeyerhoffC2001Lack of effect of repeated administration of levetiracetam on the pharmacodynamic and pharmacokinetic profiles of warfarinEpilepsy Res47556311673021
- RamaelSDe SmedtFToublancN2006Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjectsClin Ther287344416861095
- RigoJMHansGNguyenL2002The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currentsBr J Pharmacol1366597212086975
- RosenfeldWBerkovicSKnowltonR2006Efficacy and safety of levetiracetam as adjunctive treatment in adult and paediatric patients suffering from idiopathic generalized epilepsy with primary generalized tonic-clonic seizuresNeurology66Suppl 2A40
- ShannonHELovePL2004Effects of antiepileptic drugs on working memory as assessed by spatial alternation performance in ratsEpilepsy Behav58576515582833
- ShannonHELovePL2005Effects of antiepileptic drugs on attention as assessed by a five-choice serial reaction time task in ratsEpilepsy Behav7620816253568
- ShinnarSPellockJM2002Update on the epidemiology and prognosis of pediatric epilepsyJ Child Neurol17Suppl 1S41711918462
- ShoafTLLuZYeeKF2005Evaluation of the relative risk of psychiatric and behavioral adverse events in pediatric patients with refractory partial seizures treated with levetiracetam—impact of prior history and a comparison with adult dataEpilepsia46Suppl 82045
- ShorvonS2000General principles of treatment in epilepsyHandbook of epilepsy treatmentOxford, EnglandBlackwell Science Ltd3483
- TanMJAppletonRE2004Efficacy and tolerability of levetiracetam in children aged 10 years and younger: a clinical experienceSeizure13142515010050
- TongXPatsalosPN2001A microdialysis study of the novel antiepileptic drug levetiracetam: extracellular pharmacokinetics and effect on taurine in rat brainBr J Pharmacol1338677411454660
- VerdruP2005Epilepsy in children: the evidence for new antiepileptic drugsActa Neurol Scand Suppl181172016238703
- VerloesRScottoAGobertJ1988Effects of nootropic drugs in a scopalamine-induced amnesia model in micePsychopharmacology95226303137602
- WhelessJWClarkeDFCarpenterD2005Treatment of pediatric epilepsy: Expert opinion, 2005J Child Neurol20Suppl 1S15616615562
- WhelessJWNgYT2002Levetiracetam in refractory pediatric epilepsyJ Child Neurol17413512174960