Abstract
Insomnia is the leading sleep disorder in the US; however, diagnosis is often problematic. This pilot study assessed the clinical value of a novel diagnostic insomnia questionnaire. The SleepMed Insomnia Index (SMI) was administered to 543 consecutive patients and 50 normal control subjects during a pilot study. Mean SMI scores were assessed based on subsequent sleep-related diagnoses. The SMI scores for patients with sleep-related disorders were significantly higher than those for the control group (p < 0.001) and highest for the 90 patients comprising the insomnia group. Analysis of the SMI scores from the 90 insomnia patients indicates a high degree of reliability (Cronbach’s alpha: 0.7). These data support our clinical experience with this diagnostic tool which indicates a strong likelihood of disrupted nighttime sleep in patients with high SMI scores. Following further validation, the SMI may prove to be a valuable tool for evaluating sleep disorders, specifically as an aid in the diagnosis of insomnia. The Sleep Matrix is a visual tool that quantifies a sleep complaint by combining scores from the Epworth Sleepiness Scale (ESS) and the SMI. The SMI measures an insomnia component while the ESS is an accepted measure of daytime sleepiness. The Sleep Matrix visually displays the complexity of the sleep complaint in an effort to differentiate insomnia with differing etiologies from other sleep disorders and measure treatment outcomes. To pilot test the Sleep Matrix, the tool was administered to 90 patients with insomnia and to 22 normal controls. Plots from the insomnia patients were concentrated into the “insomnia zone” while scores from the normal controls were located in the “normal zone” located in the lower left quadrant. Additional research using the Sleep Matrix could provide data that the tool could be utilized to visually aid the clinician in the diagnosis of unknown sleep complaints.
Keywords:
Introduction
Estimated to affect as many as 25 million adults, chronic insomnia is the most prevalent sleep disorder in America today (CitationWalsh 2004) and is associated with significant health-related consequences. The National Institutes of Health (NIH) reports that 50% of patients seen in clinical practice today describe symptoms of insomnia; 30% of the general population report disrupted nocturnal sleep and 10% of the population has an insomnia diagnosis with impaired daytime functioning (CitationNIH 2005). In a survey of 1000 people with insomnia symptoms (CitationAncoli-Israel and Roth 1999), 72% of people responded “very often” or “sometimes” to the question: how often do you wake up feeling drowsy or tired?
The problem worsens with advancing age. Insomnia is estimated to increase by as much as 20%–50% in the elderly (>65 years) (CitationNIH 2005), especially among women and is often attributed to the onset of menopause. Compared to men, the prevalence of insomnia is 1.4 times greater in women under age 45, increasing to 1.7 times greater after age 45 (CitationOhayon 2002). The reasons for the increased frequency of insomnia in this population are many and include an increased incidence of medical and psychiatric disorders with advanced age and greater use of medications which can adversely affect sleep (CitationRichardson and Zee 2006).
Compared to people with normal sleep, patients with insomnia are more likely to experience anxiety and depression and suffer other serious consequences, such as work- and driving-related accidents (CitationMitler et al 2000) and an overall increase in mortality rate (CitationKripke et al 2002). The direct healthcare costs of insomnia in the US have been estimated to be $14 billion annually (CitationWalsh 2004). Considering the personal and societal consequences of insomnia, the need for early diagnosis and intervention is obvious, although not easily achieved.
Acute insomnia is transient short term or adjustment insomnia. The duration of acute insomnia is one night to a few weeks and may be related to environmental or occupational stress or illness. The acute insomnia typically resolves with adaptation or removal of the stressor. Acute insomnia can lead to chronic insomnia if left untreated.
Chronic insomnia is generally defined as difficulty initiating or maintaining nocturnal sleep or difficulty achieving restorative sleep for longer than one month, associated with significant distress and without obvious relationship with other disorders or substance abuse (CitationAPA 1994). Chronic insomnia can either be primary or co-morbid and waxes and wanes over time. Primary insomnia is the term applied when no co-existing disorder can be identified (CitationNIH 2005). In addition, primary insomnia often results in other health-related sequelae and patients may present to their primary care physician with major complaints other than insomnia, such as daytime sleepiness, fatigue, mood changes (CitationMoul et al 2002), or other symptoms which have a negative impact on daytime functioning.
Co-morbid insomnia may be obscured by an underlying condition, such as chronic pain, sleep disorders, medications, substance abuse, or a host of other medical and psychiatric conditions (CitationAmerican Academy of Sleep Medicine 2005). Consequently, a diagnosis of insomnia may not be obvious and the National Center on Sleep Disorders Research at the National Institutes of Health recently recommended that secondary insomnia be termed ‘co-morbid insomnia’ rather than a symptom of other disorders, emphasizing that large numbers of individuals with chronic illness suffer from fragmented sleep (CitationNIH 2005). This group has also identified the need for additional research to more clearly define the nature of chronic insomnia and to better characterize its expression among various patient populations (CitationNIH 2005).
Since insomnia can occur as a result of varied causes, it is essential to evaluate the other differential diagnoses to allow successful treatment planning (CitationChesson 2000). There is a high co-morbidity between insomnia and psychiatric disorders. Approximately 50% of patients with insomnia have a psychiatric diagnosis (CitationBonnet 1983). It is important to differentiate insomnia from these other psychiatric or mood disorders. Various sleep disorders can produce an insomnia complaint as well including periodic limb movement disorder, restless legs syndrome, sleep apnea, parasomnias, and/or sleep-related eating disorders. Many medical disorders can produce insomnia including asthma, COPD, bronchitis, GERD, dementia, Parkinson’s disease, rheumatoid arthritis, fibromylagia, and hyperthyroidism. Often patients may have poor sleep habits/hygiene which leads to sleep fragmentation. Symptoms or a history of substance or alcohol abuse can have long lasting effects on sleep quality requiring clinicians to obtain a history of current and past usage. A complete history of prescription medication use and over-the-counter medications will assess if any medication(s) could be a contributing factor in the insomnia complaint.
Circadian rhythm sleep disorders should be considered when evaluating patients with insomnia. Homeostatic and circadian processes regulate sleep wake cycles (Kilduff 1993). The homeostatic drives for sleep are driven by the need for sleep and increase with the duration of waking (CitationMonk 2003). Circadian rhythms synchronize sleep and waking to the dynamic 24-hour light-dark cycle (Kilduff 1993). Behavior affecting this rhythm or physiological abnormalities in the regulation of this pattern can lead to sleep disorders producing an insomnia complaint.
The circadian rhythm sleep disorders are jet lag syndrome, shift work sleep disorder, delayed sleep phase syndrome, advanced sleep phase syndrome, irregular sleep-wake pattern, and non-24-hour sleep-wake syndrome. Time zone change syndrome known as jet lag and shift work sleep disorder are extrinsic sleep disorders which occur as a result of a behaviorally initiated voluntary mismatch between the desired timing of the sleep-wake cycle and circadian phase (CitationWyatt 2004). While the four remaining circadian disorders can be referred to as intrinsic sleep disorders which presumably result from abnormal functioning of the circadian system itself or interaction with sleep homeostasis (CitationWyatt 2004). In considering the differential diagnosis of delayed sleep phase syndrome, patients will report an inability to sleep until several hours or awaken before several hours later than they would prefer. Therefore, when attempts are made to go to sleep earlier than biologically mandated later hour, sleep-onset insomnia is present (CitationWyatt 2004).
Given that 70% of patients with sleep problems have never discussed their symptoms with their physician (CitationAncoli-Israel and Roth 1999), it can be challenging for physicians to identify patients with insomnia. Insomnia problems are complex and multifactorial illustrating the importance of a sophisticated and thorough assessment (CitationEdinger 2005). Tools that can help identify and quantify an insomnia complaint will enable physicians to provide a higher level of care for patients. The early identification and intervention in patients with sleep disturbances may prevent the development of long-term insomnia (CitationSateia et al 2000).
Although nocturnal polysomnography is useful for diagnosing underlying sleep pathologies, such as sleep-disordered breathing, it is not indicated for the routine evaluation of insomnia (CitationLittner et al 2003). Therefore, reaching a diagnosis of insomnia must be based on clinical evaluation including patient interviews. A detailed, skilled history is the foundation of an adequate evaluation of an insomnia complaint (CitationSateia 2000). The clinician needs a working knowledge of the signs and symptoms of the spectrum of sleep disorders since these may contribute to the insomnia complaint (CitationChesson 2000). Complete medical and psychiatric histories are important to obtain to assess for any other co-morbidities impacting the insomnia compliant. A sleep log or sleep diary kept by the patient and recording their sleep/wake habits for 1–2 weeks is another tool that will aid the clinician. The sleep log can incorporate other variables such as caffeine, alcohol, nicotine, drugs, and circadian influences. Bed partner interviews regarding sleep patterns are useful.
To assist with the diagnosis of sleep disorders, including insomnia, several patient questionnaires have been developed and validated. The Pittsburg Sleep Quality Index (PSQI) is a self-rating scale intended to measure general sleep disturbances consisting of 19 items and is often used in clinical research trials (Buysse 1988). The Insomnia Severity Index is a 7-item scale used to measure perceived sleep difficulties as an outcome measure for insomnia research (CitationBastien 2001). The Athens Insomnia Scale is an 8-item scale that evaluates the severity of insomnia as well as its impact on daytime well being and functional capacity (CitationSoldatos 2000). However, there are many disadvantages to their use, such as being long and time-consuming, difficult for patients to use or having complex scoring systems (CitationBuysse et al 1989; CitationSoldatos et al 2000; CitationViolani et al 2004). In contrast, the Epworth Sleepiness Scale (ESS) questionnaire is simple to use and easy to score (CitationJohns 1991). It is used commonly to detect abnormal sleepiness and the need for further evaluation by a sleep specialist. Our goal in developing the SMI is to have a clinical tool which is simple for patient use, easy to score, and quickly provides the clinician insight into the important characteristics of the sleep complaint. In addition, the factor and global scores can be used in a database as a measure of patient outcomes.
Even in the presence of a decrease in total sleep time, patients with insomnia are not typically hypersomnolent. The current pathophysiology of insomnia is considered to be a hyperaroused state. Therefore, patients usually have normal ESS scores. The multiple sleep latency test (MSLT) is a sleep test that objectively measures excessive sleepiness or sleep need. It is series of 4–5 naps during the day in a sleep laboratory measuring latency to sleep onset by EEG. Patients who are hypersomnolent usually have shortened sleep latencies such as in narcolepsy. The MSLT is not used in clinical practice to evaluate an insomnia complaint. In previous research studies of insomnia patients when compared with normal controls, MSLT results did not show impaired alertness in the insomnia group despite having significantly less nocturnal sleep (Stepanski 1988). In the clinical environment patients present with multiple sleep complaints that include primary insomnia, co-morbid insomnia, circadian rhythm misalignment, and other sleep disorders. Since the sleep complaint is often complex and associated with co-morbidities, a sleep vital sign using the SMI and ESS as a two dimensional matrix, the Sleep Matrix, offers a quantitative and differentiating measure of the sleep complaint. It is suggested that patients with abnormal insomnia scores with elevated ESS scores should be evaluated for other medical, psychiatric, or sleep disorder diagnoses as well as behavioral factors and pharmacologic influences.
The present report describes the development of a new questionnaire, the SleepMed Insomnia Index (SMI), designed to quickly measure insomnia symptoms in a simple, standardized way. Similar to the ESS, the SMI is intended to be brief, enabling patients to quickly complete it. The SMI questionnaire provides a clinical measure of the characteristics and severity of the insomnia complaint. An additional refinement to the SMI is the simultaneous use of the ESS. Plotting the results of these two measuresl the Sleep Matrix, may enable clinicians to quickly characterize excessive sleepiness and sleep, pathology complaints. These data have previously been published in abstract form (CitationBogan and Turner 2005; CitationTurner and Bogan 2005; CitationTurner and Bogan 2005.
Materials and methods
Questionnaire development
The SMI incorporates features extrapolated from the International Classification of Sleep Disorder (ICSD) criteria for the diagnosis of insomnia (CitationAmerican Academy of Sleep Medicine 2005) and other clinical features of primary and co-morbid insomnia. The questions were formulated to address important sleep factors, including sleep latency, performance anxiety, first night effect, the frequency of awakenings, sleep re-initiation, total sleep time, perceived sleep quality and impact on next day function (). The SMI was designed to be a simple tool that can be quickly completed by patients and easily scored by clinic staff during the time period prior to the office visit.
Table 1 SleepMed Insomnia Index questionnaire
In Question 1, patients provide a global assessment of their sleep quality. Problems with sleep initiation and performance anxiety are revealed in Questions 2 and 3, respectively. The purpose of Question 4 is to determine arousal threshold and vigilance by assessing the impact of environmental factors on sleep. Question 5 provides insight into possible first night effects, accommodation abnormalities, or inadequate sleep hygiene. Sleep continuity is assessed in Questions 6 and 7, and Question 9 determines whether patients perceive their sleep as adequate. Finally, next day consequences of sleep problems are examined in greater detail in Questions 8 and 10. A weighting factor (0–4) is employed to discriminate patients with no or minimal insomnia symptoms resulting in potential total scores ranging from the 10 questions range from 0 (indicating no sleep-related problems) to 40 (suggesting significant insomnia-related complaints).
Sleep Matrix development
The Sleep Matrix is a diagnostic tool designed to visually aid in the diagnosis of unknown sleep complaints. The SMI is primarily designed to characterize the nature and restorative quality of nocturnal sleep while the ESS measures the degree of daytime sleepiness. ESS scores <10 are considered normal while scores >12 indicate severe or pathological sleepiness. Ironically, most patients with insomnia complaints display normal ESS scores despite their perceived decrease in total sleep time or quality. Pathological sleepiness is more likely to be the result of sleep disorders such narcolepsy, sleep apnea, restless legs syndrome, circadian rhythm disorders, and/or sleep deprivation. As described above, co-morbid insomnia may be the result of other disease processes and psychiatric disorders.
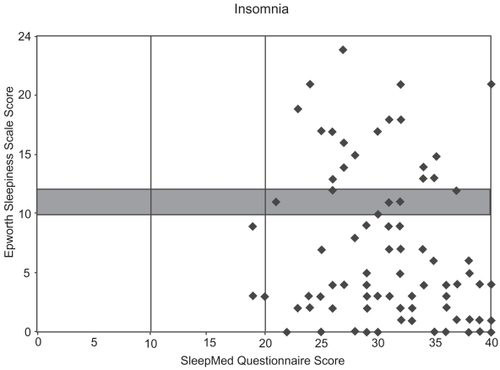
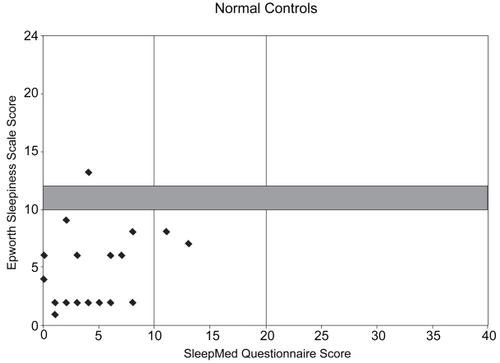
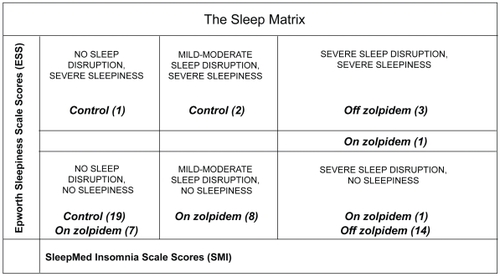
Therefore, the Sleep Matrix was developed to visually display the complexity of the sleep complaint in an effort to efficiently differentiate insomnia with differing etiologies from other sleep disorders and measure treatment outcomes. The Sleep Matrix plots the SMI scores on the x-axis against ESS scores on the y-axis. Six zones within the matrix help characterize the sleep complaint. Patients with ESS scores <10 fall into three categories in the lower half of the matrix: normal patients (SMI scores 0–10), non-sleepy patients with nonrestorative sleep (SMI scores 11–20), and insomnia patients (SMI scores >20). Patients with ESS scores >12 fall into three categories in the upper half of the matrix: sleepy patients (SMI scores of 0–10), sleepy patients with nonrestorative sleep (SMI scores of 11–20), and sleepy patients with sleep disruption/insomnia (SMI scores >20). Patients with SMI/ESS intercepts in the upper half of the matrix will need further clinical correlation for the presence of an underlying sleep disorder. As ESS scores ranging from 10–12 indicate only moderate sleepiness, patients falling into this “grey zone” require further clinical assessment.
Assessment of SleepMed Insomnia Index (SMI)
The SMI was administered to 543 consecutive adult patients who presented to our clinic for evaluation of sleep complaints as a 2-month pilot project. Each patient completed the self-administered questionnaire prior to the initial examination by a Board Certified Sleep Physician. Patients were instructed to complete the questionnaire by considering how they feel about their sleep quality generally and were encouraged to avoid focusing on a recent night of poor quality sleep. No patient was excluded from the pilot study.
Each patient underwent a complete history, physical examination and a nocturnal polysomnogram if deemed necessary by the physician. The final diagnosis was made using established ICSD criteria (CitationAmerican Academy of Sleep Medicine 2005). The physician was blinded to the results of the SMI score until the examination was completed and a patient diagnosis had been recorded by the physician on the SMI score sheet. The scores sheets were collected for later correlation of SMI scores with sleep disorder diagnoses.
For the purpose of this initial SMI assessment, evaluated patients consisted of patients with both undiagnosed sleep disorders and previously-diagnosed insomnia for which some patients were currently receiving treatment with zolpidem. The SMI was also administered to a group of 50 control subjects with no history of any sleeping problems, use of medications known to affect sleep, or variable shift work.
Statistical analysis
Means and standard deviations are reported for the groups. Analysis of variance (ANOVA) was used to measure between-group differences. Bartlett’s test for data homogeneity of variances was calculated, and SMI question reliability was tested using Cronbach’s alpha with significance was accepted at ≤0.7. Independent t-tests assessed significant differences between data sets: significance was accepted at p < 0.001.
Results
Insomnia questionnaire
The SMI scores were divided into 10 diagnostic categories to facilitate analysis and comparison. The largest subgroup in the sample was patients with obstructive sleep apnea, and this group was sub-divided into those on continuous positive airway pressure therapy (CPAP) and those not on CPAP. Patients reported a sleep complaint secondary to co-morbid processes including fibromyalgia, pain and depression/mood disorders. Due to small patient numbers, less common sleep disorders such as REM behavior disorder, nocturnal eating syndrome, and shift work sleep disorder were placed into a single category.
The demographic characteristics of the study participants are provided in . The sample was fairly homogenous in composition by age. The mean age of insomnia patinets was 51 (range 21–86 years). The mean age of other groups was similar except for patients with narcolepsy (40 years), idiopathic hypersomnia (46 years), and other sleep disorders (47 years). The mean age of the 50 control subjects controls was 38. The composition of the sample by sex follows patterns previously reported in the published literature for the various diagnoses. There was a higher percentage of women (64%) than men (36%) among patients with insomnia.
Table 2 Sample characteristics
For the primary assessment of validity, the degree to which the SMI detected clinically distinct differences between the various patient groups and normal controls was assessed. Thus, the diagnoses based upon the structured clinical interviews using ICSD criteria provide the “gold standard.”
Means and standard deviations for the SMI scores for patients with various sleep-related disorders are summarized in . Overall, SMI scores were significantly higher than the scores in the control group scores (p < 0.001). Notably, the mean SMI score for patients diagnosed with insomnia group was 31 and was significantly higher than mean scores in other groups (generally <20; p < 0.001) with the exception of the pain group which contained only small numbers. Reliability of the tool was assessed for the 90 insomnia patients with Cronbach’s alpha = 0.7. Mean scores were higher for the pain (N = 4), fibromyalgia (N = 12), and depression/mood disorder groups (N = 5), supporting an association between these illnesses and insomnia; however, the patient numbers in these groups were small and these results may not be representative of the entire patient population. Bartlett’s Test for homogeneity of variances was significant (Chi-square = 78, DF = 10, p < 0.001).
Sleep Matrix
SMI and ESS scores from the 90 patients with insomnia (some of the plots represent treated insomnia patients) were plotted on a Sleep Matrix with 22 normal controls, revealing distinctly different patterns. Plots of the insomnia patients were concentrated in the “insomnia zone” in the lower-right corner and a few revealed sleepiness with disrupted sleep (upper-right corner); however, none were in the “normal zone” (). In contrast, most all of the plots for the normal controls were located in the lower-left “normal zone” ().
Assessment of the SMI and Sleep Matrix in treated insomnia patients
To assess the potential utility of the SMI tool as an outcome measure, it was administered to 17 consecutive adult patients who were undergoing treatment for their insomnia during the same 2-month period. Each patient was previously diagnosed by a Board Certified Sleep Physician with primary (idiopathic) insomnia using ICSD criteria (CitationAmerican Academy of Sleep Medicine 2005) for which they were being treated with zolpidem. For the purpose of this preliminary SMI assessment, each patient completed the SMI questionnaire while receiving nightly zolpidem. Immediately after completing the questionnaire, patients were asked complete the SMI again while estimating the quality of their nighttime sleep and daytime functioning prior to receiving treatment for their insomnia. To permit repeat testing, a control group of 22 different subjects with no history of any sleeping problems, use of medications known to affect sleep, or variable shift work was also administered the SMI. The demographic characteristics of these patients and subjects are summarized in .
While taking zolpidem, the mean SMI and ESS scores for the 17 patients with insomnia were 11.5 and 4.9, respectively, and were similar to the 22 controls with SMI and ESS scores of 4.9 and 4.5, respectively. When the insomnia patients completed the SMI again while recalling their pre-treatment condition, the mean score was 34. The pre- and post-treatment SMI scores for the 17 insomnia patients were then plotted against their ESS scores. The pre-treatment intercepts were concentrated in the lower-right corner, indicating a high degree of sleep disruption/insomnia but little daytime sleepiness (). A few intercepts in the upper right corner suggest daytime sleepiness may have previously been worse for some patients. Treatment with zolpidem resulted in a left-ward shift in the intercepts, which now compare favorably with the 22 control subjects.
Figure 3 Example of utility of Sleep Matrix in a group of 17 treated insomnia patients. Sleep Matrix plots for 17 insomnia patients. When the SMI scores estimating the quality of nighttime sleep prior to drug treatment were plotted against ESS scores, most intercepts are located in the lower-right corner, indicating a high degree of sleep disruption but little daytime sleepiness. During drug therapy for insomnia, the SMI scores were plotted against ESS scores. The SMI/ESS intercepts shifted to the left of the matrix, indicating decreased sleep disruption and greater similarity with the plots for the 22 control patients.
Discussion
While significant advances have been made in the treatment of insomnia, the identification of patients with insomnia often remains problematic. Many insomnia patients present with an assortment of other medical complaints and physicians may not always associate their presenting symptoms with insomnia. The SMI was designed to quickly evaluate patients for insomnia and other potential sleep disturbances. Based on the patient SMI questionnaire scores, clinicians can structure patient interviews to more quickly focus on any potential sleep problems identified by the questionnaire.
All of the patients included in this report presented with an existing or subsequently diagnosed sleep disorder and demonstrated mean SMI scores which were considerably higher than the 50 control subjects. The SMI scores for the 90 insomnia patients were the highest observed with a mean of 31 while the mean scores in most of the other groups were below 20. Consistent with our clinical experience with the questionnaire, we believe the SMI identifies a high likelihood of significant sleep disruption in patients with SMI scores greater than 20. Pain, fibromyalgia and depression are also disorders characterized by disrupted sleep and patients with these diagnoses were also found to have higher mean SMI scores (range 21–31); however, since there were only 5 patients with depression and 4 with pain, we suspect these SMI scores may not be representative of the these patient groups.
The data obtained from the insomnia patients and normal control subjects also indicates the Sleep Matrix can discriminate between primary insomnia patients and normal controls with a reasonable degree of certainty. The ESS has previously been used to assess patients with insomnia (CitationJohns 1997); however, patients with idiopathic insomnia often suffer from states of hypervigilance, displaying normal ESS scores (Reidel and Lichstein 2000), as demonstrated by many of the patients in our study. For these patients, plotting the ESS and SMI scores on the Sleep Matrix may enable clinicians to quickly identify the problem and consider therapeutic options for managing their condition.
Excessive sleepiness is generally not a feature of primary insomnia and when present, frequently suggests the presence of other sleep disorders (CitationSateia et al 2000). Patients with ESS scores of greater than 10 need clinical correlation as to the presence of other underlying sleep disorders. The SMI and the Sleep Matrix may help physicians determine when another underlying sleep disorder may be present, signaling the need for a formal sleep study. Specifically, patients with ESS/SMI scores falling in the upper half of the Sleep Matrix may require referral to a sleep specialist for further evaluation, especially if initial treatment does not result in improvement.
The use of the Sleep Matrix indicates significant differences between patients treated for insomnia and estimates of their prior untreated condition. Thus, the Sleep Matrix may also be a useful indicator of treatment response. For example, the Sleep Matrix may be useful in assessing the clinical status of patients with insomnia during clinical trials, as the measure of therapeutic outcomes of these trials remains challenging (CitationMorin 2002).
Table 3 Application of the Sleep Matrix
It should be noted that normal patients generally have ESS scores of <10 while ESS scores >12 indicate pronounced, even pathological sleepiness. Thus, scores in the range of 10–12 constitute a “grey zone” indicating that the patient may have some symptoms of sleepiness but no adverse effects on quality of life. Further clinical evaluation is required for these patients as other medical conditions or poor sleep hygiene may be responsible for their sleepiness.
The goal of future, prospective studies will be to further validate the SMI and the Sleep Matrix with larger patient numbers in other geographical locations with various sleep disorders, and to correlate the results with other validated measures, such as the Clinical Global Impression of Severity and Change (CitationHajak et al 2002), ultimately permitting further assessment of sleep disorders and the measurement of treatment outcomes. Factor analysis will be examined to characterize specific sleep disorders. Although Cronbach’s alpha indicate the SMI is a reliable tool, retest reliability will also be scrutinized.
Conclusion
The results of this pilot study suggest that the SMI demonstrates sensitivity to discriminate normal from abnormals but more closely examines insomnia severity, characteristics or types. Both the SMI and the Sleep Matrix may be useful clinical tools for the diagnosis of insomnia. Indications for nocturnal polysomnography may be guided by these results. Future studies will attempt to validate these measures of sleep quality with the ultimate goal of improving the diagnosis and care of patients with insomnia and other sleep disorders.
Acknowledgment
The authors express their gratitude to the many patients who provided their cooperation, making this study possible.
References
- Ancoli-IsraelSRothTSleep1999Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation SurveySleep22S3475310394606
- [APA] American Psychiatric Association1994Diagnostic and statistical manual of mental disorders4th edAPAWashington, DC
- American Academy of Sleep Medicine2005International classification of sleep disorder2nd EdDiagnostic and coding manualRochester, MN
- BastienCHVallieresAMorinCM2001Validation of the insomnia severity index as an outcome measure for insomnia researchSleep Med229730711438246
- BonnetM1983Memory for events occurring during arousal from sleepPsychophysiology208176828616
- BoganRKTurnerJ2005A new tool to measure insomnia: The Sleepmed insomnia index (SMI)Sleep28SupplA224
- BuysseDJReynoldsCFIIIMonkTH1989The Pittsburg sleep quality index: a new instrument for the psychiatric practice and researchPsychiatry Res28193132748771
- ChessonAHartseKAndersonWM2000Practice parameters for evaluation of chronic insomniaSleep2315
- EdingerJMeansMKrygerMHRothTDementWC2005Overview of insomnia: definitions, epidemiology, differential diagnosis, and assessmentPrinciples and Practices of Sleep Medicine4th edPhiladelphiaElsevier Saunders70213
- HajakGCluydtsRDeclerckA2002Continuous versus non-nightly use of zolpidem in chronic insomnia: results of a large-scale, double-blind, randomized, outpatient studyInt Clin Psychopharmacol1791711800507
- JohnsMW1991A new method for measuring daytime sleepiness: The epworth sleepiness scaleSleep1454051798888
- JohnsMHockingB1997Daytime sleepiness and sleep habits of Australian workersSleep2084499415943
- KilduffTSKushidaCAChokorovertySDarnoffRB1989Circadian regulation of sleepSleep disorders medicine: basic science, technical considerations, and clinical aspects2nd edOxford: EnglandButterworth-Heinemann
- KimHYoungT2005Subjective daytime sleepiness: dimensions and correlates in the general populationSleep286253416171277
- KripkeDFGarfinkelLWingardDL2002Mortality associated with sleep duration and insomniaArch Gen Psychiatry59131611825133
- LittnerMHirshkowitzMKramerM2003Practice parameters for using polysomnography to evaluate insomnia: An updateSleep2665460
- MitlerMMDementWCDingesDFKrygerMHRothTDementWC2000Sleep medicine, public policy, and public healthPrinciples and practices of sleep medicine3rd edPhiladelphiaWB SaundersP5808
- MonkTHWelshDK2003The role of chronobiology in sleep disorders medicineSleep Med Rev74557315018090
- MorinCM2002Measuring outcomes in randomized clinical trials of insomnia treatmentsSleep Med Rev72637912927124
- MoulDENofzingerEAPilkonisPA2002Symptom reports in severe chronic insomniaSleep255536312150322
- National Institutes of HealthState-of-the-Science Conference Statement Bethesda, MD8182005
- OhayonMM2002Epidemiology of insomnia: what we know and what we still need to learnSleep Med Rev69711112531146
- ReidelBWLichsteinKL2006Insomnia and daytime functioningSleep Med Rev427798
- RichardsonGZeeP2006Insomnia: primary care editionClinical Symposia56335
- SateiaMJDoghramjiKHauriPJ2000Evaluation of chronic insomniaSleep232435510737342
- SoldatosCRDikeosDGPaparrigopoulosTJ2000Athens insomnia scale: validation of an instrument based on ICD-10 criteriaJ Psychosom Res485556011033374
- StepanskiEZorickFRoehrsT1987Daytime Alertness in patients with chronic insomnia compared with asymptomatic control subjectsSleep1154603363270
- TurnerJBoganRK2005Sleepmed insomnia index (SMI) as an outcome measure in treated insomniaSleep28supplA224
- TurnerJBoganRK2005The Sleep Matrix: A new tool to measure sleep vital signsSleep28SupplA224
- ViolaniCDevotoFLLombardoC2004Validity of a short insomnia questionnaire: the SDQBrain Res Bull631521
- WalshJK2004Clinical and socioeconomic correlates of insomniaJ Clin Psychiatry65suppl 813915153063
- WyattJK2004Delayed sleep phase syndrome: pathophysiology and treatment optionsSleep271195120315532214