Abstract
Drug-resistant epilepsy can sometimes be treated by surgery. In these cases, an accurate identification of the epileptogenic area must be addressed before resection. Ictal SPECT is one of the presurgical evaluations that can be performed, but usually, the increase in the regional cerebral perfusion observed is produced by diffusion of ictal activity. Here we describe a patient studied with v-EEG and foramen ovale electrodes that suffered a seizure after intravenous infusion of etomidate. The sequence of etomidate administration, followed by radiotracer and seizure was good enough for us to suspect that a true initial ictal SPECT was observed. We have implemented a kinetic model with four compartments, previously described (CitationAndersen 1989), in order to estimate the fraction of hydrophilic radiotracer in the brain during the pre-ictal and ictal periods. This model has shown that the fraction of hydrophilic radiotracer during the seizure into the brain would be between 18.9% and 42.3% of total infused. We show the first true initial ictal SPECT demonstrated by bioelectrical recordings of the brain activity, obtained by a correct succession of events and compatible with theoretical data obtained from the kinetic model.
Introduction
Epilepsy is one of the most common neurological disorders, affecting almost 0.5%–1.0% of the general population (CitationTheodore et al 2006). Patients usually respond well to pharmacological treatment. However, approximately, 25%–30% of patients are drug-resistant (CitationEngel 1996a). For this group, surgery can be a suitable therapeutic option (CitationEngel 1992, Citation1996b; CitationWiebe et al 2001). Surgical treatment requires an exhaustive presurgical evaluation in order to identify the epileptogenic area and minimize the side effects of the operation. Presurgical evaluation usually includes, among others (CitationPastor et al 2005; CitationSola et al 2005), electroencephalography (EEG), video-electroencephalography (v-EEG), magnetic resonance imaging (MRI) and single photon emission computerized tomography (SPECT).
Interictal or basal SPECT is a presurgical study not performed systematically in the majority of epilepsy-surgery units because it is affected by a number of drawbacks that limits its results (CitationHenry and Heertum 2003). In order to overcome these problems, some epilepsy-surgery units perform ictal SPECT, in which a radiotracer is intravenously administered after a seizure onset is identified (CitationSpencer et al 2000; CitationVan Paessen et al 2000; CitationHenry and Heertum 2003; CitationCascino et al 2004). Ictal SPECT has been shown to be an accurate means of localization of temporal lobe seizures when the tracer is injected immediately after the seizure (CitationCatafau 2001; CitationVan Paessen 2004). The localizing information was better when the injection was given soon after seizure onset (CitationNewton et al 1992; CitationAvery et al 1999; CitationVan Paessen et al 2000).
Sometimes, during v-EEG recording and in order to increase the probability of seizures, pharmacologically induced stimulation has been performed using different drugs; ie, clonidine, metohexital, etc (CitationKofke et al 1993; CitationBrockhaus et al 1997; CitationSchmitt et al 1999). Among these, the use of etomidate, a nonbarbiturate hypnotic, short-lived imidazol derivate, has been rare (CitationEbrahim et al 1986; CitationModica et al 1990).
In the Hospital La Princesa, currently, we are characterizing the response to etomidate in patients with mesial temporal lobe epilepsy (MTLE) and undergoing evaluation for surgery while they are monitored with v-EEG using foramen ovale electrodes (FOE). It is not our objective to describe this method in detail (see Dominguez-Gadea et al 2006), but to show a fortunate result obtained in the context of that study, describing the case of a patient that suffered a seizure during etomidate infusion after the radiotracer injection for activated SPECT. This allowed us to obtain that which is, to our knowledge, the first true initial ictal SPECT (recorded during the true beginning of the seizure and not during the propagated ictal activity) described in the literature. We have implemented a numerical model previously published (CitationAndersen 1989) for the analysis and discussion.
Case report
A 14-year-old, right handed girl with a diagnosis of refractory temporal lobe epilepsy (TLE) was referred to the Epilepsy Unit, Hospital Universitario “La Princesa”, Madrid, Spain for evaluation and surgical treatment of epilepsy.
Pregnancy and birth were normal. No familial epilepsy history was reported. Seizures began at age 2 years, after a viral vaccination. Nowadays, she complains of daily seizures.
Previously tried anti-epileptic drugs (AED) included phenitoin, carbamacepine, etosuximide, zonisapride, and levetiracetam. At the present time pharmacological treatment included (mg/d): topiramate 300; oxcarbamazepine 1500; valproate 600; and clobazam 10.
Methods
Presurgical evaluation, following the previously described La Princesa Hospital protocol (CitationPastor et al 2005; CitationSola et al 2005), included neurological and neuropsychological examinations, scalp EEG (Nihon Kohden®) with 19 electrodes according the 10–20 international system, 1.5 T magnetic resonance imaging (MRI; General Electric®) and interictal single photon emission computerized tomography (SPECT, General Electric®) with 99mTc-D,L-Hexamethylene-propyleneamine oxime (99mTc-HmPAO) and video-electroencephalography (v-EEG; Easy II Cadwell®) using 19 scalp electrodes and foramen ovale electrodes (CitationPastor et al 2006) removing AED from the second to day of recording.
This research was approved by the Ethical Committee of the Hospital la Princesa and informed consent was obtained from the parents because the patient was under 18 years.
SPECT imaging studies were performed using 740 MBq of 99mTc-HmPAO, with a low-energy high-resolution collimator, simple-head camera (Starcam 3200, General Electric®) and 96 projections of 22 s each using a 64 × 64 matrix. Slices were reconstructed by filtered back projection using a Butterworth filter (order 10 with a 0.6 cut-off).
V-EEG recording was performed with a bandwidth of 1–70 Hz for scalp electrodes and 1–100 Hz for FOE recording, with a sampling rate of 200 Hz.
Etomidate (Janssen-Cilag®) was intravenously applied in the resting condition, with the patient resting in bed, face up, under continuous supervision of an expert anesthesiologist at a dose of 0.1 mg/kg. Perfusion was performed in approximately 1 min. Electrocardiogram and capillary oxygen saturation (pulsioximetry) were continuously monitored throughout the process. Immediately after the etomidate infusion, a bolus of 99mTc-HmPAO was intravenously injected. Brain SPECT was acquired in the 30 min after the complete recovery of the patient.
Kinetic model
To analyze the theoretical kinetic behavior of 99mTc-HmPAO, we used a numerical model including four compartments (see ) and four parameters (CitationAndersen 1989). It is important to keep in mind that the kinetic parameters are taken from biological data, but not adjusted for our patient. The basic assumption was that 99mTc-HMPAO behaves according to the microsphere model and exhibits high rate extraction (76%) by the brain tissue during the first microcirculatory pass (CitationLassen et al 1988). Numerical solutions of the model were obtained by developing a home-made program using the Runge-Kutta method for solving differential equations, implemented in MATLAB R2007a (MathWorks, Natic, USA) and running on a personal computer (Pentium IV, 1 Gb RAM).
Results
The scalp EEG showed the presence of frequent right temporal sharp waves, usually grouped in patterns, intermingled with irregular slow waves. MRI was normal and interictal SPECT showed inconclusive results, with left temporal antero-mesial hypoperfusion doubtful.
Etomidate was intravenously applied by an anesthesiologist (MLM) whereas the patient stayed in the v-EEG monitoring unit. Ten seconds later, 99mTc-HmPAO was iv perfused (LD-G) in order to obtain a pharmacologically activated SPECT. Fifty two seconds after 99mTc-HmPAO injection, a seizure begun with head version to left, followed by left arm hypertony, oral automatisms and mild disconnection. Fifty-two seconds thereafter, the seizure finished and the patient recovered consciousness. No stupor or paralysis was observed, but a mild disorientation and agitation persisted for 25 seconds.
v-EEG recording showed a beta/gamma low voltage pattern in the right posterior mesial area (right FO electrodes #1 to #3), as we can observe in . A focal desynchronization was observed in the right temporo-occipital area of the scalp.
Figure 1 Recording of v-EEG during administration of etomidate and the subsequent seizure observed. Discontinuous arrow marks the end of etomidate infusion. Continuous arrow shows the 99mTc-HmPAO infusion. Arrowhead indicates the start of seizure and double arrowhead marks the end of it.
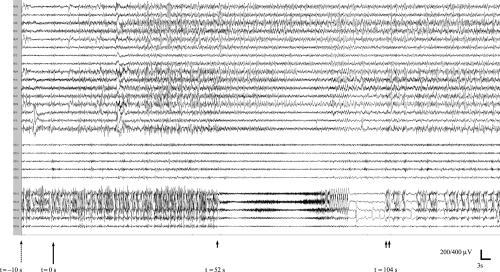
Figure 2 SPECT results and mathematical model of blood-brain 99mTc-HmPAO distribution. A) Basal (left) and etomidate-activated (right) SPECT recordings. The arrow shows the hyperperfusion on the right temporo-occipital area (around fusiform and lingual gyri) that corresponds to the most occipital FO electrodes. B) Kinetic model for numerical solution of distribution for lipophilic and hydrophilic 99mTc-HmPAO in blood and brain, according to a four-compartment model. The first-order rate constants are (min−1) k1 = 0.81; k2 = 0.35; k3 = 0.92 and k4 = 0.25 -CitationAndersen 1989; C) Kinetic evolution of the model during the first 3.5 min after 99mTc-HmPAO iv perfusion that shows the distribution in the different compartments; the broken-blue line represents the evolution of intracellular hydrophilic radio-tracer. The first arrow (from left) marks the start of seizure and the second one indicates the end.
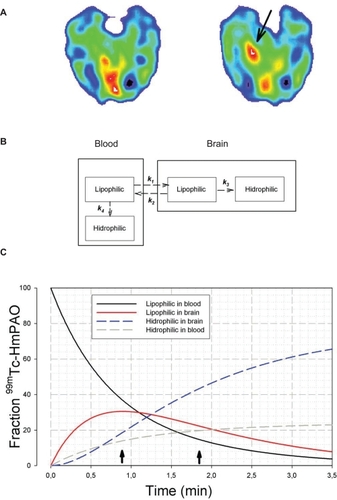
Injection of the radiotracer began immediately after etomidate and 52 sec. before a seizure started. A localized region of intense hyperperfusion was observed only in the right posterior mesial temporal lobe (, arrow). This region fits well with right FOE 1–3, which were involved in the origin of the seizure. No changes were observed in other regions compared with interictal SPECT performed after a 24-h seizure-free period.
The numeric simulation of the model (see ) showed that, in theory, at least, the hydrophilic brain concentration at the beginning of the seizure was approximately 18.9% of the total radiotracer injected, with only 36.6% remaining lipophilic in the blood. At the conclusion of the seizure, the theoretical brain hydrophilic concentration was 42.3% and only 15.3% of the radio-tracer persisted in its lipophilic form in the blood. This implies that practically all the available 99mTc-HmPAO had been extracted by the end of seizure. Of course, we are conscious that these results only show the compatibility between the theoretical model and the time course of events observed in our patient.
Discussion
This paper is the first report of an instance where the authors have knowledge of a true-early ictal SPECT, with the radiotracer injection performed at an adequate instant, demonstrated by means of bioelectrical recording.
An intense increase in regional cerebral blood flow is one of the hallmarks of partial-onset seizures. This is probably due to an increase in regional synaptic activity and changes in neurotransmission. Usually, the 99mTc-HMPAO infusion is performed briefly after the seizure starts and, moreover, a delay must be overcome before the radiotracer gets to the brain. So, ictal SPECT, although it is the most sensitive and reliable neuroimaging procedure for delineating the epileptogenic area in patients with medically intractable epilepsy (CitationBernal and Altman 2003), probably shows an increase in regional cerebral perfusion that is surely related to the seizure (CitationVan Paesschen 2004), but is almost certainly indicating propagation from the area of ictal onset.
What is more important, if the delay between seizure onset and injection is big enough, a false lateralization or localization on ictal SPECT due to the “postictal switch” phenomenon can be observed (CitationNewton et al 1992). Thus, the early injection of radiotracer has been considered the most important factor for seizure localization.
Taking into account that a majority of the radiotracer will be taken up in the first pass and that it takes around 30 seconds for the tracer to reach the brain, we can reasonably assume that a small amount of the ligand (<19%) probably would be absorbed previous to the seizure onset (pre-ictal state). However, as we can observe from , the most important amount of radiotracer will be taken up by the brain during the seizure (period between both arrows).
The safest and most reproducible way to control the moment of injection could be through the use of a pharmacological activation of the seizures. However, this possibility is far from being ready to use (CitationDiekman et al 1998; CitationKirchberger et al 1998). In the model that we are developing in order to overcome that problem, the infusion of etomidate induces important changes in the cerebral regional perfusion, but usually with no associated seizures (CitationDomínguez-Gadea et al 2007). Indeed, this case was the only one presenting a crisis out of 46 patients.
In summary, we are conscious that the case reported here was a lucky occurrence, in which the correct order of events allowed to us to demonstrate that an increase in regional cerebral perfusion would be produced in the cerebral area that started the seizure. The systematic methodology in pharmacological activated SPECT with etomidate could produce reproducible and consistent results even without pharmacologically activated seizures. However, more studies are needed before this point will be definitely established.
Acknowledgements
We thank American Journal Experts LLC for help with the English edition. This work was supported by a grant from the Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I), Instituto de Salud Carlos III, Subdirección General de Evaluación y Fomento de la Investigación PI060349.
References
- AndersenAR 99mTc-D,L-Hexamethylene-propyleneamine oxime (99mTc-HMPAO): basic kinetic studies of a tracer of cerebral blood flowCerebrovasc Brain Metab Rev198912883182701656
- AveryRASpencerSSSpanakiMVEffect of injection time on postictal SPECT perfusion changes in medically refractory epilepsyEur J Nucl Med199926830610436195
- BernalBAltmanNREvidence-based medicine: neuroimaging of seizuresNeuroimaging Clin N Am2003132112413677802
- BrockhausALehnertzKWiebruchCPossibilities and limitations of magnetic source imaging of methohexital-induced epileptiform patterns in temporal lobe epilepsy patientsElectroencephalog Clin Neurophysiol199710242336
- CascinoGDSoELBuchhalterJRThe current place of single photon emission computed tomography in epilepsy evaluationsNeuroimaging Clin N Am2004145536115324864
- CatafauAMBrain SPECT in clinical practice. Part I: PerfusionJ Nucl Med2001422597111216525
- DiekmanVBeckerWJürgensRLocalisation of epileptic foci with electric, magnetic and combined electromagnetic modelsElectroencephalogr Clin Neurophysiol19981062973139741758
- Domínguez-GadeaLPastorJMeilánMLSPECT de perfusión cerebral con etomidato en el estudio prequirúrgico de la epilepsia temporalRev Esp Med Nucl20072628
- EbrahimZYDeBoerGELudersHEffect of etomidate on the electroencephalogram of patients with epilepsyAnesth Anal19866510046
- EngelJJrRecent advances in surgical treatment of temporal lobe epilepsyActa Neurol Scand1992140Suppl 57180
- EngelJJrIntroduction to temporal lobe epilepsyEpilepsy Res1996a26141508985696
- EngelJJrCurrent concepts: surgery for seizuresN Engl J Med1996b1064752
- HenryTRvan HeertumRLPositron emission tomography and single photon emission computed tomography in epilepsy careSemin Nucl Med2003338810412756642
- KirchbergerKSchmittHHummelCClonidine- and Methohexital-induced epileptiform discharges detected by magnetoencephalography (MEG) in patients with localization-related epilepsiesEpilepsia1998391104129776332
- KofkeWADasheiffRMDongMLAnaesthetic care during thipental test to evaluate epileptic patients for surgical therapyJ Neurosurg Anaesthesiol1993516470
- LassenNAAndersenARFribergLThe retention of (99mTc)-d,l-HM-PAO in the human brain after intracarotid bolus injection: a kinetic analysisJ Cereb Blood Flow Metab198881320
- ModicaPATemplehoffRWhitePFPro-and anticonvulsant effects of anestheticsAnesth Analg199070433442180345
- NewtonMRBerkovicSFAustinMCPostictal switch in blood flow distribution and temporal lobe seizuresJ Neurol Neurosurg Psychiatr19925589141431952
- PastorJde la PridaLMHernandoVVoltage sources in mesial temporal lobe epilepsy recorded with foramen ovale electrodesClin Neurophysiol200611726041417029955
- PastorJHernando-RequejoVDominguez-GadeaLImpact of experience on improving the surgical outcome in temporal lobe epilepsyRev Neurol200547091616355354
- SchmittHDruschkyKHummelCDetection of an epileptic mirror focus after oral application of clonidineBr J Anaesth1999833495110618959
- SolaRGHernando-RequejoVPastorJPharmacoresistant temporal-lobe epilepsy. Exploration with foramen ovale electrodes and surgical outcomesRev Neurol20054141615999323
- SpencerSSBautistaREFunctional neuroimaging in localization of the ictal onset zoneAdv Neurol2000832859610999211
- TheodoreWHSpencerSSWiebeSEpilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health OrganizationEpilepsia20064717002217054693
- Van PaesschenWDupontPvan HeerdenBSelf-injection ictal SPECT during partial seizuresNeurology2000541994199710822442
- Van PaesschenWIctal SPECTEpilepsia200445Suppl 4354015281956
- WiebeSBlumeWTGirvinJPEffectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsyN Engl J Med20015311811484687