Abstract
Experiments using male CD1 mice were carried out to investigate the effects of subchronic (daily administration for 8 days) pretreatments with drugs enhancing GABAergic transmission (diazepam, 10 mg/kg, ip; gabapentin, 100 mg/kg, po; or vigabatrin, 500 mg/kg, po) on pentylenetetrazol (PTZ)-induced seizures, 24 h after the last injection. Subchronic administration of diazepam reduced latencies to clonus, tonic extension and death induced by PTZ. Subchronic vigabatrin produced enhanced latency to the first clonus but faster occurrence of tonic extension and death induced by PTZ. Subchronic gabapentin did not modify PTZ-induced seizures. Autoradiography experiments revealed reduced benzodiazepine receptor binding in several brain areas after subchronic treatment with diazepam or gabapentin, whereas subchronic vigabatrin did not induce significant receptor changes. The present results indicate differential effects induced by the subchronic administration of diazepam, vigabatrin, and gabapentin on the susceptibility to PTZ-induced seizures, benzodiazepine receptor binding, or both.
Introduction
The increasing brain GABA (gamma-amino butyric acid) content and administration of centrally active GABA-mimetic agents have been used as an effective therapeutic approach for the treatment of epilepsy (CitationOlsen and Avoli 1997). However, it has also been suggested that the use of drugs that continually alter synaptic transmission does not represent the best strategy to control seizure expression (CitationGale 1992). Prolonged administration of drugs enhancing GABA action can lead to reduced GABAergic function (CitationGyenes et al 1988) and alterations in GABAA receptors (CitationCalkin and Barnes 1994; CitationYu and Ticku 1995) as well as tolerance and dependence, especially to sedative and anticonvulsant effects of drugs such as benzodiazepines (BDZ) (CitationBraestrup et al 1979).
The increase of GABAergic transmission can induce excitatory effects (CitationGale 1992). Chronic administration of BDZ can produce decreased sensitivity to GABA (CitationGallager et al 1984) and reduced ability of this amino acid to enhance BDZ binding (CitationBrett and Pratt 1995). Indeed, withdrawal symptoms or spontaneous seizures have been reported to occur in nonepileptic BDZ abusers upon drug discontinuation (CitationLukas and Griffith 1984).
The present study was designed to determine the effects of subchronic pretreatment with drugs enhancing GABAergic transmission on pentylenetetrazol (PTZ)-induced seizures and their correlation with BDZ receptor binding. We used vigabatrin, gabapentin, and diazepam because these are antiepileptic compounds activating the GABAergic system. Vigabatrin is a GABA structural analogue, which irreversibly blocks GABA transaminase (GABA-T), the enzyme responsible of GABA degradation (CitationLippert et al 1977; CitationSchechter et al 1979). The mechanisms of action of gabapentin, an analogue of GABA (CitationSuman-Chauhan et al 1993), appear to be a complex synergy between increased GABA synthesis and function (CitationTaylor et al 1998), non-NMDA receptor antagonism and binding to the α2δ-subunit of voltage dependent calcium channels. The latter action inhibits the release of excitatory neurotransmitters (CitationBennet and Simpson 2004). Diazepam, as a BDZ agonist, increases the channel opening frequency in presence of GABA increasing the effects of this amino acid (CitationHaefely et al 1995). Autoradiography studies using [3H]flunitrazepam were carried out to evaluate BDZ receptor binding in specific brain areas of mice treated subchronically with these antiepileptic drugs.
Material and methods
Animals
The experimental subjects were adult male CD-1 mice, weighing 25–30 g at the beginning of the experiments. Animals were housed in groups of 5–6 per cage (24 × 17 × 12 cm) under controlled environmental conditions (22 ± 1 °C; 12; 12-h light–dark cycle, lighting at 8:00 h; food and water ad libitum). Experimental procedures were conducted according to the Mexican Official Standard NOM-062-ZOO-1999 and the Ethical Committee of the Center for Research and Advanced Studies (Mexico, Project 222/04).
Evaluation of PTZ-induced seizures
Mice received a daily administration of saline (0.1 ml/10 g, per oral [po] or intraperitoneal [ip]) for 1 week. This procedure allows the animals to habituate to manipulation, avoiding downregulation of BDZ binding induced by acute handling (CitationAndrews et al 1992). Thereafter, they were daily treated with vigabatrin (500 mg/kg po, n = 10), gabapentin (100 mg/kg po, n = 10), or diazepm (10 mg/kg ip, n = 10) for 8 days. Immediately after each drug administration, animals were kept in individual cages for 30 min for behavioral evaluation. Preliminary experiments from our group indicated that a single administration of diazepam, gabapentin, or vigabatrin, at the doses and routes described above, was able to reduce PTZ-induced seizures and Fos expression in the mouse brain. For the present study, we chose the oral route for gabapentin and vigabatrin administration because it is how these drugs are used in patients. Indeed, vigabatrin exerts similar anticonvulsant potency at oral and ip administration (CitationLoscher and Frey 1987). A solution of PTZ (Sigma Aldrich Ltd., St. Louis, MO, USA) freshly prepared was applied (90 mg/kg, ip) 24 h after the last drug administration. Latencies to the first clonic seizure, tonic extension, and convulsion-induced death were evaluated during 30 min. The criterion of death was considered when animals spent 1 min or more with breathing and heart arrest. For each experimental drug, one control group (n = 10) was monitored in parallel. Control animals were manipulated as described above, except that they received daily saline administration instead of antiepileptic drugs. Diazepam was dissolved in vehicle (0.5% Tween 80 and saline), whereas gabapentin, vigabatrin, and PTZ were dissolved in saline.
Evaluation of [3H]flunitrazepam binding
After daily injection of saline (0.1 ml/10 g, po or ip) for 1 week, mice received daily administration with vigabatrin, gabapentin, or diazepam for 8 days, at the doses and routes described above (n = 10 per drug). For each drug, a control group (n = 10) was monitored in parallel, in which animals received saline instead of antiepileptic drugs. Mice were sacrificed by decapitation 24 h after the last administration and their brains were quickly removed, frozen in pulverized dry ice and stored at −70 °C. Frozen coronal sections of 20 μm were cut in a cryostat, thaw-mounted on gelatin-coated slides, and stored at −70 °C until the day of incubation.
Tris-HCl buffer (170 mM, pH 7.4) was used. Initially, brain sections were prewashed for 30 min at 25 °C. Then, brain sections were incubated during 45 min at 4 °C in a 2 nM [3H]flunitrazepam solution (88 Ci/mmol), in absence or presence of 1 μM of chlordiazepoxide. Binding obtained in presence of chlordiazepoxide was considered non-specific. Incubation was completed with 2 consecutive washes (1 min each) and a distilled water rinse (2 s) at 4 °C. The sections were then quickly dried under a gentle stream of cold air.
Slides were arrayed in X-ray cassettes with tritium standards (Amersham Biosciences, Piscataway, NJ, USA) and apposed to tritium-sensitive film (Amersham Ultrafilm) for 3 weeks at room temperature. Films were developed using standard Kodak D11 and fixer. Optical densities were determined using a video-computer enhancement program (JAVA Jandel Video Analysis Software; Jandel Scientific, Corte. Madera, CA, USA). Brain regions were localized by reference to the mouse brain atlas of CitationWilliam and colleagues (1999). For each structure, 10 optical density readings were taken from at least 5 sections and they were averaged. The optical density readings of the standards were used to determine tissue radioactivity values (dpm/mm2) for the accompanying tissue sections. Then, dpm/mm2 values were converted to fmol/mg protein by dividing dpm by the specific activity of [3H]flunitrazepam (88 Ci/mmol) by 2.22. The value 2.22 represents the conversion factor from Ci to dpm.
[3H]flunitrazepam binding was analyzed in the following structures: motor, sensorimotor, and cingulate cortices; caudate putamen, nucleus accumbens, dentate gyrus, and CA1–3 fields of hippocampus; medial, and basolateral amygdala nuclei; complete thalamus and hypothalamus.
Statistical analysis
The values obtained from PTZ-induced seizures and receptor binding were examined statistically by Student’s t tests.
Results
Saline group
All control animals (100%) pretreated with saline presented clonic seizures, and tonic extension after PTZ administration. The incidence of death was from 60% to 80%. Latencies (mean ± SD) for these PTZ-induced changes were as follows: clonic seizures, 50 ± 4 s; tonic extension, 527 ± 86 s; death, 636 ± 74 s (). Concerning BDZ binding, [3H]flunitrazepam binding was robust throughout the following evaluated brain areas: motor, sensorimotor, and cingulate cortices, dentate gyrus, CA1–3 fields of hippocampus, medial, and basolateral amygdala nuclei. [3H]flunitrazepam binding was mild to moderate at the level of caudate putamen, nucleus acumbens, thalamus and hypothalamus (-).
Figure 1 Effects of subchronic administration with diazepam (upper panel), vigabatrin (medium panel) and gabapentin (lower panel) on PTZ-induced convulsions. Results show the latency in seconds from the injection of PTZ (90 mg/kg ip) to first clonus, tonic extension and death.
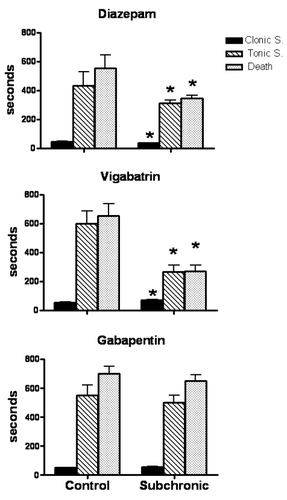
Table 1 [3H]Flunitrazepam binding (fmol/mg of protein) in brain areas of mice treated with subchronic administration of saline and diazepam (10 mg/kg ip)
Table 3 [3H]Flunitrazepam binding (fmol/mg of protein) in brain areas of mice treated with subchronic administration of saline and gabapentin (100 mg/kg po)
Effects of subchronic administration with diazepam
Mice pretreated subchronically with diazepam showed reduced latency to the first PTZ-induced clonus (21%, p < 0.05), tonic extension (27%, p < 0.05) and death (37%, p < 0.05) (). Mortality rate was similar to that found in the control group (80%).
Subchronic administration with diazepam induced a significant decrease of [3H]flunitrazepam binding in motor (25%, p < 0.05), sensorimotor (27%, p < 0.05) and cingulate (38%, p < 0.05) cortices, dentate gyrus (33%, p < 0.05), and CA1–3 fields of hippocampus (34%, p < 0.05), medial (41%, p < 0.05) and basolateral (40%, p < 0.05) amygdala nuclei, and hypothalamus (35%, p < 0.05) ().
Effects of subchronic administration with vigabatrin
Animals pretreated subchronically with vigabatrin presented enhanced latency to the first clonus (32%, p < 0.05), but shorter latencies for tonic extension (55%, p < 0.05) and death (58%, p < 0.05) (). Mortality rate was not significantly modified (60%) when compared with control group (70%).
Subchronic administration with vigabatrin tended to decrease [3H]flunitrazepam binding in motor (10%) and cingulate (6%) cortices; caudate putamen (18%) and nucleus accumbens (11%) ().
Table 2 [3H]Flunitrazepam binding (fmol/mg of protein) in brain areas of mice treated with subchronic administration of saline and vigabatrin (500 mg/kg po)
Effects of subchronic administration with gabapentin
Mice treated subchronically with gabapentin did not show significant changes in latency to the different components of PTZ-induced convulsions (). However, their mortality rate was lower (40%) when compared with control animals (60%).
Subchronic administration with gabapentin reduced [3H]flunitrazepam binding in the motor (20%, p < 0.05), sensorimotor (34%, p < 0.05) and cingulate (20%, p < 0.05) cortices; dentate gyrus (22%, p < 0.05) and CA1–CA3 fields of hippocampus (34%, p < 0.05), medial (27%, p < 0.05) and basolateral (33%, p < 0.05) amygdala nuclei, thalamus (45%, p < 0.05) and hypothalamus (38%, p < 0.05) ().
Discussion
There is growing awareness that antiepileptic drugs can sometimes worsen epileptic disorders (CitationPerucca et al 1998) by disrupting the equilibrium between excitatory and inhibitory circuits (CitationLoiseau 1998). In the present study, we found that mice pretreated subchronically with antiepileptic drugs enhancing GABAergic neurotransmission showed an augmented susceptibility to PTZ-induced seizures, reduced BDZ receptor binding, or both. These effects may be associated with a withdrawal-like syndrome as consequence of the sudden cessation of antiepileptic drugs.
PTZ is a noncompetitive GABA receptor antagonist that produces generalized seizures at high doses. The forebrain is involved in the expression of clonic seizures, whereas the activation of brainstem structures participates in the expression of the tonic component (CitationYonekawa et al 1980). We found that there is an aggravation of PTZ-induced seizures following the repetitive administration of diazepam or vigabatrin. The shorter latency to all the components of the PTZ-induced seizures after subchronic administration with diazepam could result from a higher activation of forebrain areas, while the facilitation of the tonic extension component and mortality after subchronic treatment with vigabatrin could be restricted to an overactivation of brainstem areas. These results support previous studies indicating that the abrupt discontinuation of long-term use of BDZ may produce status epilepticus (CitationGatzonis et al 2000) and a higher seizure susceptibility to bicuculline (CitationGallager et al 1985). Regarding vigabatrin, its repetitive administration leads to decreased electroconvulsive threshold (CitationLoscher 1982) and does not prevent the development of secondary spontaneous seizures as consequence of status epilepticus (CitationHalonen et al 2001).
Diazepam produces its overt effects by interacting with a specific recognition site on GABAA receptors in the mammalian central nervous system. Diazepam enters brain rapidly, and is also cleared rapidly, yielding desmethyldiazepam and oxazepam as metabolites in plasma and brain (CitationGreenblatt and Sethy 1990). However, the repetitive daily injection of diazepam is associated with a peak concentration at 2 h after injection and a progressive drug accumulation in brain (CitationFernandes et al 1999). This situation represents a marked fluctuation in receptor occupancy by diazepam over a 24-h period that may induce a pharmacological kindling effect (CitationArnot et al 2001), a critical condition for the higher susceptibility to PTZ-induced seizures observed in the present study.
The reduction in the BDZ receptor binding detected after the subchronic administration with diazepam is in agreement with the results obtained from other authors in mouse brain (CitationRosenberg and Chiu 1979, Citation1981a, Citation1981b; CitationGrimm and Hershkowitz 1981; CitationTietz et al 1986). Chronic treatment with diazepam has been shown to alter GABAA receptor affinity for the agonist GABA and some BDZ binding characteristics (CitationTietz et al 1989; CitationWu et al 1994). Indeed, repetitive daily injection of diazepam decreases GABA enhancement of BDZ binding (CitationArnot et al 2001). These receptor changes could be involved in the lower BDZ binding and the higher vulnerability to PTZ-induced convulsions detected after the repetitive administration with diazepam.
The pharmacodynamic effects of vigabatrin (inhibition of central and peripheral GABA-T and increase of brain GABA levels) remain after the drug has been eliminated from plasma (CitationBolton et al 1989). However, when treatment is continued for 8 days, more marked effects of vigabatrin on GABA-T and GABA, a more severe toxicity and higher vigabatrin plasma concentrations are observed (CitationValdizán and Armijo 1992; CitationValdizán et al 1999), and tolerance developed to the anticonvulsant effect of the treatment (CitationLoscher and Frey 1987). We found that the subchronic administration of vigabatrin did not modify the [3H]flunitrazepam binding, a finding that is in agreement with others authors (CitationHalonen et al 1991; CitationJackson et al 1994; CitationVerhoeff et al 1999). Probably, the magnitude of the GABA level increase by vigabatrin is not sufficient to induce changes in the BDZ receptor (CitationMaloteaux et al 1987). However, subcronic treatment with vigabatrin facilitated PTZ-induced seizures, an effect that could be explained by receptor desensitization or toxic effects. Indeed, in vigabatrin-treated animals, diazepam at high doses displayed lower anticonvulsant effects (CitationSchmid et al 1996).
Gabapentin was originally developed as a chemical analogue of GABA. However, different mechanisms are known to be involved in different therapeutic actions of this drug (CitationCheng and Chiou 2006). Its mechanisms of action appear to be a complex synergy between increased GABA synthesis, non-NMDA receptor antagonism and binding to the α2δ subunit of voltage dependent calcium channels. The latter action inhibits the release of excitatory neurotransmitters (CitationBennett and Simpson 2004). Gabapentin fails to influence GABA concentrations following both single and repeated administration in mice (CitationLeach et al 1997), but increases aminooxyacetic acid-induced GABA accumulation in several brain regions (CitationLoscher et al 1991). The plasma gabapentin half-life after oral administration in rodents is only about 2 h, and there is no evidence for accumulation of active metabolites (CitationVollmer et al 1986). Gabapentin exerts potent anticonvulsant effect in rats after 2 h, but this effect is completely lost after 9 h (CitationLoscher and Schmidt 1988). Our experiments revealed that subchronic administration with gabapentin did not modify the generalized seizures induced by PTZ, suggesting that its repetitive administration does not induce subsequent withdrawal-like effects.
It is known that the chronic exposition of an agonist produces an adaptation response that could be associated with receptor down-regulation or phosphorylation with a subsequent decrease in the response of involved system (CitationAlberts et al 1994). These mechanisms could explain the decreased BDZ binding after the repetitive administration of antiepileptic drugs enhancing the GABAergic system. The differential effects on PTZ-induced seizures produced after subchronic diazepam, vigabatrin, and gabapentin may result from changes in specific subunits that regulate the GABAA or BDZ binding. For example, mutations in the γ2 subunit may cause incorrect assembly of subunits with the receptor complex reducing sensitivity to BDZ, which would lead to an increase in neuronal excitability (CitationWu et al 2004). Similarly, deletion of GABAA receptor α1-subunit alters receptor subtype assembly, pharmacological and behavioral responses to BDZ (CitationKralic et al 2002). Concerning diazepam, its repetitive daily injection increases α1-subunit mRNA levels, an effect that may result in an increase in diazepam insensitive GABAA receptor subtypes (CitationArnot et al 2001). More experiments should be carried out to determine how the repetitive administration of antiepileptic drugs is associated with changes in GABAA receptor subunits leading to facilitation of seizure activity.
The mechanisms underlying the facilitation in seizure activity by antiepileptic drugs are poorly understood, but they may include the unmasking of secondary pharmacodynamic effects at high dosage or an undesired extension of the drug primary action to additional cell populations or neurotransmitters. Studies support the interactions between GABA and cannabinoid systems (CitationPertwee et al 1988; CitationWarnault et al 2007). In fact, activation of presynaptic cannabinoid type 1 (CB1) receptors induces robust inhibition of local GABAergic afferents or depolarization-induced suppression of inhibition (DSI) (CitationWilson et al 2001; CitationWilson and Nicoll 2001). It is possible that the abrupt cessation of GABAergic drugs induces a withdrawal syndrome associated with sensitized GABAergic synapses to the presynaptic effect of cannabinoid CB1 receptor stimulation (CitationCentonzene et al 2007). This situation may facilitate the DSI and the reduced threshold to the PTZ-induced seizures.
Our results provide evidence of a relationship between subchronic exposition of an antiepileptic drug and the decrease in the BDZ receptor binding, a phenomenon that could be involved in the mechanisms of pharmacoresistant epilepsy (CitationAicardi 1998; CitationJallon 1997; CitationLoscher et al 1997).
Acknowledgements
The author thanks Magdalena Briones, Leticia Neri-Bazán and Héctor Vázquez for their excellent technical assistance. This study was partially supported by CONACyT grant 45943-M.
References
- AicardiJClinical approach to the management of intractable epilepsyDev Med Child Neurol198830429403049187
- AlbertsBBrayDLewisJMolecular Biology of the Cell1994New York; USAGarland Publishing Inc72185
- AndrewsNZharkovskyAFileSEAcute handling stress downregulates benzodiazepine receptors: reversal by diazepamEur J Pharmacol1992210247511319335
- ArnotMIDaviesMMartinILGABA(A) receptor gene expression in rat cortex: differential effects of two chronic diazepam treatment regimesJ Neurosci Res2001646172511398186
- BennettMISimpsonKHGabapentin in the treatment of neuropathic painPalliat Med20041851114982201
- BoltonJBRimmerEWilliamsJThe effect of vigabatrin on brain and platelet GABA-transaminase activitiesBr J Clin Pharmacol198927Suppl135S42S2757907
- BraestrupCNielsenMSquiresRFNo change in rat benzodiazepine receptors after withdrawal from continuous treatment with lorazepam and diazepamLife Sci1979243475034764
- BrettRRPrattJAChanges in benzodiazepine-GABA receptor coupling in an accumbens-habenula circuit after chronic diazepam treatmentBr J Pharmacol19951162375848581272
- CalkinPABarnesEMGABAA agonists down-regulate GABAA/benzodiazepine receptor polypeptides from the surface of chick cortical neuronsJ Biol Chem19942691548538288621
- CentonzeDRossiSDe ChiaraVChronic cocaine sensitizes striatal GABAergic synapses to the stimulation of cannabinoid CB1 receptorsEur J Neurosci20072516314017408430
- ChengJKChiouLCMechanisms of the antinociceptive action of gabapentinJ Pharmacol Sci20061004718616474201
- FernandesCArnotMIIrvineEEThe effect of treatment regimen on the development of tolerance to the sedative and anxiolytic effects of diazepamPsychopharmacology (Berl)1999145251910494573
- GaleKGABA and epilepsy: basic concepts from preclinical researchEpilepsia199233Suppl 5S3S121330509
- GallagerDWLakoskiJMGonsalvesSFChronic benzodiazepine treatment decreases postsynaptic GABA sensitivityNature19843087476322004
- GallagerWDMalcolmBAAndersonASContinuous release of diazepam: electrophysiological, biochemical and behavioral consequencesBrain Res198534226362994822
- GatzonisDSAngelopoulosKEDaskalopoulouGEConvulsive status epilepticus following abrupt high-dose benzodiazepine discontinuationDrug Alcohol Depend20005995710706979
- GreenblattDJSethyVHBenzodiazepine concentrations in brain directly reflect receptor occupancy: studies of diazepam, lorazepam, and oxazepamPsychopharmacology (Berl)199010237381979181
- GrimmEVHershkowitzMThe effect of chronic diazepam treatment on discrimination performance and 3H-flunitrazepam binding in the brains of shocked and nonshoked ratsPsychopharmacology19817413266115444
- GyenesMFarrantMFarbDH“Run down” of GABAA receptor function during whole-cell recording- a possible role for phosphorylationMol Pharmacol198834719232849041
- HalonenTPitkanenASaanoVEffects of vigabatrin (gamma-vynil GABA) on neurotransmission-related amino acids and on GABA and benzodiazepine receptor binding in ratsEpilepsia19913224291672276
- HalonenTNissinenJPitkanenAChronic elevation of brain GABA levels beginning two days after status epilepticus does not prevent epileptogenesis in ratsNeuropharmacol20014053650
- HaefelyWKyburzEGereckeMRecent advances in the molecular pharmacology of benzodiazepine receptors and in the structure-activity relationship of their agonist and antagonistAdv Drug Res199514165322
- JacksonFMDennisTEsplinBAcute effects of gamma-vynil GABA (vigabatrin) on hippocampal GABAergic inhibition in vitroBrain Res199465185917922593
- JallonPThe problem of intractability: the continuing need for new medical therapies in epilepsyEpilepsia199738Suppl 9S37S429578544
- KralicJEO’BuckleyTKKhistiRTGABA(A) receptor alpha-1 subunit deletion alters receptor subtype assembly, pharmacological and behavioral responses to benzodiazepines and zolpidemNeuropharmacology2002436859412367614
- LeachJPSillsGJButlerENeurochemical actions of gabapentin in mouse brainEpilepsy Res199727175809237051
- LippertBMetcalfBJungMJ4-amino- hex-5-enoic acid, a selective catalytic inhibitor of 4- aminobutyric acid aminotransferase in mammalian brainEur J Biochem1977744415856582
- LoiseauPDo antiepileptic drugs exacerbate seizures?Epilepsia199839249578006
- LoscherWAnticonvulsant and biochemical effects of inhibitors of GABA amitransferase and valproic acid during subchronic treatment in miceBiochem Pharmacol198231837426805473
- LoscherWHönackDTaylorCPGabapentin increases aminooxyacetic acid-induced GABA accumulation in several regions of rat brainNeurosci Lett199112815041945036
- LoscherWAnimal models of intractable epilepsyProg Neurobiol199753239589364612
- LoscherWFreyHHOne to three day dose intervals during subchronic treatment of epileptic gerbils with gamma-vinyl GABA: anticonvulsant efficacy and alterations in regional brain GABA levelsEur J Pharmacol1987143335423691661
- LoscherWSchmidtDWhich animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerationsEpilepsy Res19882145813058469
- LukasSEGriffithRRPrecipitated diazepam withdrawal in baboons: effects of dose and duration of diazepam exposureEur J Pharmacol1984100163716428921
- MaloteauxJMOctaveJNGossuinAGABA induces down-regulation of the benzodiazepine-GABA receptor complex in the rat cultured neuronsEur J Pharmacol1987144173832830123
- OlsenWRAvoliMGABA and epileptogenesisEpilepsia1997383994079118844
- PeruccaEGramLAvanziniGAntiepileptic drugs as a cause of worsening seizuresEpilepsia1998395179578007
- PertweeRGGreentreeSGSwiftPADrugs which stimulate or facilitate central GABAergic transmission interact synergistically with delta-9-tetrahydrocannabinol to produce marked catalepsy in miceNeuropharmacology1988271265702854226
- RosenbergCHChiuHTDecreased3H-diazepam binding is a specific response to chronic benzodiazepine treatmentLife Sci197924803836541
- RosenbergCHChiuHTTolerance during chronic benzodiazepine treatment associated with decreased receptor bindingEur J Pharmacol1981a70453607238571
- RosenbergCHChiuHTRegional specificity of benzodiazepine receptor down-regulation during chronic treatment of rats with flurazepamNeurosci Lett1981b2449526267524
- SchechterPJTranierYGroveJMandelPDeFeudesFVAttempts to correlate alterations in brain GABA metabolism by GABA-T inhibitors with their anticonvulsant effectsGABA- biochemistry and CNS functions1979New YorkPlenum Pr4357
- SchmidLBottlaenderMBrouilletEVigabatrin modulates benzodiazepine receptor activity in vivo: a positron emission tomography study in baboonJ Pharmacol Exp Ther1996276977838786578
- Suman-ChauhanNWebdaleLHillDRCharacterization of 3H-gabapentin binding to a novel site in rat brain: homogenate binding studiesEur J Pharmacol19932442933018384570
- TaylorPCGeeSNSuTZA summary of mechanistic hypotheses of gabapentin pharmacologyEpilepsy Res199829233499551785
- TietzIERosenbergCHChiuHTAutoradiographic localization of benzodiazepine receptor downregulationJ Pharmacol Exp Ther1986236284923001290
- TietzEIChiuTHRosenbergHCRegional GABA/benzodiazepine receptor/chloride channel coupling after acute and chronic benzodiazepine treatmentEur J Pharmacol198916757652476326
- ValdizánEMArmijoJAEffects of single and multiple increasing doses of vigabatrin on brain GABA metabolism and correlation with vigabatrin plasma concentrationBiochem. Pharmacol199228214350
- ValdizánEMGarcíaAPArmijoJATime course of the GABAergic effects of vigabatrin: is the time course of brain GABA related to platelet GABA-transaminase inhibition?Epilepsia1999401062910448817
- VerhoeffGLPNPetroffCAOHyderFEffects of vigabatrin on the GABAergic system as determined by 123I-Iomazenil SPECT and GABA MRSEpilepsia1999401433810528940
- VollmerKOvon HodenbergAKölleEUPharmacokinetics and metabolism of gabapentin in rat, dog and manArzneimittelforschung19863683093730018
- WarnaultVHouchiHBarbierEThe lack of CB1 receptors prevents neuroadapatations of both NMDA and GABA(A) receptors after chronic ethanol exposureJ Neurochem20071027415217442049
- WilliamsRWWilliamsAGCapraTAtlas of the mouse brain [online]1999 Accessed on November 12, 2007.http://www.mbl.org/atlas/atlas.php
- WilsonRIKunosGNicollRAPresynaptic specificity of endocannabinoid signaling in the hippocampusNeuron2001314536211516401
- WilsonRINicollRAEndogenous cannabinoids mediate retrograde signalling at hippocampal synapsesNature20014105889211279497
- WuYRosenbergHCChiuTHRamsey-WilliamsVRegional changes in [3H]zolpidem binding to brain benzodiazepine receptors in flurazepam tolerant rat: comparison with changes in [3H]flunitrazepam bindingJ Pharmacol Exp Ther1994268675828113978
- WuJEllsworthKEllsworthMAbnormal benzodiazepine and zinc modulation of GABAA receptors in an acquired absence epilepsy modelBrain Res200410132304015193533
- YonekawaWDKupferbergHJWoodburyDMRelation between pentylenetetrazol-induced seizures and brain pentylenetetrazol levels in miceJ Pharmacol Exp Ther1980214589937400961
- YuRTickuMKChronic neurosteroid treatment produces functional heterologous uncoupling at the GABAA/benzodiazepine receptor complex in mammalian cortical neuronsMol Pharmacol199547603107700257