Abstract
Risperidone is a commonly used medication for the treatment of bipolar disorder and schizophrenia in children and adolescents. It has been studied as a monotherapy treatment in early onset schizophrenia and as both monotherapy and combination therapy for pediatric bipolar disorder. Studies to date indicate that risperidone is an effective treatment for positive and negative symptoms of schizophrenia and mania symptoms of bipolar disorder. In young patient populations, side effects such as weight gain, extrapyramidal side effects, and prolactin elevation require consideration when evaluating the risk benefit ratio for individual patients. Here we review published studies of risperidone for the treatment of bipolar disorder and schizophrenia in children and adolescents to provide practitioners with an overview of published data on the efficacy and safety of risperidone in these patient populations.
Introduction
Risperidone is a commonly used second generation antipsychotic agent (SGA) recently approved for the treatment of schizophrenia in adolescents, ages 13–17, and for the short-term treatment of manic or mixed episodes of bipolar I disorder in children and adolescents ages 10–17. It has additional indications for bipolar mania and schizophrenia in adults, and irritability associated with autism in children and adolescents 5–16 years of age. Risperidone or similar SGAs, either alone or in combination with the mood stabilizers lithium, valproic acid carbamazepine, serve as first line treatment options for the treatment of pediatric bipolar disorder based on severity and acuteness of the illness (CitationPavuluri et al 2004b; CitationKowatch and DelBello 2005). Risperidone or similar SGAs are also the mainstay of therapy for persons with schizophrenia (CitationMiller et al 2004). From 1996 to 2000, risperidone was the most prescribed antipsychotic agent in one large study sample of children and adolescents (CitationPatel et al 2005). Although more recent data in pediatric populations have not been released, risperidone was the second most prescribed antipsychotic agent in the United States after quetiapine by both sales and prescription number in 2006 (CitationLamb 2007) and continues to be widely used in a variety of disorders prevalent in childhood and adolescence. Given such wide utilization, risperidone, along with other pharmacologic agents used to treat bipolar disorder and schizophrenia in the pediatric and adolescent populations, need to be reviewed in detail to allow prescribers, patients, and families to weigh the risks and benefits of treatment. The purpose of this article, therefore, is to review available data on the efficacy, effectiveness, and safety of risperidone in children and adolescents with bipolar disorder or schizophrenia.
This review was conducted using the publication databases of PubMed, Medline, EMBASE, Psych Abstracts, and 2006–2007 annual meeting abstracts for the American Psychiatric Association, American Association of Child and Adolescent Psychiatry, American College of Neuropsychopharmacology, College of Psychiatric and Neurologic Pharmacists, and International Congress on Schizophrenia Research. The terms of search included “risperidone”, “risperidone and adolescent”, “risperidone and pediatric”, “risperidone and bipolar”, and “risperidone and schizophrenia” for studies of children and adolescents with bipolar disorder or schizophrenia. Where indicated, the references from pertinent articles were tracked further. This method allowed the presentation of our most up to date findings on risperidone in these populations. Studies are limited, and therefore, despite small sample sizes, findings are described in detail along with tabulation to present a comprehensive review to the readers.
Neuropharmacology
Risperidone is an antipsychotic agent with a benzisoxazole chemical structure that has potent dopamine-2 (D2), serotonin-2A (5HT2A), and alpha-1 receptor antagonism (CitationGardner et al 2005). Risperidone is classified by most as a second generation or “atypical” antipsychotic agent due to its relatively lower propensity to induce extrapyramidal side effects at lower doses as compared with first generation counterparts like haloperidol. It is a major substrate of cytochrome-P450 (CYP450) 2D6 and a minor substrate of CYP3A4, with major and mild inhibition of those two metabolic enzyme systems, respectively. The half life of risperidone is approximately 20–30 hours, with the half-life of the active 9-OH-risperidone metabolite being 21–30 hours. The antipsychotic and antimania effects of risperidone are postulated to result from potent dopamine-2 and serotonin-2A antagonism in the mesolimbic dopamine pathway and cerebral cortex, respectively. Risperidone has been shown to be effective in the acute and maintenance treatment of schizophrenia and bipolar disorders, with emerging evidence in younger patient populations reviewed here.
Efficacy/effectiveness
Adolescents with schizophrenia
Risperidone has been studied in seven published trials of adolescents with schizophrenia – two open label monotherapy studies, two comparisons with olanzapine and haloperidol, two comparisons with olanzapine, and one randomized double blind placebo-controlled study of two fixed doses. The studies summarized in were conducted in adolescent and young adult populations, with ages ranging from 9 to 28 years and the majority of participants below 20 years of age. Primary outcomes are summarized in .
Table 1 Summary of risperidone treatment studies in children and adolescents with bipolar disorder or schizophrenia
In 1997, one of the first formal studies of risperidone in adolescents with schizophrenia was published (CitationArmenteros et al 1997). This was an open-label, 6-week study of 10 adolescents ages 11–18 with schizophrenia who had undergone a 2-week washout prior to the initiation of risperidone titrated to 0.05–0.104 mg/kg/day (4–10 mg/day). Seven of the 10 participants had been previously treated with at least one first generation antipsychotic agent. These subjects were a mix of paranoid, disorganized, and undifferentiated schizophrenia diagnoses with Positive and Negative Symptoms Scale (PANSS) total scores averaging 70.7 at the beginning of the study. After 6 weeks, total scores were reduced by 19.9 points. Positive and negative subscale components improved 5.6 and 7.0 points respectively (all p-values <0.01). Clinical Global Impression (CGI) scores improved from an average of 4.5 at baseline to 3.1 at endpoint.
Somnolence was reported in eight out of 10 subjects, two had dystonic reactions, one had mild orofacial dyskinesia, and three required benztropine to treat extrapyramidal symptoms (EPS). Weight gain was common, occurring in 8 out of 10 participants, with details summarized in . Despite the arguably high doses and side effects, none of the subjects discontinued the study due to adverse effects. Changes in prolactin were not reported.
Table 2 Weight gain
Zalsman et al published one of the few studies of risperidone in predominantly drug naïve patients (CitationZalsman et al 2003). This was an open-label, 6-week study of 11 adolescents ages 15.5–20 with schizophrenia who were treated with 1–4.5 mg/day risperidone (mean maximum dose = 3.14 ± 1.6 mg/day). After 6 weeks, the initial average PANSS total scores were reduced from 103.54 to 77. Positive and negative subscale components improved an average of 11.31 and 2.86 points respectively over the course of the study, which was a statistically significant improvement for the positive subscale measure only (p < 0.0001). CGI and BPRS improvements are summarized in . Most subjects reported significant somnolence (n = 8/11) and depression (n = 7/11). Akathisia was reported in 4 out of 11 participants, dystonia in 2, and orthostasis in 5 participants of the study. Eight of 11 reported significant weight gain, ranging from 1 to 19 kg (see ) over the course of the 6-week trial. Changes in prolactin were not reported.
CitationGothelf et al (2003) reported an open-label, 8-week study of 43 adolescents ages 14–20 with schizophrenia (CitationGothelf et al 2003). This was a mixed sample of paranoid, disorganized, and undifferentiated subtypes. Thirty-six of these patients had been previously treated with antipsychotic agents, differentiating this sample from the previously described predominantly treatment-naïve group. Participants in this investigation were treated with risperidone (3.3 ± 1.1 mg/d), olanzapine (12.9 ± 3.1 mg/day), or haloperidol (8.3 ± 3.8 mg/day). Thirty-nine of the 43 subjects completed the trial, with discontinuations due to non-response or non-compliance. Baseline PANSS total scores averaged 90.2, 71.6, and 86.1 for the risperidone, olanzapine, and haloperidol treatment groups respectively. Improvements averaged 16.9, 13.9, and 19.7 points for the three groups. Positive symptoms improved 4.6, 3.3, and 7.2 points, with negative symptoms improving 3.9, 4.3, and 4.3 points for risperidone, olanzapine and haloperidol, respectively. Although all treatments significantly improved symptoms over time, there were no differences in improvement across the three medication groups.
Approximately 3–5 subjects reported inner restlessness, increased need for sleep, increased salivation, micturition disturbances, polyuria or polydypsia, and dizziness or orthostasis. A separate publication was reported on the weight gain incurred in these subjects after 12 weeks of treatment (CitationRatzoni et al 2002). Increases in weight were 3.9 ± 4.8 kg, 7.2 ± 6.3 kg, and 1.1 ± 3.3 kg for risperidone, olanzapine, and haloperidol-treated subjects. Olanzapine subjects gained significantly more weight than risperidone and haloperidol groups. Extreme weight gain, defined according to FDA criteria as ≥ 7% increase from baseline was greatest in the olanzapine group (90.5% of olanzapine group), followed by risperidone (42.9% of risperidone group) and haloperidol (12.5% of haloperidol group). On further analysis, these investigators determined that the majority of weight gained from the second generation antipsychotics occurred in the first four weeks of treatment (6.45% for olanzapine and 4.5% for risperidone).
A subsequent study aimed to further compare risperidone and olanzapine in early onset schizophrenia was published in 2006 (CitationMozes et al 2006). This was a 12-week, open label, randomized study of risperidone (maximum dose range 0.5–4.5 mg/day) versus olanzapine (maximum dose range 5–20 mg/day) in 25 children with child-onset schizophrenia ages 9–14 years. Ten subjects had diagnoses of schizophreniform disorder, 7 disorganized schizophrenia, 6 paranoid, and 2 with diagnoses of schizophrenia with unspecified subtypes. One olanzapine subject and 4 risperidone treated subjects dropped out of the trial. Three of the risperidone discontinuations were due to lack of improvement with the other due to severe hyperprolactinemia (55 ug/L). Baseline PANSS total scores averaged 93.85 and 92.75 for the risperidone and olanzapine groups respectively. Improvements in PANSS total scores averaged 30.41 and 42.25 points for the two groups. Positive scores improved an average of 11.61 and 13.3 points for risperidone and olanzapine-treated participants, respectively. Negative symptoms improved an average of 4.23 and 8.75 points for the two groups. All PANSS measures, along with Brief Psychiatric Rating Scale (BPRS) and Children’s Global Assessment Scale (CGAS) scores improved significantly (p < 0.001) for both treatment groups, with no statistical differences observed between groups for this study when continuous change scores were considered. When the two treatment groups were compared to examine differences between groups improving at least 50% on PANSS negative scores, there was a trend towards greater improvement in the olanzapine group (p = 0.047), but this was not significant after correcting for multiple comparisons. The risperidone treatment group reported more extrapyramidal side effects than the olanzapine group, with >30% of risperidone-treated subjects assessed as having changes in gait, arm dropping, elbow rigidity, and wrist rigidity according to the Barnes Akathisia Rating Scale and the Simpson Angus Scale. Mean weight gain was not significantly different between the risperidone and olanzapine groups respectively, with both groups gaining significant weight from baseline as summarized in .
In another randomized open-label study, symptom response and side effects of risperidone and olanzapine were compared in 44 children and adolescents ages 16–28 years with schizophreniform, schizophrenia, or schizoaffective disorders (Citationvan Bruggen et al 2003). Subjects were assigned to risperidone (mean endpoint dose = 4.4 ± 2.9 mg/day) or olanzapine (mean endpoint dose = 15.6 ± 3.1 mg/day) in a flexible titration dosing scheme for this 6-week study. Baseline PANSS total scores were 89.2 and 90.7 for the risperidone and olanzapine groups, respectively. Improvements in PANSS total scores averaged 15.0 and 15.1 points for the two groups. On average, positive symptoms improved 5.5 and 3.2 points for the risperidone and olanzapine groups. Negative symptoms improved 2.4 and 4.3 points, respectively. There were not statistically significant differences in clinical outcomes as assessed by the PANSS total or subscale measures, percent of patients achieving remission, or time to treatment response between the two medication groups.
A significant number of side effects were reported in this study. Greater than 30% of risperidone-treated subjects reported somnolence (68.4%), excessive thirst (52.6%), decreased libido (52.6%), excessive appetite (42.1%), akathisia (31.6%), and dizziness (31.6%). Somnolence, thirst, and libido were adverse events reported more often in risperidone-treated participants than olanzapine-treated subjects. Headache was reported significantly more often in the olanzapine (33.3%) than the risperidone (5.3%) group. Weight gain averaged 4.5 ± 5.2 kg in the risperidone group and 3.6 ± 3.8 kg in the olanzapine group. There were no significant differences in weight gain observed. Prolactin was not examined in this investigation. Furthermore, the relationship between adjunctive medications and side effects was not reported. However, 16% of the risperidone group and 0% of the olanzapine group were taking antidepressants, 32% of risperidone and 39% of olanzapine group were taking benzodiazepines, and 28% of risperidone and 11% of olanzapine participants were taking anticholinergic medications. The side-effect profiles of these medications in addition to the antipsychotics may have accounted for the unusually high rates of some of the reported adverse events and warrants consideration when interpreting the results of this study.
Fifty psychotic youths, 52% with schizophrenia spectrum disorders and 42% with a psychotic mood disorder, were treated in a randomized double blind 8-week trial of risperidone (4.0 ± 1.2 mg), olanzapine (12.3 ± 3.5 mg), and haloperidol (5.0 ± 2.0 mg) (CitationSikich et al 2004). Participants ranged from 8–19 years of age. Dosing was done in a randomized, fixed dose, parallel design for the 8-week treatment period. Because of the mix of schizophrenia spectrum and mood disorders, the PANSS was not administered. Baseline BPRS-C scores averaged 54, 50, and 49 for the risperidone, olanzapine, and haloperidol treatment groups respectively. Mean BPRS-C improvements were 27, 28, and 16 points for the three groups. Child Psychiatric Rating Scale (CPRS) positive-symptom scores improved 13, 19, and 14 points for the three groups, while CPRS-negative symptom scores improved 12, 6, and 7 points for risperidone, olanzapine, and haloperidol respectively. Changes in scores over time, percent responders at week 8, and percent of subjects in each treatment group have a CGI value of “1”at the end of the study were all similar across treatments.
Fifty-three percent of the risperidone-treated group required low dose benztropine for the management of EPS. This was similar to the 53% and 67% of olanzapine and haloperidol-treated participants who also required anticholinergic treatment. At the end point of the study, sedation (26%) and headache (31%) were the two most commonly reported side effects for the risperidone group. However, over 30% of risperidone-treated subjects reported sedation (89%), blurry vision (42%), dry mouth (32%), nervousness (53%), headache (62%), drooling (32%), light headedness (37%), nausea (53%), weakness (32%), constipation (32%), musculoskeletal pain (42%), or sweats/chills (32%) at one point or another throughout the course of the trial. Significant differences between treatment groups in side effects at end point included the haloperidol group reporting more dry mouth and decreased coordination at any point, lightheadedness, and sweats/chills at termination. Adjunctive medications were allowed in this study including anticholinergics, beta blockers, benzodiazepines, antidepressants, and mood stabilizers but did not differ significantly between treatment groups. As mentioned in the context of the previous study, the number of concomitant medications may have artificially elevated the percentage of subjects reporting various adverse events at various points throughout the course of the study. Weight gain averaged 4.9 ± 3.6 kg, 7.1 ± 4.1 kg, and 3.5 ± 3.7 kg for the risperidone, olanzapine, and haloperidol groups, respectively, which was statistically different across groups. Prolactin levels for all groups were mildly elevated at baseline (34.1 ± 21.11 ng/mL risperidone, 31.5 ± 25.8 olanzapine, and 28.8 ± 27.4 haloperidol). Prolactin elevations did not significantly vary from baseline after the 8-week treatment period for any of the three treatment groups, although prior treatments that may have influenced the elevated levels at baseline were not described.
The most recent research regarding the efficacy and safety of risperidone in the treatment of schizophrenia in adolescents was reported at the 2007 American Psychiatric Association Annual Meeting (CitationHaas et al 2007). This was a double blind, randomized, placebo-controlled study of 2 fixed-dose strata of risperidone (1–3 mg/day and 4–6 mg/day). A total of 269 patients 13–17 years of age were randomized to placebo (n =160), risperidone 1–3 mg/day (n = 55), or risperidone 4–6 mg/day (n = 54) for 6 weeks. PANSS total score improvements averaged 21.3, 21.2, and 8.9 points for the risperidone 1–3 mg, 4–6 mg, and placebo groups, respectively. No differences were observed between the two dose strata and the authors concluded that the lower dose range had the best risk benefit profile for use in this population. Details of adverse events were not given in detail in the published abstract.
Children and adolescents with bipolar disorder
Risperidone has been studied in 4 prospective studies in pediatric bipolar disorder study samples, with additional data on safety and effectiveness published in retrospective and case studies. The studies summarized in include those done in adolescent and young adult populations, with ages ranging from 4–18 years. As is the case with the treatment of adults with bipolar disorder, the majority of children and adolescents with bipolar 1 or bipolar disorder not otherwise specified require more than one medication for symptom management. Therefore it is not surprising that two of the four prospective studies involve the analysis of risperidone in combination with other mood stabilization medications. Primary outcomes are summarized in .
In 1999, the first study of using risperidone to treat juveniles with bipolar disorder was published (CitationFrazier et al 1999). This was a retrospective chart review of a well characterized population of 28 youths ages 4–17 years treated for an average of 6.1 ± 8.5 months (range = 0.25–34 months) with an average dose of 1.7 ± 1.3 mg/day of risperidone. As is commonly observed in clinical practice, the number of patients with comorbid diagnoses was high, with an average of 2.6 ± 0.8 additional psychiatric diagnoses per person. This included attention deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), pervasive developmental disorder (PDD), language disorder, anxiety disorder, conduct disorder, obsessive compulsive disorder (OCD), and post traumatic stress disorder (PTSD). Accordingly, many other concomitant medications were also being used in this population, including stimulants (71%), alpha agonists or beta blockers (71%), mood stabilizers (64%), serotonin reuptake inhibitors (43%), tricyclic antidepressants (43%), first generation antipsychotics 32%), or benzodiazepines (7%). The primary assessment of effectiveness in these patients was the CGI stratified by specific syndromes (eg, mania, ADHD, psychosis, or aggression). After the addition of risperidone to the treatment regimens of these patients, the CGI-mania scale scores decreased 2.5 points from the baseline mean of 5.4 ± 1.0, the CGI-aggression scores decreased 2.1 points from the baseline mean of 5.1 ± 1.2, psychosis symptoms declined 2.1 points from the baseline mean scores of 3.6 ± 0.4, and the ADHD component scores decreased an average of 0.7 points from the baseline mean of 5.0 ± 0.8. All changes in subscale measures significantly improved (p < 0.01), although the improvements in ADHD-specific symptoms were not as clinically robust as those observed on other subscales.
Weight gain was reported for 18% of the patients, although the specific amounts gained were not reported. In this sample, sedation was the other side effect, reported at 18%. Prolactin levels were available for 11 of the children, nine of whom had elevations above normal limits, with an average of 32.8 ± 12.05 ng/mL (reference 0–15 ng/mL).
Recently, Saxena et al published a case series of 4 boys and 2 girls ages 5–15 with pediatric bipolar disorder (CitationSaxena et al 2006). The goal of this study was to evaluate the effectiveness of risperidone in this population of patients who had failed to adequately respond to other mood stabilizers, primarily divalproex sodium. The clinical scenarios of the cases presented are representative of many patients seen in clinical practice where initial treatments fail to adequately treat aggressive symptoms. In these patients, the mean dose of risperidone was 0.85 mg/day and was added on to therapeutic doses of existing medications that included mood stabilizers, low dose antidepressants, and low dose stimulants. In all of the patients included in this case series, symptoms of aggression were greatly reduced after the addition of risperidone. One patient had akathisia at a dose of 0.25 mg in the morning, which was relieved after removing the medication and re-starting at different a.m./p.m. dosing schedule. One patient had the dose reduced due to possible cognitive impairment. No subjects in this report permanently discontinued risperidone due to side effects.
CitationBiederman et al (2005a) recently conducted an open label 8-week monotherapy trial of risperidone (1.25 ± 1.5 mg/day) in 30 children and adolescents 6–17 years of age with bipolar disorder (CitationBiederman et al 2005a). Additional treatment with antidepressants, mood stabilizers, other antipsychotics, or anticonvulsants was not permitted for this investigation. The Young Mania Rating Scale (YMRS), BPRS, and CDRS were the primary outcomes reported. Twenty-two of the 30 participants completed the entire 8-week study, with dropouts primarily due to lack of efficacy and lack of follow-up (7 participants) and one due to orthostatic hypotension. The baseline YMRS total scores were in the severe range at 27.9 ± 9.1 and decreased significantly (p < 0.0001) from an average of 14.4 points to 13.5 ± 9.7 at endpoint. BRPS total scores decreased an average of 12.6 points over the course of study, with significant improvements on resistance (mania symptoms) and positive symptoms, but not negative symptoms or anxiety/dayepression components of the scale. CDRS scores decreased 10.2 points, from baseline scores of 40.9 ± 11.5 to 30.7 ± 11.0, which was a significant improvement (p < 0.0001).
This research group also conducted a similar study in a younger patient population with an open-label, 8-week trial comparing risperidone (1.4 ± 0.5 mg/day) to olanzapine (6.3 ± 2.3 mg/day) in 31 children 4–6 years of age with a diagnosis of pediatric bipolar disorder (CitationBiederman et al 2005b). This study did not allow the utilization of other antimanic, mood stabilizing, or antidepressant medications. The YMRS and CGI mania scales were the primary outcome measures for the study. Of the 16 risperidone and 15 olanzapine-treated subjects, 6 olanzapine and 1 risperidone subjects discontinued before the 8-week endpoint. Discontinuations were largely due to lack of efficacy (4/7 subjects). The baseline YMRS total scores for the risperidone and olanzapine groups were 35.2 ± 8.2 and 34.2 ± 6.4 and decreased significantly (p < 0.001) by an average of 18.3 and 12.1 points over the course of 8 weeks. The difference between improvements seen in the two treatment groups was not statistically significant for YMRS measures. Improvement on CGI measures was subsequently assessed categorically, by examining the number of patients in each treatment arm who were rated as “much” or “ very much” improved. The proportion of subjects meeting this criteria was 69% and 53% (p = 0.9) for the risperidone and olanzapine treatment groups, respectively. Spontaneously reported side effects were not statistically different between the two treatment groups. Side effects occurring in greater than 30% of participants in one or both treatment groups were increased appetite, cold/allergies/infection, and headache. Both groups gained weight, with an average of 2.2 ± 0.4 kg in the risperidone group and 3.2 ± 0.7 kg in the olanzapine group. The groups did not differ significantly in the amount of weight gained. Of the vitals biochemical measures collected, including lipid panel, glucose, and prolactin, the extent of prolactin elevation was greater in the risperidone group (mean increase of 35.7 ng/mL) than the olanzapine group (mean increase of 11.9 ng/mL).
Pavuluri et al (2004) conducted an open-label trial of 37 pediatric bipolar subjects with mania ages 5–18 who were sequentially assigned to risperidone (0.75 ± 0.75 mg/day) plus divalproex sodium (106 μg/dayL) or lithium (0.9 mEq/L) for 6 months (CitationPavuluri et al 2004a). Primary outcomes were assessed via improvements on the YMRS and CGI-Severity mania scales. Baseline YMRS scores were 28.8 ± 4.35 and 30.0 ± 3.69 for the risperidone + lithium and risperidone + divalproex sodium groups, respectively. These scores improved significantly over time for both groups, with YMRS score reductions averaging 23.53 and 22.0 points for the risperidone + lithium and risperidone + divalproex sodium groups, respectively. Differences between groups were not statistically significant. Baseline CGI-BP Mania subscales averaged 5.35 ± 1.41 and 5.70 ± 0.87 with mean improvements of 3.56 and 3.82 points for the two groups. Weight gain was the only side effect observed in ≥30% of participants (35.3% lithium + risperidone; 40% divalproex sodium + risperidone). Other notable side effects were sedation (23.5%, 20%), nausea (23.5%, 15%), increased appetite (23.5%, 20%), and stomach pain (23.5%, 10%).
Side effects and tolerability
Risperidone is classified by most as a second generation or “atypical” antipsychotic agent, a class so named for their lower propensity to induce movement-related side effects like EPS and tardive dyskinesia than older first generation agents. Although specific criteria for antipsychotic “atypicality” are still debated, significant affinity of these agents to 5HT2A receptors in the mesocortical and nigrostriatal dopamine tracks is one common feature, which is thought to facilitate the release of dopamine in these areas, thus counteracting the negative effects resulting from D2 blockade in these regions. This is one proposed mechanism for lower EPS rates in these agents as compared to their first generation counterparts. Of the second generation agents, risperidone has the highest affinity to D2 receptors and as mentioned in the previous study reviews of risperidone in young patients with schizophrenia and bipolar disorder some patients do develop EPS, resulting in medication discontinuation or adjunctive treatment with anticholinergic or beta-blocking agents for EPS. Potent D2 blockade also results in prolactin elevation, which is more significant after risperidone administration than for any other first or second generation agent. Finally, one of the most problematic side effects of risperidone, as well as other second generation agents like olanzapine, quetiapine, and clozapine is weight gain. The following section will briefly summarize possible mechanisms of action for these three broad classes of side effects that are highly relevant for risperidone. While other adverse effects such as somnolence, sedation, and upset stomach are also important, EPS, prolactin elevation, and weight gain may have long term health consequences for persons having to take medications like risperidone indefinitely.
EPS
Early-onset EPS usually appear within the first 4 weeks of antipsychotic treatment and include dystonia, akathisia, and parkinsonism. The dopamine-acetylcholine balance hypothesis is one theory of antipsychotic-induced EPS. An equilibrium of dopaminergic (inhibitory) and cholinergic (excitatory) neuronal activity in the corpus striatum is required for normal motor functioning. Dopamine blockade by antipsychotics leads to a decrease of dopamine and a subsequent relative increase in acetylcholine that results in signs and symptoms of EPS (CitationMarsden and Jenner 1980). EPS is a dose-dependent phenomenon. In adults taking risperidone, the rates of EPS at doses ≥6 mg/day approach those seen with haloperidol, which is why clinical recommendations for adults suggest not dosing above 4–6 mg/day. This specific relationship has not been formally described in children and adolescents, although data presented earlier in this review suggests a similar dose-response correlation. In the previously presented studies, the percentage of patients reporting dystonic reactions ranged from 0%–20%, with the highest rate (20%) reported in the small study sample where doses averaged 6.6 mg/day. The percent of patients presenting with EPS, including parkinsonism, akathisia, or dyskinesias, ranged from 0%–61.5% in these studies. Interestingly, the highest rate of overall EPS, 61.5% was reported in a study where the average dose was 1.62 mg/day risperidone. Ascertainment with the SAS and BARS scales may have increased the recognition of subtle effects in these participants that were perhaps not as recognized in other studies relying on generic side effect assessment scales or spontaneous report. Concomitant anticholinergic medications often confound our abilities as clinicians to recognize the true rate of EPS, although in these studies, anticholinergics were generally administered after the observation of such effects. presents a rough assessment of this relationship by plotting mean daily dose of risperidone by percentage of patients receiving anticholinergic medications in monotherapy trials. Recognizing the limitations that age differences, duration of treatment, and previous medications may have on the likelihood of a given patient needing adjunctive treatment for EPS, we can see that doses above 3 mg/day result in 18%–67% of patients requiring anticholinergic medications. This is quite robust given the “atypical” designation of risperidone.
Figure 1 Percent of subjects receiving anticholinergic medications in risperidone monotherapy trials of children and adolescents.
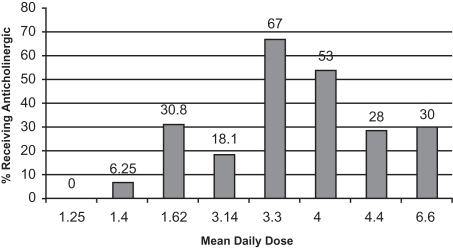
The potential for EPS from risperidone is a significant consideration for patients and families. EPS can lead to stigmatization of children and adolescents, which may affect their ability to interact with peers. Tremors and akathisia may disrupt learning if not adequately managed, due to interference with writing and ability to perform fine motor movements as well as the ability to sit still in class. Anticholinergic medications are well known for their propensity to cause dry mouth, constipation, confusion, and cognitive impairment. Therefore these adverse effects of the medications used to manage adverse effects of risperidone also warrant close monitoring. Pre-treatment and routine follow-up monitoring of EPS as well as tardive dyskinesia assessments are warranted before starting any antipsychotic agent and risperidone is no different. The studies presented here were all relatively short-term trials, where tardive dyskinesia was not observed. However, the high incidence of EPS suggests that the long term monitoring for this late-onset movement side effect is warranted.
Prolactin elevation
Prolactin is secreted by the anterior pituitary and is involved in many physiological processes. Prolactin elevation inhibits the hypothalamic-pituitary axis, suppresses gonadotropin-releasing hormone release from the hypothalamus, and eventually reduces estrogen and testosterone in males and females (CitationPetty 1999). Modest elevations induced by antipsychotic agents have been associated with amenorrhea, hirsutism, galactorrhea, decreased libido, and hypogonadism in women and decreased libido and reduced spermatogenesis in males (CitationHowes and Smith 2002).
Dopamine signaling through D2 receptors located on the lactotrophs of the anterior pituitary, slows prolactin gene transcription and modulates prolactin release. Reducing D2 transmission results in a disinhibition of this negative feedback mechanism, which elevates prolactin. D2 receptor blockade is the primary mechanism of action for antipsychotic medications like risperidone. Agents with the highest binding affinities for D2 (eg, risperidone) result in the most substantial increases in prolactin levels as a result of therapy (CitationDickson and Glazer 1999).
Prolactin elevation occurs in patients irrespective of psychiatric diagnosis. When patients first begin conventional APs or risperidone, a substantial spike in prolactin levels is commonly seen that persists for 4–6 weeks until stabilization occurs (CitationKinon et al 2003). For some, levels will return to just above normal. For others levels remain significantly elevated, putting them at risk for prolacin-related sequelae. Investigations in patients taking risperidone ≥6 weeks consistently show a wide range of variability in prolactin levels, with standard deviations ranging from 15–60 ng/mL and some subjects having levels >200 ng/mL. The range of values seen in these patients is intriguing and commands further investigation.
Few studies have investigated the safety and efficacy of risperidone-associated prolactin elevations in pediatric and adolescent patient populations treated for long periods of time. CitationFindling et al (2003), conducted a pooled analysis of 5 clinical trials which included 592 subjects 5–15 years of age treated with risperidone (mean = 1.25 mg/day) and had at least 1 post dose prolactin level drawn (CitationFindling et al 2003). Prolactin levels were followed over the course of 48 weeks and found to peak at weeks 4–7. There was an overall 40% attrition rate over the 48-week study period, but in those who were followed long-term, prolactin levels at endpoint averaged above baseline for both genders levels of 28.8 ± 16 ng/mL; 21.4 ± 22.7 ng/mL (for males and females respectively) in the 358 subjects who continued to week 48. Prolactin elevations of the 40% of subjects who dropped out were not reported.
Masi et al published a 3-year study of 53 preschool children (4.6 ± 7 years of age) who were treated with 0.55 ± −0.2 mg/day of risperidone (CitationMasi et al 2003). The discontinuation rate was 52.8% in this study, with 22.6% due to adverse effects. Elevated prolactin levels occurred in 65% of subjects, with average levels of 28.38 ± 22.45) reported for those who remained in the study (normal range for their assay was reported as ≤15 ng/mL) with elevations that exceeded 60 ng/mL occurring in 16% of their subjects.
Saito et al conducted a prospective study of prolactin elevation in adolescent subjects treated with risperidone, olanzapine, or quetiapine for an average of 11.2 weeks (CitationSaito et al 2004). Subjects treated with risperidone (mean dose = 2 mg/day) had endpoint prolactin values of 46.8 ± 33.3 ng/mL with 25% of the risperidone subjects complaining of prolactin-related side effects such as breast tenderness, menstrual irregularities, galactorrhea, or erectile dysfunction.
Finally, only 1 published study has looked at risperidone-related prolactin increases and sexual maturation in a pooled analysis of 5 randomized controlled studies of children (5–15 years of age) treated with 1.31 ± 0.70 mg/day of risperidone for 12 months (CitationDunbar et al 2004). Of the 700 children analyzed, there were no adverse outcomes at 1 year for height, growth, Tanner stage, or maturation. To our knowledge, data beyond one year have not been published.
In summary, treatment trials of children and adolescents show acute elevations in prolactin, which peak around 4–6 weeks after initiation and then regress to elevated steady-state levels after approximately 6–8 weeks of therapy, with the degree of elevation being quite variable, and often occurring in >50% of subjects. Significant elevations (>60 ng/mL) are not uncommon. These long-term studies had drop-out rates that approached 50%, with 20%–25% of the attrition due to adverse effects. These reports indicate that prolactin increases were not associated with visible adverse effects in persons who did not drop out although the potential effects of extended periods of prolactin elevation on bone development and homeostasis have not been defined and warrant future investigation.
Weight gain
Obesity is a growing epidemic in the United States as well as many other countries around the world. Obesity and weight gain predispose individuals to diabetes mellitus, cardiovascular disease, and overall increased mortality. Recent prevalence estimates report that from 2003–2004, 18.2% of males and 16.0% of females ages 2–19 in the United States are classified as overweight (defined as BMI ≥ 95th percentile for age) (CitationOgden et al 2007). When this criterion is relaxed to also include children and adolescents at risk for obesity (defined as BMI ≥85th percentile for age) these numbers approximately double to 34.8% of males and 32.4% females. Children and adolescents with schizophrenia or bipolar disorder face additional challenges, as the majority of mood stabilizing agents like lithium, divalproex sodium, and carbamazepine and antipsychotic agents like olanzapine, risperidone, quetiapine, and clozapine are all associated with significant weight gain. Recently, weight gain and metabolic effects in pediatric bipolar disorder were reviewed with a pooled analysis done on available trial data in this population (CitationCorrell 2007). Monotherapy with second generation agents such as olanzapine, quetiapine, risperidone, clozapine, and aripiprazole resulted in an average of 3.4 kg of weight gained in studies lasting up to 12 weeks. Combining a mood stabilizing agent like lithium, lamotrigine, oxcarbazepine, or divalproex sodium with a second generation antipsychotic agent resulted in an average of 5.5 kg of weight gained.
The mechanisms behind antipsychotic-associated weight gain have not been fully elucidated but have been postulated to result from specific aspects of antipsychotic pharmacology as well as systemic effects potentially altering insulin sensitivity and satiety. The second generation agents most commonly associated with weight gain are those with significant antihistaminic properties, which are thought to increase thirst and hunger drive (CitationMatsui-Sakata et al 2005). Among second generation antipsychotic agents, histamine-1 (H1) dissociation constants (Kd) have been reported to be 0.2 for olanzapine, 1.0 for clozapine, 6 for quetiapine, 8 for ziprasidone, and 12 for risperidone, with lower Kd values indicating more significant receptor binding. Additional receptor-binding associated with weight gain include serotonin-2C (5HT2C) receptors, as well as muscarinic acetylcholine (mAch) receptors (CitationMatsui-Sakata et al 2005). Risperidone Kd values for mAch receptors are relatively high, with modest 5HT2C binding properties. Despite receptor dissociation constants similar to relatively weight neutral agents like ziprasidone, weight gain is a clinically problematic side effect for children and adolescents taking risperidone. Weight gain, like EPS and prolactin elevation, is not specific to disease state, although concomitant medications that are relatively disease state-specific may influence the likelihood of weight gain or the extent of weight gained (CitationCorrell 2007).
summarizes the weight gained in trials of pediatric and adolescent participants of efficacy and effectiveness trials. Selected studies of children and adolescents with diagnoses other than schizophrenia or bipolar disorder are included for comparison. Weight gained over the course of 6- to 12-week studies of children and adolescents with a variety of psychiatric disorders ranged approximately from 1 to 8 kg. No clear relationship with dose and duration can be extrapolated from these studies due to heterogeneity in previous medication treatments that may have caused weight gain through similar mechanisms, dose, or concomitant medications allowed in the studies. Nonetheless, the amount of weight gained in this short amount of time demands vigilance as well as appropriate monitoring. summarizes monitoring parameters developed for our Pediatric Mood Disorders Clinic for risperidone with other similar recommendations in place for other antipsychotic agents. These monitoring parameters were developed to incorporate 2005 American Psychiatric Association and American Diabetes Association monitoring guidelines for metabolic effects from second generation agents and then modified to fit the needs of our patient population.
Table 3 Monitoring recommendations for risperidone (modified recommendations developed from CitationADA, APA [2004])
Conclusion
Risperidone is a commonly used and effective medication for the treatment of youths with schizophrenia or bipolar disorders. In children and adolescents with schizophrenia, all of the published treatment trials have resulted in significant improvements in psychopathology as assessed by PANSS, BPRS, and CGI rating scales. Comparison trials with olanzapine and haloperidol have not yielded any clear differences in efficacy or effectiveness between agents. In children and adolescents with bipolar disorder, risperidone is highly effective, primarily according to YMRS, BPRS, and CGI rating scales (as well as others); although the number of monotherapy trials is less than those done in the schizophrenia population. Doses used in the schizophrenia trials were on average higher than those used in the bipolar studies, although the latter largely enrolled younger participants and often studied risperidone in combination with other psychotropic medicines to address common comorbidities.
Risperidone appears to be well-tolerated, when considering that the number of participants in these studies who dropped out due to adverse effects was low. However, somnolence, EPS, prolactin elevation, and weight gain were commonly observed and representative of what is seen in clinical practice. Somnolence is an adverse effect that often attenuates over time and is minimized by bed time administration of the drug. EPS rates in these studies were relatively high, with 18%–67% of subjects taking ≥3 mg/day requiring an anticholinergic medication to manage these symptoms. Both EPS and anticholinergic medications used to manage them require close monitoring by clinicians, parents, and patients. Rates of EPS appear to be higher than with olanzapine although further studies need to be done to compare risperidone to other commonly used agents in youths like quetiapine, aripiprazole, and ziprasidone. EPS and tardive dyskinesia assessments should be done prior to beginning treatment, periodically during the first six months of therapy and every 6 to 12 months thereafter.
Prolactin elevation is nearly always significant in young patients taking risperidone, with 3- to 4-fold increases resulting in steady-state prolactin levels in the 30–50 ng/mL range common. However, the relationship between the extent of prolactin elevation and side effects is not clear and differs from patient to patient. The relationship of extended periods of antipsychotic-associated prolactin elevation and bone development is not clear and warrants additional research. Morning prolactin levels should be assessed prior to treatment and again after six weeks of treatment to determine whether elevations are clinically significant and warrant further evaluation.
Weight gain is also unfortunately common in risperidone-treated youths with increases from baseline roughly averaging 6–19 pounds (2.75–8.65 kg) in 6- to 12-week treatment trials. No clear relationship between weight gain and dose or treatment duration has been established, although the most significant rates of gain appear to occur in the first 6 weeks of treatment. Weight gain along with other metabolic parameters needs to be monitored closely in patients treated with risperidone, just as standard of practice dictates for other antipsychotic agents dictates (CitationADA, APA 2004). Understandably, strictly adhering to monitoring guidelines is not always logistically feasible due to the intricacies of the patient population, school schedules, etc. Therefore, we have developed our own monitoring guidelines for risperidone in our pediatric mood disorder population which is based on the recommendations set forth by the American Diabetes Association and the American Psychiatric Association, which are summarized in . We feel strongly that efforts to conduct monitoring roughly within the scope of presented recommendations or documentation of attempting to do so is clinically prudent to ensure the long term safety of patients as best as possible.
Risperidone is therefore a widely used and effective medication for the treatment of schizophrenia and bipolar disorders in youths. Additional research needs to be conducted to assess comparisons with other second generation antipsychotic medications that are also treatment options. Monitoring and counseling on common side effects from risperidone is essential, just as it is with other medications. Future research on clinical and biological predictors of treatment response and side effect risk will allow us to optimize treatments with antipsychotic agents in these populations to further optimize the risk benefit ratio in the treatment of these hard-to-treat patients.
Disclosures
JRB has received grant/research support from University of Illinois, Vahlteich Foundation, and American College of Clinical Pharmacy, and has given an industry-sponsored talk for Eli Lilly.
MNP has received grant/research support from National Institutes of Health, NARSAD, Janssen Pharmaceuticals, GlaxoSmithKline, Abbott Labs; is a consultant to Janssen Pharmaceuticals, GlaxoSmithKline, Bristol Myers Squibb, Shire, Inc., and Abbott Labs; and is on the Speakers' Bureau for Janssen Pharmaceuticals, GlaxoSmithKline, Bristol Myers Squibb, Shire, Inc., Abbott Labs, and Astra Zeneca.
References
- ADA, APAConsensus development conference on antipsychotic drugs and obesity and diabetesDiabetes Care20042759660114747245
- AmanMGDe SmedtGDerivanADouble-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligenceAm J Psychiatry200215913374612153826
- ArmenterosJLWhitakerAHWeliksonMRisperidone in adolescents with schizophrenia: an open pilot studyJ Am Acad Child Adolesc Psychiatry1997366947009136505
- BiedermanJMickEHammernessPOpen-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age childrenBiol Psychiatry2005a585899416239162
- BiedermanJMickEWozniakJAn open-label trial of risperidone in children and adolescents with bipolar disorderJ Child Adolesc Psychopharmacol2005b15311715910215
- CorrellCUWeight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systematic review and pooled analysis of short-term trialsJ Am Acad Child Adolesc Psychiatry20074668770017513981
- DicksonRAGlazerWMNeuroleptic-induced hyperprolactinemiaSchizophr Res199935SupplS758610190228
- DunbarFKusumakarVDanemanDGrowth and sexual maturation during long-term treatment with risperidoneAm J Psychiatry20041619182015121661
- FindlingRLKusumakarVDanemanDProlactin levels during long-term risperidone treatment in children and adolescentsJ Clin Psychiatry2003641362914658952
- FindlingRLMcNamaraNKBranickyLAA double-blind pilot study of risperidone in the treatment of conduct disorderJ Am Acad Child Adolesc Psychiatry2000395091610761354
- FrazierJAMeyerMCBiedermanJRisperidone treatment for juvenile bipolar disorder: a retrospective chart reviewJ Am Acad Child Adolesc Psychiatry199938960510434487
- GaffneyGRPerryPJLundBCRisperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndromeJ Am Acad Child Adolesc Psychiatry200241330611886028
- GardnerDMBaldessariniRJWaraichPModern antipsychotic drugs: a critical overviewCMAJ200517217031115967975
- GothelfDApterAReidmanJOlanzapine, risperidone and haloperidol in the treatment of adolescent patients with schizophreniaJ Neural Transm20031105456012721815
- HaasMUnisASCopehhaverMEfficacy and Safety of Risperidone in Adolescents with SchizophreniaAmerican Psychiatric Association Annual Meeting2007221
- HowesOSmithSHyperprolactinaemia caused by antipsychotic drugs. Endocrine antipsychotic side effects must be systemically assessedBMJ2002324127812028991
- KinonBJGilmoreJALiuHHyperprolactinemia in response to antipsychotic drugs: characterization across comparative clinical trialsPsychoneuroendocrinology200328Suppl 2698212650682
- KowatchRADelBelloMPPharmacotherapy of children and adolescents with bipolar disorderPsychiatr Clin North Am2005283859715826738
- LambETop 200 Prescription Drugs of 2006Pharmacy Times2007
- MaloneRPMaislinGChoudhuryMSRisperidone treatment in children and adolescents with autism: short- and long-term safety and effectivenessJ Am Acad Child Adolesc Psychiatry200241140711837403
- MarsdenCDJennerPThe pathophysiology of extrapyramidal side-effects of neuroleptic drugsPsychol Med19801055726104342
- MasiGCosenzaAMucciMA 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidoneJ Clin Psychiatry20036410394714628979
- Matsui-SakataAOhtaniHSawadaYReceptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitusDrug Metab Pharmacokinet2005203687816272755
- McCrackenJTMcGoughJShahBRisperidone in children with autism and serious behavioral problemsN Engl J Med20023473142112151468
- McDougleCJHolmesJPBronsonMRRisperidone treatment of children and adolescents with pervasive developmental disorders: a prospective open-label studyJ Am Acad Child Adolesc Psychiatry199736685939136504
- MillerALHallCSBuchananRWThe Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 updateJ Clin Psychiatry200465500815119912
- MozesTEbertTMichalSEAn open-label randomized comparison of olanzapine versus risperidone in the treatment of childhood-onset schizophreniaJ Child Adolesc Psychopharmacol20061639340316958565
- OgdenCLYanovskiSZCarrollMDThe epidemiology of obesityGastroenterology2007132208710217498505
- PatelNCCrismonMLHoagwoodKTrends in the use of typical and atypical antipsychotics in children and adolescentsJ Am Acad Child Adolesc Psychiatry2005445485615908837
- PavuluriMNHenryDBCarbrayJAOpen-label prospective trial of risperidone in combination with lithium or divalproex sodium in pediatric maniaJ Affect Disord2004a82Suppl 1S1031115571784
- PavuluriMNHenryDBDevineniBA pharmacotherapy algorithm for stabilization and maintenance of pediatric bipolar disorderJ Am Acad Child Adolesc Psychiatry2004b438596715213587
- PettyRGProlactin and antipsychotic medications: mechanism of actionSchizophr Res199935Supp lS677310190227
- RatzoniGGothelfDBrand-GothelfAWeight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective studyJ Am Acad Child Adolesc Psychiatry2002413374311886029
- SaitoECorrellCUGallelliKA prospective study of hyperprolactinemia in children and adolescents treated with atypical antipsychotic agentsJ Child Adolesc Psychopharmacol200414350815650492
- SaxenaKChangKSteinerHTreatment of aggression with risperidone in children and adolescents with bipolar disorder: a case seriesBipolar Disord200684051016879141
- SikichLHamerRMBashfordRAA pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trialNeuropsychopharmacology2004291334514583740
- SnyderRTurgayAAmanMEffects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQsJ Am Acad Child Adolesc Psychiatry20024110263612218423
- van BruggenJTijssenJDingemansPSymptom response and side-effects of olanzapine and risperidone in young adults with recent onset schizophreniaInt Clin Psychopharmacol200318341614571154
- ZalsmanGCarmonEMartinAEffectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label studyJ Child Adolesc Psychopharmacol2003133192714642020