Abstract
Hypnotic effects of melatonin and melatoninergic drugs are mediated via MT1 and MT2 receptors, especially those in the circadian pacemaker, the suprachiasmatic nucleus, which acts on the hypothalamic sleep switch. Therefore, they differ fundamentally from GABAergic hypnotics. Melatoninergic agonists primarily favor sleep initiation and reset the circadian clock to phases allowing persistent sleep, as required in circadian rhythm sleep disorders. A major obstacle for the use of melatonin to support sleep maintenance in primary insomnia results from its short half-life in the circulation. Solutions to this problem have been sought by developing prolonged-release formulations of the natural hormone, or melatoninergic drugs of longer half-life, such as ramelteon, tasimelteon and agomelatine. With all these drugs, improvements of sleep are statistically demonstrable, but remain limited, especially in primary chronic insomnia, so that GABAergic drugs may be indicated. Melatoninergic agonists do not cause next-day hangover and withdrawal effects, or dependence. They do not induce behavioral changes, as sometimes observed with z-drugs. Despite otherwise good tolerability, the use of melatoninergic drugs in children, adolescents, and during pregnancy has been a matter of concern, and should be avoided in autoimmune diseases and Parkinsonism. Problems and limits of melatoninergic hypnotics are compared.
Introduction
Insomnia is a highly common disorder, which is experienced by almost everybody, at least at advanced age, and becomes chronic in about 10% of the population. Because of the transient nature of its milder forms, its importance is frequently underrated. On the other hand, the treatment of severe sleep disturbances, such as primary chronic insomnia, is challenging and frequently complicated by comorbid symptoms.Citation1–Citation3 The etiology of insomnia is obviously divergent. It is sometimes related to psychiatric or neurologic diseases that may develop already in younger or middle-aged subjects. Moreover, it may be acquired as a consequence of neurodegenerative disorders including Alzheimer’s disease,Citation4 especially when the circadian pacemaker, the suprachiasmatic nucleus (SCN), or its downstream connections are affected.Citation5–Citation7 Circadian rhythm sleep disorders (CRSDs) may be present or develop independently of neurodegeneration. In particular, familial advanced sleep phase syndrome (FASPS) and delayed sleep phase syndrome (DSPS) are characterized by exceptionally short or long spontaneous circadian period lengths. Other circadian disorders are related to weak coupling with external time cues, eg, in some blind subjects. Typically, CRSDs cause transient or periodically occurring forms of insomnia.Citation8–Citation10 For the circadian system, a possible mode of intervention is that of favoring synchronization with the environment. Apart from bright light in the morning, ie, enhancement of Zeitgeber strength to reinforce coupling with light onset, melatonin may be administered in the evening to make use of the re-synchronizing, chronobiotic as well as sleep onset-promoting properties of this molecular mediator of the darkness signal. In fact, melatonin was shown to be effective in the treatment of various forms of CRSDs.Citation11–Citation14
While the use of the chronobiotic melatonin in CRSD is plausible for mechanistic reasons, its application in other types of insomnia does not warrant immediate success, but has been worthy of exploration. In neurobiological terms, the actions of melatonin on sleep are largely of a chronobiological nature. High densities of the membrane-bound, G protein-coupled melatonin receptors MT1 and MT2 are found in the SCN, where the pineal hormone acts in a dual way, by resetting the clock – mainly via MT2 – and by suppressing neuronal firing – mainly via MT1.Citation15–Citation19 Leaving aside some complexities of the signaling mechanisms,Citation19 the MT1-mediated effects of melatonin on the SCN favor sleep initiation especially, but perhaps not exclusively via the hypothalamic sleep switch. This structure exhibits on-off responsesCitation20–Citation22 and suppresses, under the influence of melatonin, the wake-related neuronal downstream pathways (“off”) and promotes the sleep-related ones (“on”).Citation23,Citation24 However, sleep is a complex phenomenon that involves numerous brain regions. Melatonin receptors have been detected in various parts of the brain, but receptor densities are considerably lower than in the SCN.Citation25–Citation28 The thalamus has been assumed to be also involved in soporific actions of melatonin.Citation29,Citation30 Melatonin receptors are expressed in this region, and spindle formation is promoted by the indoleamine.Citation29–Citation31 Spindles are characteristics of non-REM (rapid eye movement) sleep, and are involved in the transition from stage 2 sleep to deeper sleep stages. However, a major problem for judging the relative importance compared to the primary SCN-mediated effects results from the complexity of the neuronal connections. Apart from the thalamocortical interplay, which is necessary for spindle formation, the thalamus also influences the SCN. Inputs to the SCN are known from various other brain areas, too, especially from the intergeniculate leaflet,Citation30 which is connected to many parts of the brain and also receives a photic input.Citation32 At the present state of our knowledge, the problem remains as to what extent the thalamus and other brain areas may assist the SCN by transmitting melatonin-dependent responses, and whether SCN-independent actions of melatonin are sufficient for sleep promotion. In individuals with severe SCN dysfunction and melatonin deficiency, exogenous melatonin was found to be insufficient for substantially mitigating sleep difficulties.Citation33 However, SCN destruction, which causes sleep fragmentation and losses of circadian rhythmicity, still allows spindle formation.Citation30 Another source of complexity results from the necessary integration of primarily chonobiotic and homeostatic components of sleep regulation. The homeostatic mechanism also comprises a circadian component,Citation24,Citation34,Citation35 and the existence of a separate homeostatic oscillator has been proposed.Citation36 The extent of melatonin’s influences on homeostatic sleep may deserve further attention. At least, melatonin has been reported to be useful under conditions of an insufficient homeostatic drive to sleep.Citation37 Despite the highly complex interplay of brain areas during sleep, and the existence of presumably multiple inputs from melatonin, primary and secondary actions have to be distinguished. The phase-resetting effects are relatively well understood and a participation of the SCN in sleep initiation cannot be denied. Melatoninergic actions in other brain areas and their contribution to sleep require further elucidation. With regard to the high receptor density and the knowledge of SCN-mediated actions, the influence of melatonin on the circadian pacemaker will be the focus of our considerations.
Melatonin differs in its mode of action from other hypnotics such as benzodiazepines and z-drugs (zolpidem, zaleplon, zopiclone, eszopiclone), which lead to a more generalized central nervous depression via GABAA receptors. Melatonin is capable of indirectly influencing GABAergic mechanisms involved in sleep-related routes downstream of the SCN.Citation20–Citation22 Indirect GABAergic effects in other brain areas may, possibly, play an additional role. Only at strongly elevated pharmacological concentrations can melatonin exert more generalized sedative or even narcotic effects, which are, however, mediated by other mechanisms, such as antiexcitatory suppression of calcium signaling and inhibition of neuronal NO synthase.Citation38 Moreover, melatonin contrasts with benzodiazepines and z-drugs with regard to sleep architecture, ie, the relative duration of sleep stages (stages 1–4), which differ in sleep depth and undergo an ultradian REM/nonREM cycle of about 90 minutes duration. While sleep architecture can be considerably changed by GABAergic drugs, the ultradian cycle is usually poorly influenced by melatonin, perhaps because this periodicity is generated by another, the pontine sleep switch,Citation39,Citation40 which does not seem to be a major target of melatonin. However, melatonin was reported to increase REM sleep duration in a subgroup of patients with reduced REM sleep.Citation41 In this context, the SCN is, again, not independent of inputs from other brain areas, since certain SCN neurons were found to fire more rapidly during REM than nonREM phases,Citation24 notwithstanding the primarily suppressive MT1 signaling. Therefore, these changes within the REM/nonREM cycle do not reflect direct melatoninergic actions, although they are relevant to sleep and may be indirectly influenced by the hormone.
In CRSDs, a melatonin surge of relatively short duration can be sufficient for resetting the circadian clock, at least when applied in a suitable phase of the phase-response curve. However, in primary chronic insomnia, the major obstacle for the use of melatonin as a clinically efficient hypnotic drug was assumed to result from its extremely short half-life in the circulation, which is mostly in the range of 20 to 30 minutes, sometimes even less, but maximally about 45 min.Citation20–Citation22,Citation42 Although a short-acting compound may promote sleep initiation, it can improve sleep maintenance only marginally. Theoretically, this problem has two solutions. One is a prolonged-release formulation of melatonin, the other the development of long-acting melatoninergic agonists. Both possibilities have been studied and given rise to the production of approved or investigative drugs. Their relative advantages will be discussed and, where appropriate, also compared to the nonmelatoninergic, primarily GABAergic, hypnotics that are currently in use.
Signaling and pharmacology of melatoninergic agonists
At therapeutic doses, the hypnotic actions of melatonin and synthetic melatoninergic drugs are mediated by the membrane receptors MT1 and MT2, as outlined above. In addition to the first-discovered agonist-dependent decreases in cAMP, a more complex system of signaling routes has been identified that contributes to the cellular effects.Citation19 These include phospholipase C activation, in the case of MT2, and control of inward rectifier K+ (Kir) channels, with secondary effects on voltage-gated Ca2+ channels, by MT1. Citation19 These last actions may be particularly relevant to the suppression of neuronal firing and, thereby, contribute to sleep induction via the hypothalamic sleep switch.
While phase shifting and neuronal suppression in the SCN represent a basis of hypnotic actions of all melatoninergic drugs, sleep research literature frequently ignores the fact that the membrane-bound melatonin receptors are not restricted to the SCN. Even though receptor density may be lower in other target tissues or cells, any melatoninergic agonist has to be expected to exert additional effects via these receptors, eg, in the immune system, the gastrointestinal tract, the vasculature, other central nervous structures and various hormonal subsystems.Citation18,Citation19,Citation43–Citation45 Therefore, by contrast with other hypnotics, any of the melatoninergic drugs is, for fundamental reasons, not only a soporific agent, but also a regulator of other physiological functions. These additional effects, which are frequently disregarded, may not always be beneficial, especially in patients suffering from autoimmune diseases or Parkinson’s disease (see following sections).
While signaling and distribution of MT1 and MT2 receptors discriminate melatoninergic agonists from GABAergic hypnotics, melatonin also differs from its synthetic analogs in the spectrum of binding sites. Several other melatonin-binding sites beyond the G protein-coupled MT1 and MT2 receptors have been identified,Citation19,Citation44,Citation45 which either display negligible affinity to the synthetic analogs, or have not yet been tested. These additional binding sites include quinone reductase 2 (formerly believed to represent a third membrane receptor), nuclear receptors belonging to the retinoic acid receptor superfamily, in particular, RORα1, RORα2, RZRα and RZRβ, calcium-binding proteins such as calmodulin (presumably requiring pharmacological levels because of low affinity), calreticulin, nuclear calreticulin analogs, and two mitochondrial binding sites, one of which is located at the amphipathic ramp of complex 1 and displays high affinity to the indoleamine.Citation19,Citation21,Citation43–Citation45 The majority of synthetic agonists has not been tested for these binding sites, with the exception of ramelteon, which has a low affinity to quinone reductase 2,Citation46 and does not seem to act via calmodulin.Citation21
In addition to its direct actions, melatonin is metabolized to various bioactive compounds, including indolic (eg, 5-methoxytryptamine, N-acetylserotonin) and kynuric [N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK)] substances and their derivatives (Figure ).Citation43–Citation45 For reasons of chemical dissimilarity, no homologs of these melatonin metabolites can be formed from nonindolic drugs. Among the hypnotics tested, only the investigative drug β-methyl-6-chloromelatonin might lead to some homologous derivatives (Figure ).Citation47 In conclusion, the full spectrum of actions known from melatonin, which also comprises various beneficial effects,Citation38,Citation44 cannot be expected to be found with nonindolic hypnotics. On the other hand, those drugs showing selectivity towards MT1 and MT2 receptors also exert effects beyond sleep promotion.
Figure 1 Chemical structures and main metabolic routes of melatonin and synthetic melatoninergic hypnotics. Flashes = sites of metabolic reactions.
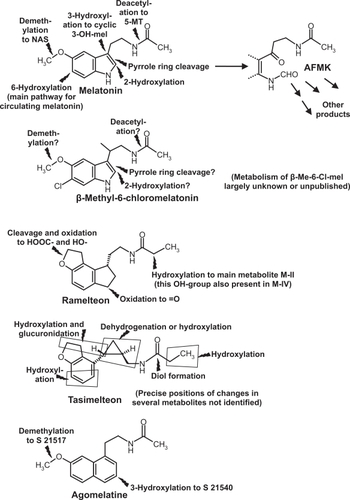
The various melatoninergic agonists tested for soporific effects exhibit substantial differences in receptor affinity, half-life, metabolism, and contribution of metabolites to sleep promotion. Melatonin itself has different affinities to human MT1 and MT2 receptors (Ki = 80.7 and 383 pM, respectively).Citation21,Citation47 Its physiological half-life in the circulation is, as mentioned, usually less than half an hour, mainly because of rapid hepatic 6-hydroxylation by cytochrome P450 monooxygenase subforms, in particular, CYP1A2, but also CYP1A1 and CYP1B1.Citation48,Citation49 6-Hydroxymelatonin is conjugated and excreted. In other tissues, especially the brain, melatonin can be metabolized differently.Citation43 No soporific effects are known from any of melatonin’s natural metabolites, except for 5-methoxytryptophol.Citation50 The sleep-related effects of this compound are presumably without physiological significance. However, the indolic, partially serotoninergicCitation43 metabolites, 5-methoxytryptamine and N-acetylserotonin, should be tested in more detail for possible interferences with sleep or wakefulness.
All these considerations are relevant to immediate and prolonged-release melatonin as well, with a main difference in bioavailability. Among the various formulations used in different studies, the brand Circadin® will be particularly considered, because of its approval by the European Medicines Agency (EMEA). Circadin® has been developed by Neurim, Israel and UK (marketing authorization holder) and is now also provided by Lundbeck and by Nycomed. In April 2007, it received marketing authorization by EMEA for the treatment of insomnia in patients aged 55 years and over. It was licensed for the combination of improvement of sleep quality and next-day feeling. The pharmacokinetics of Circadin®, which requires more detailed future investigation, has been tested in 8 healthy male subjects receiving 1, 2, 4 or 8 mg of the prolonged-release formulation at 10 am, either in conjunction with fasting or with a standard meal.Citation51 The unusual time of administration was obviously chosen to better demonstrate the efficacy of the slow-release formulation and to avoid interference with the endogenous melatonin peak. Normally, melatonin is given shortly (30 minutes) before bedtime, according to the time profile of the natural hormone, which exhibits, at least, in healthy nonelderly subjects a pronounced nocturnal peak. However, the pharmacokinetics of melatonin may differ between daytime and nighttime hours, according to data from rats which received continuous infusions of melatonin.Citation52 This possibility should be considered in humans, too. Under the conditions tested in humans, 2 mg led to a shift of Tmax from about 4 pm to about 11.30 am (without meal) or 12.30 pm (with meal).Citation51 These values should not be overinterpreted, since peaks resulting from the drug should not be compared with the physiological nocturnal maximum. Cmax values, as presented, have to be regarded as preliminary, since they showed considerable variation among the relatively few volunteers [without drug: range 30 to 126 pg/mL (median 51 pg/mL); drug and fasting: range 180 to 855 pg/mL (median 393 pg/mL); drug with meal: 205 to 1020 pg/mL (median: 390 pg/mL)]. In the 24 h-AUC values, a considerable interindividual variation was, again, observed [basal: range 150 to 1017 pg ×h/mg (median 375 pg × h/mg); drug and fasting: range 823 to 4478 pg × h/mg (median 2257 pg × h/mg); drug with meal: range 618 to 5252 pg × h/mg (median 2010 pg × h/mg)]. These data merely show that this melatonin formulation causes increases in blood levels, what had to be expected, but the improvements in duration of elevated bioavailability, compared to immediate-release melatonin, are not sufficiently evident from published data. Tmax and Cmax values are not suitable for judging the advantage of prolonged release. Elimination time has been inferred to be the same (t1/2 about 40 to 50 minutes) as with conventional melatonin preparations,Citation51 although this may be dose-dependent. In another study on healthy volunteers of both genders, aged 55 to 69 years, the effect of food on AUC after 2 mg Circadin® revealed only minor changes.Citation51 A major difficulty in interpreting the pharmacokinetic data results from the very high interindividual variability. This is not uncommon with melatonin in general and is usually explained by differences in the rapid first-path hepatic metabolism of the hormone. Whether this is really so, especially when authors are claiming a more than 80% elimination via 6-hydroxylation and conjugation,Citation51 may be debated. Additional variation may result from the gut, which is both a source and sink of melatonin,Citation53–Citation56 allows enterohepatic cycling of this compound,Citation55–Citation58 contains by two orders of magnitude more melatonin than the pineal gland,Citation54 and can release melatonin in terms of a postprandial response.Citation54,Citation55,Citation59 Gastrointestinal release of melatonin in response to tryptophan was associated with profound sleep promoting effects.Citation53 Because of these complexities and difficulties concerning pharmacokinetics, the advantages of prolonged-release melatonin should be judged rather from the effects on sleep.
Sustained-release formulations different from Circadin® have been also tested with regard to their pharmakokinetics, including coated sugar spheresCitation60 and solid lipid nanoparticles.Citation61 However, their clinical use is not sufficiently established, so that they will not be considered here in detail.
Among the synthetic analogs that have been clinically tested, the investigative drug β-methyl-6-chloromelatonin (LY 156735) is that one most related to melatonin. This agonist developed by Eli Lilly also diplays a high affinity towards MT1 und MT2 receptors (Ki = 81 pM for MT1 bzw. 42 pM for MT2). The preferential binding to MT2 is typical for the 6-chlorinated melatonin derivatives, and also seen with 6-chloromelatonin.Citation47 Because of the substitution at C-atom 6, this drug cannot be converted by the respective CYP isoforms to 6-hydroxymelatonin and its half-life in the circulation is, therefore, extended.Citation47,Citation62 Although β-methyl-6-chloromelatonin was effective in phase-shifting the circadian rhythm and showed sleep latency-reducing properties similar to those of melatonin, its effects on sleep maintenance remained marginal, even at doses of 20 or 100 mg,Citation47,Citation62,Citation63 so that this drug will not be further considered in this review.
Much more detailed information is available on a structurally dissimilar melatoninergic agonist, ramelteon {= Rozerem® = TAK-375 = (S)-N-[2-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-yl)ethyl]propionamide}, produced by Takeda Pharmaceuticals Inc. This compound has been approved in 2005 by the FDA for treatment of insomnia in the USA. After a negative recommendation by EMEA,Citation64 Takeda has withdrawn its European marketing authorization application in September 2008.Citation65 The affinities of ramelteon to MT1 und MT2 receptors (Ki = 14 pM and 112 pM, respectively) are higher than those of melatonin (cf Ki = 80,7 and 383 pM).Citation46,Citation66,Citation67 Contrary to melatonin and other indolic analogs, such as N-acetylserotonin, it does not bind to quinone reductase 2, at therapeutic doses (Ki = 2.65 μM; cf melatonin: Ki = 24 pM).Citation46 It displays very moderate binding to the serotonin receptor 5-HT1A (Ki = 5.6 μM), but virtually none to other 5-HT receptor subtypes. Numerous other receptors, eg, for neurotransmitters, neuropeptides including endorphins have been tested and reported to have no substantial affinity.Citation67 However, other melatonin binding sites, as mentioned above, have not been investigated.
Pharmacokinetics and metabolism of ramelteon have been studied in detail. It is rapidly taken up, reaching Tmax between 0.75 and 0.94 h, over a considerable dose range.Citation68 Its half-life in the circulation amounts to about 1 to 2 h and is, therefore, considerably longer than that of melatonin.Citation68 After escalating doses of 4, 8, 16, 32, and 64 mg, Cmax values of 1.15, 5.73, 6.92, 17.4, and 25.9 ng/mL, and AUC values of 1.71, 6.95, 9.88, 22.5, and 36.1 ng ×h/mL were obtained.Citation68 Because of its structural dissimilarity, the metabolism of ramelteon fundamentally deviates from that of melatonin.Citation21,Citation43,Citation47,Citation68 Although it is substrate to cytochrome P450 enzymes, including the melatonin-hydroxylating CYP1A2, the products are substantially different. Four metabolites formed by CYP1A2, CYP2C and CYP3A are usually referred to as M-I, M-II, M-III und M-IV. Apart from cleavage of the tetrahydrofuran ring in M-I, hydroxylations and oxidations take place in positions not accessible in melatonin, either at a C-atom corresponding to the nitrogen in melatonin’s pyrrole ring (M-III, M-IV) or at C-atom 2 of the propionyl residue of the aliphatic side chain (M-II, M-IV) (Figure ).Citation43,Citation47 Because of the absence of a pyrrole ring, no kynuric metabolism is possible in the case of ramelteon.Citation43,Citation47
The properties of metabolite M-II are rather unusual and substantially contribute to the pharmacological activity of the parent compound. This is not particularly surprising, since M-II differs from ramelteon only by the hydroxyl group in the aliphatic side chain. On the one hand, this change reduces the affinities to MT1 und MT2 receptors by a factor of about 10, but, on the other hand, M-II itself is much less metabolized, has a half-life 2 to 5 h longer than the parent compound and can attain concentrations by 20- to 100-fold higher than ramelteon.Citation68 Therefore, any pharmacokinetic consideration of ramelteon cannot be made without considering the long-lasting contribution of M-II. Consequently, judgments on the time course of action should not be restricted to Tmax, Cmax and AUC values of ramelteon alone.
Another newly introduced synthetic melatoninergic agonist is tasimelteon (= N-{[(1R,2R)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl}propanamide = VEC-162; earlier research codes: BMS-214778 and MA-1). This investigative drug is produced and being further developed by Vanda Pharmaceuticals, under license from Bristol-Myers Squibb Co. Binding and pharmacokinetic properties in humans have been disclosed only in part, although the company may possess more detailed information. According to unpublished information cited elsewhere,Citation69 tasimelteon is selective for MT1 and MT2 receptors. In a web appendix, the following affinity data were presented: pKi = 9.45 ± 0.04 (0.35 nM) for MT1, and pKi = 9.8 ± 0.07 (0.17 nM) for MT2, Citation70 without experimental details. After single oral doses between 10 and 100 mg, mean Tmax values varied from 1.9 to 3.0 h, mean Cmax from 59.1 to 417.1 ng/mL, and AUC from 171.1 to 1916.1 ng ×h/mL, however, with considerable interindividual deviations.Citation70 More detailed pharmacokinetic data have been presented for rats and monkeys (Macaca fascicularis).Citation71 These data indicate rapid uptake (monkeys, at moderate dose, Tmax in the range of 1 h) and longer half-life than melatonin (t1/2 about 2 to 3 h). A longer half-life mentioned elsewhere, without precise values,Citation72 may have referred to the human. The metabolism, studied in rat, monkey and human liver, showed degradation by CYP1A1, CYP1A2, CYP2D6, and CYP2C9, and also some conjugation with glucuronic acid.Citation71,Citation73 Most of the metabolites have only been partially characterized and are, again, nonhomologous to those of melatonin (Figure ), for fundamental reasons. Properties and kinetics of the tasimelteon metabolites are either unknown or not disclosed.
Agomelatine {= Valdoxan® = N-[2-(7-methoxynaphth-1-yl)ethyl]acetamide = S20098}, developed by Servier, is also an agonist at MT1 and MT2 receptors (Ki = 61.5 pM and 268 pM, respectively), but additionally acts as a 5-HT2C receptor antagonist (IC50 = 270 nM), with low affinities to other 5-HT receptor subforms.Citation21,Citation43,Citation47,Citation74 5-HT2C inhibition is largely responsible for the additional antidepressant action of this drug. The metabolism involving CYP1A1, CYP1A2, and CYP2C9 is partially different from that of melatonin (Figure ). One main metabolite (S22153) is hydroxylated at the second ring carrying the long side chain, another one demethylated, corresponding to the formation of N-acetylserotonin from melatonin. The demethylated compound (S21517) resembles serotonin with regard to the presence of the hydroxyl group and, in fact, displays affinity to 5-HT2C. Citation21,Citation43 Since agomelatine is not exclusively a melatoninergic drug, it should not be regarded simply as a sleep-promoting compound suitable for treating an average insomniac, but may be of specific value in depressed patients. In February 2009, Valdoxan® was approved by EMEA for the treatment of major depressive episodes (MDE) in adults,Citation75 but not generally as a hypnotic agent. Therefore, it will not be discussed here in any detail, despite its undoubtedly existing soporific actions.
Efficacy of melatoninergic hypnotics
All drugs mentioned in the previous section have been tested for their soporific potential. First of all, one has to distinguish between sleep-promoting effects in patients with CRSDs and others suffering from primary chronic insomnia. Any of the melatoninergic drugs is effective in phase-shifting circadian rhythms and, thus, seems suitable for treating jet lag and CRSDs, at least from the hypnotic point of view, but not necessarily under aspects of long-term safety.Citation21,Citation22,Citation47,Citation69 Since acute phase shifting and facilitation of sleep onset are also achieved by immediate-release melatonin, advantages of prolonged-release melatonin or longer-acting synthetic analogs should rather be sought in the treatment of primary chronic insomnia. β-Methyl-6-chloromelatonin was only marginally efficient in sleep maintenance,Citation47,Citation63,Citation63 and, in terms of published evidence, tasimelteon has been only tested after artificial light-dark shifts.Citation69 For reasons mentioned, the use of agomelatine should be only considered in conjunction with depression. Therefore, it seems primarily important to compare prolonged-release melatonin, such as Circadin®, and its synthetic analog, ramelteon (Rozerem®), with a focus on primary chronic insomnia.
Studies on prolonged/extended-release melatonin have been conducted in the past using different preparations, sometimes referred to as controlled, sustained or slow release. Different doses had been used and some formulations contained combinations fast (1 mg) and controlled-release components (4 mg). Citation76 The different studies are highly diverse, frequently of exploratory nature, or related to various disorders. Trials on larger numbers of subjects were only based on subjective measures. As compared to study design and detailed information on other hypnotics, the evidence is often circumstantial and sometimes contradictory. A conceptual diversity is even apparent in the material summarized by EMEA on Circadin®.Citation51 This material includes various exploratory and extended studies, including a phase III trial. The larger clinical studies on Circadin®,Citation51,Citation77,Citation78 conducted on several hundred elderly patients (55 years and older) with primary insomnia are randomized, placebo-controlled and double-blind, but not generally with crossover design, and mainly based on questionnaires only (Leeds Sleep Evaluation Questionnaire = LSEQ; sometimes also Pittsburgh Sleep Quality Index = PSQI, WHO-5 well being index, and Clinical Global Improvement scale = CGI). Taken together with exploratory studies also using polysomnography (PSG) or wrist actigraphy,Citation51,Citation79 the data collectively show that prolonged-release melatonin/Circadin® significantly reduces sleep onset latency (SOL), whereas direct evidence for the support of sleep maintenance and total sleep time is poor. Changes in awakenings from sleep are sometimes not statistically demonstrable, but may be deduced from patients’ reports on improvements of sleep quality.Citation51,Citation77,Citation78 More direct support, based on objective measures, for this important aspect of primary insomnia would be welcome. It should be also mentioned that the percentage of nonresponders to melatonin was substantial.Citation51 In this context, the improvements obtained by the prolonged-release formulation should be decisive. Reductions in sleep latency are well known for fast-release melatonin, and have to be also expected for prolonged-release pills, especially as the amounts required for promoting sleep initiation are relatively low.Citation80,Citation81 Since the development of prolonged-release melatonin was aiming to support sleep maintenance, especially in patients with primary chronic insomnia, the most relevant parameters should be reductions in number or duration of awakenings from sleep and improvements of total sleep time. To convincingly demonstrate efficacy in sleep maintenance, more data on objective measures are required. However, there is a good reason for assuming that improvements in sleep quality and efficiency will be also demonstrable according to hard criteria, insofar as the subjective improvements were particularly evident in patients with severe or very severe forms of primary insomnia as well as in a subpopulation of poor melatonin secretors, as identified by low urinary 6-sulfatoxymelatonin levels.Citation51,Citation78 Therefore, Circadin® or other prolonged-release formulations of melatonin may be suitable for replacement therapy, eg, in patients with age-related decreases in nocturnal melatonin secretion.
Additional information on prolonged-/controlled-release melatonin is available from studies on treatment jet lag, shift work and various disorders, sometimes including comparisons with fast-release melatonin. In jet lag,Citation80–Citation82 not unexpectedly, either formulation proved to be effective. A meta-analysis of 10 studies revealed, however, a superiority of fast-release melatonin.Citation81 A study on aircrews on transatlantic flights, based on both subjective measures and wrist actigraphy, reported a relatively good efficacy of 2 mg sustained-release melatonin.Citation82 In addition to reductions in sleep latency, improvements concerning number and duration of awakenings after sleep onset, quality of sleep and facilitation of returning to sleep were demonstrated.
Studies on simulated shift workCitation83,Citation84 were affected by the problem of melatonin administration in unfavorable circadian phases and are, thus, difficult to compare. The efficacy of sustained-release melatonin was also studied in children and young adults with CRSDsCitation76,Citation85 and with neurodevelopmental disabilities.Citation76,Citation86–Citation94 Improvements were reported, but data on sleep initiation were either not provided or, in part, insufficient. The clearest results were obtained in the most recent study using 5 mg controlled-release melatonin tablets.Citation86 With this higher dosage, reductions in sleep latency and rises in night-time sleep duration were demonstrated by both subjective measures and wrist actigraphy. In children with autism, an open-label study, based on the Children’s Sleep Habits Questionnaire and diary, improvements were obtained with controlled-release melatonin.Citation95 Some circumstantial evidence for sleep improvements were reported for depressed patients,Citation96,Citation97 but without changes in the Hamilton Rating Score for Depression.Citation97 Positive results were also obtained in intensive-care patients with chronic obstructive pulmonary disease or pneumonia.Citation98 In a subpopulation of schizophrenics, improvements of sleep were reported,Citation99 but not after sleep disturbance by the so-called first night effect in a sleep laboratory.Citation100 Although some smaller studies indicated sleep improvements by melatonin in patients with Alzheimer’s disease, as previously summarized,Citation6 neither 2.5 mgCitation4 nor 6 mgCitation101 sustained-release nor 10 mg immediate-releaseCitation4 melatonin resulted in statistically significant improvements, perhaps an indication for the heterogeneity of these populations.
Compared to prolonged-release melatonin, the outcome of trials on ramelteon is much more uniform, as becomes evident from recent summaries.Citation20–Citation22,Citation102,Citation103 Collectively, all the data unanimously show that ramelteon, at doses of 4 or 8 mg, not only reduces sleep onset latency, but also improves total sleep time and sleep efficiency/sleep quality. This has also been demonstrated in several double-blind, placebo-controlled studies on a total of more than a thousand adult or elderly subjects with primary chronic insomnia.Citation104–Citation107 All the effects were statistically significant, but the improvements of sleep maintenance remained moderate. In accordance with the higher receptor affinities of ramelteon compared to melatonin, no further improvements were obtained with 16 or 32 mg daily.Citation105,Citation107 Moreover, ramelteon did not worsen sleep apnea,Citation108 in accordance with the lack of generalized central nervous suppression, as would occur with GABAergic agonists. According to the available data for recommended doses (usually 8 mg), ramelteon seems to be somewhat more effective than prolonged-release melatonin in the treatment of primary chronic insomnia, as far as sleep maintenance is concerned. Several factors should contribute to this finding: (i) higher receptor affinities to both melatonin receptors, especially to MT1; (ii) higher bioavailability because of longer half-life; (iii) a long-lasting contribution of the metabolite M-II; and (iv) the higher recommended doses of 4 or 8 mg ramelteon vs 2 mg Circadin®. Nevertheless, EMEA found the efficacy of ramelteon in improving sleep maintenance insufficient for a marketing authorization.Citation64
Safety, tolerability, withdrawal
In full agreement with numerous findings on immediate-release melatonin, all studies on the prolonged-release formulation unanimously show that the recommended dose does not cause next-day hangover, but rather favors morning alertness – although some exceptions have been described in other investigations using different doses. It does not lead to dependence, early or late withdrawal effects after discontinuation.Citation51,Citation77–Citation79 The development of tolerance is usually absent with melatonin, although a few exceptions have been reported, especially in some children with neurological disorders.Citation91–Citation94 Should the development of tolerance turn out to be a consequence of altered metabolism, which remains to be demonstrated, other melatoninergic agonists might be tested. A recent randomized, double-blind, placebo-controlled crossover study on prolonged-release melatonin confirmed the absence of next-day impairments of psychomotor functions, driving skills and memory recall, in contrast to 10 mg zolpidem.Citation109 Controlled-release melatonin (2 mg) was successfully used even for facilitating benzodiazepine discontinuation.Citation110
Like melatonin, ramelteon did not cause next-day hangover (as revealed by subjective feeling, psychomotor and cognitive tests, and ability to concentrate),Citation105 rebound insomnia or other withdrawal effects, or development of tolerance or addiction.Citation20–Citation22,Citation105 Under these conditions, both prolonged-release melatonin and ramelteon appear safe in short-term treatment, as may be assumed for other exclusively melatoninergic drugs in general.
For subjective criteria of adverse effects, such as reports of nausea, digestive difficulties, headache or other pain, dizziness, and mood, no substantial differences were detected between Circadin® and placebo, and frequently trends were detected even towards fewer subjective side effects in the melatonin groups.Citation51 In this context, it should be also noted that considerably higher doses of melatonin, 300 mg/day enterally, were administered for up to 2 years to amyotrophic lateral sclerosis patients and found to be safe.Citation111 Subjective reports of adverse effects showed that ramelteon 4 or 8 mg was also well tolerated, with similar outcomes as for placebo.Citation20,Citation21,Citation104–Citation107
Precautions should be taken with both melatonin and ramelteon for other reasons. First, the use of a melatoninergic agonist should be restricted to appropriate circadian phases in the evening, since it may cause drowsiness when taken during daytime and, in this case, may in fact impair psychomotor functions, including driving skills. While the use of hypnotics should anyway be restricted to bedtime, more specific precautions are related to the pleiotropyCitation19,Citation45 of melatonin. It is of utmost importance to keep in mind that melatonin is not just a hormone transmitting the darkness signal, and not only a regulator affecting the SCN, but rather influences numerous additional functions.Citation19,Citation43–Citation45,Citation49 Even for ramelteon, which may exclusively act on MT1 and MT2 receptors (although this selectivity has not been demonstrated for the newly discovered binding sites), various effects beyond SCN modulaton and sleep promotion have to be expected. This would include influences on other hormones, and on the immune system, vasculature, and the gastrointestinal system. The possibility of undesired melatoninergic effects on the reproduction system may be a controversial issue. The respective influences of the hormone are without any doubt not comparable to those in seasonal breeders, but, on the other hand, earlier attempts to use melatonin as a contraceptive,Citation112–Citation114 suppressive effects on the GnRH pulse generatorCitation115 and deviations of melatonin in reproductive disordersCitation116–Citation118 have been seen as a caveat in the opinion of some investigators and also of EMEA.Citation51 Especially in reproductive disorders, changes in melatonin may not be causative, but rather consequences of other anatomical or physiological disturbances. In perimenopausal women, effects of melatonin on LH, FSH and thyroid hormones were observed,Citation119 whereas no changes were detected in LH, FSH, testosterone and inhibin-β in normal men subjected to long-term treatment with the pineal hormone.Citation120 However, melatonin was also reported to decrease semen quality in two healthy men,Citation121 but this study was conducted with a very small number of volunteers. Concerns because of changes in the reproductive system may be taken as a contraindication for treating children, adolescents and pregnant women with melatonin, as did EMEA in the case of Circadin®.Citation51 On the other hand, children, adolescents and young adults have been treated for considerable periods of time with the pineal hormone, without reports of undesired effects in the reproductive system.Citation14,Citation76,Citation85,Citation95,Citation122–Citation124 The position of EMEA, which has approved Circadin® only for subjects of 55 years and older,Citation51 may appear unduly cautious, but EMEA intends to be cautious. Nevertheless, melatonin formulations or other melatoninergic drugs should be an option for children with severe and otherwise intractable neurological disorders.
Precautions are necessary in subjects with immunological disorders, since melatonin is also a mainly stimulatory immunomodulator.Citation19,Citation45,Citation49 Thus, melatoninergic drugs should generally not be prescribed to patients with autoimmune diseases.Citation51 With both melatonin and ramelteon, another caveat concerns drugs influencing cytochrome P450 enzymes, especially inhibitors of CYP1A2,Citation20,Citation47,Citation48,Citation51 such as fluvoxamine, which would lead to substantial rises in circulating melatonin and ramelteon as well. Additional specific precautions are listed for Circadin®, such as LAPP lactase deficiency and glucose-galactose malabsorption,Citation51 and for ramelteon concerning alcohol, high-fat diet and renal impairment.Citation20,Citation125
Another disorder that may be regarded as a contra-indication against the use of melatoninergic agonists is Parkinson’s disease (PD). Contrary to findings in various animal models, melatonin has not been generally beneficial in PD patients, especially for disease progression, as summarized earlier.Citation6 More recently, PD has been interpreted as an endocrine disorder characterized by melatonin hyperplasia.Citation126 Correspondingly, clinical improvements have been obtained by melatonin antagonist treatment.Citation127 Melatonin hyperplasia may also deserve attention in other diseases, eg, irritable bowel syndrome type II, in which an enhanced proliferation of melatonin and serotonin producing cells is observed, in conjunction with losses of other cell types.Citation128
At recommended dosesCitation51 and even higher, melatonin is devoid of mutagenicity or carcinogenicity, but instead appears to be protective in this regard.Citation38,Citation44,Citation49,Citation55,Citation129 The absence of genotoxicity and carcinogenicity is also reported for ramelteon.Citation125 However, some reservations seem appropriate with this drug,Citation20,Citation21,Citation47 since the no-effect level for induction of hepatic tumors in male mice was only three times the concentration of the metabolite M-II measured after the therapeutic dose.Citation125 Moreover, micronuclei formations were observed in Chinese hamster lung cells after metabolic activation.Citation125 The naphthalenic compound agomelatine may require further toxicological studies.Citation21,Citation47 In this place, it should be emphasized that safety studies also have to consider the properties of the metabolites, which is not generally sufficiently done. For the metabolites of melatonin, one can state that they never attain high concentrations. Kynuric products, which may be relevant in tissues, have been reported to be protective rather than deleterious.Citation38,Citation44,Citation129 More extensive studies on properties of metabolites are necessary for any of the synthetic melatoninergic drugs, including β-methyl-6-chloromelatonin, tasimelteon and agomelatine, in the last case also for the serotonin analog S 21517,Citation21 and, most importantly, for the ramelteon metabolite M-II, because of the high concentrations attained and its long half-life in the circulation.
In summary, both prolonged-release melatonin and ramelteon are well tolerated and safe in the populations indicated by the respective approvals, and acceptable for short-term treatment. Experience with the extended high-dose melatonin treatment in ALS patientsCitation111 indicates that Circadin® may be safe even for prolonged treatment, whereas more studies would be required for ramelteon to be sure about this point.Citation20,Citation21,Citation47
Conclusions, place in therapy
Melatonin and all synthetic melatoninergic drugs discussed here are capable of phase shifting the circadian pacemaker, and all of them can be expected to reduce sleep onset latency, with the exception of a certain number of nonresponders. In terms of toxicology, beyond the subjective reports on absence or presence of adverse effects, β-methyl-6-chloromelatonin, ramelteon, tasimelteon and agomelatine need further investigation for long-term safety, particularly for tasimelteon, which is administered in relatively high doses of 20 or 50 mg,Citation69 compared with the much lower doses of ramelteon (8 mg) or melatonin (2 mg). The nonselective drug agomelatine may be useful in major depressive disorder,Citation21,Citation47 but, alternatively, combinations of classic antidepressants with z-drugs such as zolpidem extended-release may be likewise effective.Citation130
With these reservations, all the chronobiotics, but more in particular, the approved hypnotics Circadin® (melatonin prolonged release) and Rozerem® (ramelteon), but presumably also the investigative drug tasimelteon,Citation69 should be suitable for treating jet lag or other phase shifts, and also tractable forms of CRSDs, such as DSPS and FASPS. Beyond phase resetting, facilitation of sleep onset can be expected in mild types of CRDS-related insomnia. In this regard, one might, however, ask whether a prolonged-release formulation or a drug of longer half-life and higher receptor affinity is really needed. Sleep onset can be even promoted by 0.1 or 0.3 mg immediate-release melatonin,Citation20 so that a higher dose may not be required in these cases, nor prolonged release, longer half-life or higher receptor affinity. Circadin® or Rozerem® may be tested, if immediate-release melatonin fails.
The situation is different in primary chronic insomnia, in which a substantial support of sleep maintenance is required. In this disorder, statistically significant but still moderate effects of ramelteon have been reported,Citation20–Citation22,Citation47,Citation102–Citation107 whereas prolonged-release melatonin would require substantiation of its efficacy. Such a comparison should, however, consider the differences in recommended doses. Although ramelteon has considerably higher receptor affinities and a relatively longer half-life, 4 or 8 mg are recommended, whereas only 2 mg of melatonin are present in a Circadin® tablet. It seems inappropriate to be extremely cautious with the natural compound melatonin, which is exceptionally well tolerated in the majority of individuals, but not to apply the same criteria to a longer-acting synthetic analog with higher receptor affinity.Citation47
Treatment with melatoninergic agonists seems to be promising in another disorder, Smith-Magenis syndrome, which is characterized, apart from developmental and neurobehavioral abnormalities, by a largely inverted melatonin rhythm and sleep difficulties.Citation87,Citation88 In this case, a combination of a β1-adrenergic blocker in the morning, to suppress diurnal melatonin secretion, and melatonin in the evening has been applied with some success.Citation89,Citation90 In this congenital disease, a sustained high nocturnal level of melatonin would be of particular importance, which indicates the use of a prolonged-release formulation. Whether or not melatonin may be replaced by synthetic agonists such as ramelteon remains to be clarified and may depend on long-term safety. Other neurodevelopmental and neuropsychiatric disorders associated with sleep difficulties or CRSDs, which have been studied in children and young adults and are sometimes otherwise intractable,Citation76,Citation85,Citation86,Citation91–Citation94 may be seen as an additional area of treatment, despite the reservations of EMEA.
Nevertheless, caution should go beyond the risks mentioned in the previous section, such as autoimmune diseases, Parkinson’s disease, coadministration of CYP inhibitors, and hepatic and renal diseases. Surveillance seems to be appropriate for the development and function of reproductive organs. Pregnancy would be another condition under which benefits and possible risks have to be weighed. These considerations should equally apply to melatonin prolonged or immediate-release, ramelteon and other melatoninergic drugs. Therefore, the decision by EMEA to approve Circadin® for subjects older than 54 years, along with a list of specific precautions, is a responsible one, although it may appear unduly cautious. The same criteria should be applied to ramelteon, and to other melatoninergic drugs that may be evaluated for approval.
Nevertheless, all melatoninergic drugs discussed are well tolerated in short-term treatment, and for the natural compound, melatonin, the same should be valid for long-term administration, except for the precautions mentioned above.
Melatonin and its synthetic analogs may be helpful even in other disorders, such as relieving sleep difficulties caused by benzodiazepine discontinuationCitation110 and in chronic obstructive pulmonary disorder, in which ramelteon has been shown to be effective,Citation131,Citation132 and for which the same may be valid in the case of melatonin.Citation98,Citation133
In practical terms, sleep difficulties should first be tested for causes related to circadian dysfunction, in which immediate-release melatonin may already be effective, and Circadin® should be tried if the immediate-release formulation does not suffice. In cases of chronic primary insomnia, ramelteon seems, according to current knowledge, slightly more promising than prolonged-release melatonin. If melatoninergic drugs fail, z-drugs may be the better option. In patients of appropriate age and not belonging to a risk group, the general strategy may be to first test the natural compound, melatonin, because of its remarkable tolerability and safety, before other options are used.
Disclosures
The author has no conflicts of interest to declare.
References
- American Psychiatric AssociationDiagnostic and statistical manual of mental disorders4th edition(DSM-IV), text revision. Washington, DCAmerican Psychiatric Association2000
- DrakeCLRoehrsTRothTInsomnia causes, consequences, and therapeutics: an overviewDepress Anxiety20031816317614661186
- National Institutes of HealthNIH statement regarding the treatment of insomnia. State of the Science Conference Statement: Manifestations and management of chronic insomnia ind adultsSleep2005281049105716268373
- SingerCTractenbergREKayeJSchaferKGamstAGrundmanMA multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s diseaseSleep20032689390114655926
- SkeneDJSwaabDFMelatonin rhythmicity: effect of age and Alzheimer’s diseaseExp Gerontol20033819920612543278
- SrinivasanVPandi-PerumalSRCardinaliDPPoeggelerBHardelandRMelatonin in Alzheimer’s disease and other neurodegenerative disordersBehav Brain Funct200621516674804
- WuYHSwaabDFDisturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer’s diseaseSleep Med2007862363617383938
- LockleySWSkeneDJButlerLJArendtJSleep and activity rhythms are related to circadian phase in the blindSleep19992261662310450596
- EbisawaTCircadian rhythms in the CNS and peripheral clock disorders: human sleep disorders and clock genesJ Pharmacol Sci200710315015417299246
- OkawaMUchiyamaMCircadian rhythm sleep disorders and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndromeSleep Med Rev20071148549617964201
- SkeneDJLockleySWArendtJUse of melatonin in the treatment of phase shift and sleep disordersAdv Exp Med Biol1999467798410721041
- KameiYHayakawaTUrataJUchiyamaMShibuiKKimKMelatonin treatment for circadian rhythm sleep disordersPsychiatry Clin Neurosci200054391392
- ZisapelNCircadian sleep disorders: pathophysiology and potential approaches to managementCNS Drugs20011531132811463135
- SzeinbergABorodkinADaganYMelatonin treatment in adolescents with delayed sleep phase syndromeClin Pediatr (Phila)20064580981817041168
- LiuCWeaverDRJinXShearmanLPPieschlRLGribkoffVKMolecular dissection of two distinct actions of melatonin on the suprachiasmatic clockNeuron199719911029247266
- von GallCStehleJHWeaverDRMammalian melatonin receptors: molecular biology and signal transductionCell Tissue Res200230915116212111545
- JinXvon GallCPieschlRLGribkoffVKStehleJHReppertSMTargeted disruption of the mouse Mel1b melatonin receptorMol Cell Biol2003231054106012529409
- DubocovichMLMarkowskaMFunctional MT1 and MT2 melatonin receptors in mammalsEndocrine20052710111016217123
- HardelandRMelatonin: Signaling mechanisms of a pleiotropic agentBioFactors20093518319219449447
- Pandi-PerumalSRSrinivasanVPoeggelerBHardelandRCardinaliDPDrug insight: the use of melatonergic agonists for the treatment of insomnia – focus on ramelteonNat Clin Pract Neurol2007322122817410109
- HardelandRPoeggelerBSrinivasanVTrakhtIPandi-PerumalSRCardinaliDPMelatonergic drugs in clinical practiceArzneimittel-forschung20085811018368944
- SrinivasanVPandi-PerumalSRTrahktISpenceDWPoeggelerBHardelandRMelatonin and melatonergic drugs on sleep: possible mechanisms of actionInt J Neurosci200911982184619326288
- SaperCBScammellTELuJHypothalamic regulation of sleep and circadian rhythmsNature20054371257126316251950
- FullerPMGooleyJJSaperCBNeurobiology of sleep-wake cycle, sleep architecture, circadian regulation and regulatory feed backJ Biol Rhythms20062148249317107938
- ReppertSMGodsonCMahleCDWeaverDRSlaugenhauptSAGusellaJFMolecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptorProc Natl Acad Sci U S A199592873487387568007
- MazzucchelliCPannacciMNonnoRLuciniVFraschiniFStankovBMThe melatonin receptor in the human brain: cloning experiments and distribution studiesBrain Res Mol Brain Res1996391171268804720
- ThomasLPurvisCCDrewJEAbramovichDRWilliamsLMMelatonin receptors in human fetal brain: 2-[125I]iodomelatonin binding and MT1 gene expressionJ Pineal Res20023321822412390504
- Ambriz-TututiMRocha-GonzálezHICruzSLGranados-SotoVMelatonin: A hormone that modulates painLife Sci20098448949819223003
- DijkDJRothCLandoltHPWerthEAeppliMAchermannPMelatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activityNeurosci Lett199520113168830301
- JanJEReiterRJWasdellMBBaxMThe role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disordersJ Pineal Res2009461718761566
- De GennaroLFerraraMSleep spindles. An overviewSleep Med Rev2003742344014573378
- MorinLPAllenCNThe circadian visual system, 2005Brain Res Rev20065116016337005
- JanJEWasdellMBReiterRJWeissMDJohnsonKPIvanenkoAMelatonin therapy of pediatric sleep disorders: recent advances, why it works, who are the candidates and how to treatCurr Pediatr Rev20073214224
- CajochenCMünchMKnoblauchVBlatterKWirz-JusticeAAge-related changes in the circadian and homeostatic regulation of human sleepChronobiol Int20062346147416687319
- GorfineTZisapelNLate evening brain activation patterns and their relation to the internal biological time, melatonin, and homeostatic sleep debtHum Brain Mapp20093054155218095278
- DijkDJvon SchantzMTiming and consolidation of human sleep, wakefulness, and performance by a symphony of oscillatorsJ Biol Rhythms20052027929016077148
- CajochenCKräuchiKWirz-JusticeARole of melatonin in the regulation of human circadian rhythms and sleepJ Neuroendocrinol20031543243712622846
- HardelandRAntioxidative protection by melatonin – Multiplicity of mechanisms from radical detoxification to radical avoidanceEndocrine20052711913016217125
- LuJShermanDDevorMSaperCBA putative flip-flop switch for control of REM sleepNature200644158959416688184
- FullerPMSaperCBLuJThe pontine REM switch: past and presentJ Physiol200758473574117884926
- KunzDMahlbergRMüllerCTilmannABesFMelatonin in patients with reduced REM sleep duration: two randomized controlled trialsJ Clin Endocrinol Metab20048912813414715839
- ClaustratBBrunJChazotGThe basic physiology and pathophysiology of melatoninSleep Med Rev20059112415649735
- HardelandRPoeggelerBActions of melatonin, its structural and functional analogs in the central nervous system and the significance of metabolismCent Nerv Syst Agents Med Chem10077289303
- HardelandRPoeggelerBMelatonin beyond its classical functionsOpen Physiol J20081123
- HardelandRMelatonin, hormone of darkness and more – occurrence, control mechanisms, actions and bioactive metabolitesCell Mol Life Sci2008652001201818344019
- KatoKHiraiKNishiyamaKUchikawaOFukatsuKOhkawaSNeurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonistNeuropharmacology20054830131015695169
- PoeggelerBHardelandRMelatoninerge Chronobiotika: Wirkungen und ProblemeHardelandRFacetten der Chronobiologie Abh Leibniz-Soz23BerlinTrafo20087187
- MaXIdleJRKrauszKWGonzalezFJMetabolism of melatonin by human cytochromes p450Drug Metab Dispos20053348949415616152
- Pandi-PerumalSRSrinivasanVMaestroniGJMCardinaliDPPoeggelerBHardelandRMelatonin – Nature’s most versatile biological signal?FEBS J20062732813283816817850
- FeldsteinAChangFHKucharskiJMTryptophol, 5-hydroxytryptophol and 5-methoxytryptophol induced sleep in miceLife Sci197093233295444013
- EMEA. Assessment report for Circadin200752 p. Available from: http.//www.emea.europe.eu/humandocs/PDFs/EPAR/Circadin/H-695-eu6.pdf
- HuetherGMessnerMRodenbeckAHardelandREffect of continuous melatonin infusions on steady-state plasma melatonin levels in rats under near physiological conditionsJ Pineal Res1998241461519551851
- HajakGHuetherGBlankeJBlömerMFreyerCPoeggelerBThe influence of intravenous L-tryptophan on plasma melatonin and sleep in menPharmacopsychiatry19912417202011617
- BubenikGAGastrointestinal melatonin: localization, function, and clinical relevanceDig Dis Sci2002472336234812395907
- HardelandRPandi-PerumalSRMelatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrugNutr Metab (Lond)22005article no 22 [DOI 10.1186/1743-7075-2-22].
- PoeggelerBCornélissenGHuetherGHardelandRJózsaRZemanMChronomics affirm extending scope of lead in phase of duodenal vs pineal circadian melatonin rhythmsBiomed Pharmacother200559S220S22416275498
- TanDXManchesterLCReiterRJQiWHanesMAFarleyNJHigh physiological levels of melatonin in the bile of mammalsLife Sci1999652523252910622237
- MessnerMHuetherGLorfTRamadoriGSchwörerHPresence of melatonin in the human hepatobiliary-gastrointestinal tractLife Sci20016954355111510949
- HuetherGMelatonin synthesis in the gastrointestinal tract and the impact of nutritional factors on circulating melatoninAnn N Y Acad Sci19947191461588010590
- LeeBJRyuSGChoiHGKimCKParrottKAAyresJWBatch variation and pharmacokinetics of oral sustained release melatonin-loaded sugar spheres in human subjectsArch Pharm Res19972055555918982259
- PrianoLEspostiDEspostiRCastagnaGDe MediciCFraschiniFSolid lipid nanoparticles incorporating melatonin as new model for sustained oral and transdermal delivery systemsJ Nanosci Nanotechnol200773596360118330178
- MulchaheyJJGoldwaterDRZemlanFPA single blind, placebo controlled, across groups dose escalation study of the safety, tolerability, pharmacokinetics and pharmacodynamics of the melatonin analog β-methyl-6-chloromelatoninLife Sci2004751843185615302228
- ZemlanFPMulchaheyJJScharfMBMaylebenDWRosenbergRLankfordAThe efficacy and safety of the melatonin agonist β-methyl-6-chloromelatonin in primary insomnia: a randomized, placebo-controlled, crossover clinical trialJ Clin Psychiatry20056638439015766306
- EMEAQuestions and answers on recommendation for the refusal of the marketing authorisation for ramelteon. Press Release, May 30, 2008. http://www.emea.europa.eu/pdfs/human/opinion/Ramelteon_26821608en.pdf
- EMEATakeda withdraws its marketing authorisation application for Ramelteon. Press Release, September 25, 2008. http://www.emea.europa.eu/humandocs/PDFs/EPAR/ramelteon/50471608en.pdf
- MiyamotoMNishikawaHOhtaHUchikawaOOhkawaOOhkawaSBehavioural pharmacology of TAK-375 in small animalsAnn Neurol200354S4612891653
- McGechanAWellingtonKRamelteonCNS Drugs2005191057106516332146
- KarimATolbertDCaoCDisposition kinetics and tolerance of escalating single doses of ramelteon, a high affinity MT1 and MT2 melatonin receptor agonist indicated for the treatment of insomniaJ Clin Pharmacol20064614014816432265
- RajaratnamSMPolymeropoulosMHFisherDMRothTScottCBirznieksGMelatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trialsLancet200937348249119054552
- KlermanEBMelatonin agonist tasimelteon (VEC-162) for transient insomnia after sleep-time shift: two randomised controlled multicentre trials. Web Appendix to online prepublication of ref. 53. 2008www.thelancet.com
- VachharajaniNNYeleswaramKBoultonDWPreclinical pharmacokinetics and metabolism of BMS-214778, a novel melatonin receptor agonistJ Pharm Sci20039276077212661062
- IDdb Author. Associated Professional Sleep Societies – 20 Annual Meeting (Part I), Hypnotic and Wake-promoting Drugs, Salt Lake City, UT, USA. IDdb Meeting Report; 2006, June 17–22.
- ZhuMMitrokaJShawAALukeGMVachharajaniNKripalaniKJIdentification of in vitro and in vivo metabolites of BMS-214778 in monkeys by ion trap LC/MS and LC/NMRDrug Metab Rev200032Suppl 2260
- MillanMJGobertALejeuneFDekeyneANewman-TancrediAPasteauVThe novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathwaysJ Pharmacol Exp Ther200330695496412750432
- Servier PharmaValdoxan® (agomelatine), a novel antidepressant, receives European marketing authorizationServier Press Release2009http://www.agomelatine.org/2009-valdoxan.html
- CarrRWasdellMBHamiltonDWeissMDFreemanRDTaiJLong-term effectiveness outcome of melatonin therapy in children with treatment-resistant circadian rhythm sleep disordersJ Pineal Res20074335135917910603
- WadeAGFordICrawfordGMcMahonADNirTLaudonMEfficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomesCurr Med Res Opin2007232597260517875243
- LemoinePNirTLaudonMZisapelNProlonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effectsJ Sleep Res20071637238018036082
- HaimovILaviePLaudonMHererPVigderCZisapelNMelatonin replacement therapy of elderly insomniacsSleep1995185986038552931
- SuhnerASchlagenhaufPJohnsonRTschoppASteffenRComparative study to determine the optimal melatonin dosage form for the alleviation of jet lagChronobiol Int1998156556669844753
- HerxheimerAPetrieKKJMelatonin for the prevention and treatment of jet lagCochrane Database Syst Rev20022article no. CD001520. DOI: 10.1002/14651858.CD001520.
- PaulMAGrayGSardanaTMPigeauRAMelatonin and zopiclone as facilitators of early circadian sleep in operational air transport crewsAviat Space Environ Med20047543944315152897
- SharkeyKMFoggLFEastmanCIEffects of melatonin administration on daytime sleep after simulated night shift workJ Sleep Res20011018119211696071
- CrowleySJLeeCTsengCYFoggLFEastmanCICombinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift workJ Biol Rhythms20031851352314667152
- JanJEHamiltonDSewardNFastDKFreemanRDLaudonMClinical trials of controlled-release melatonin in children with sleep-wake cycle disordersJ Pineal Res200029343910949538
- WasdellMBJanJEBombenMMFreemanRDRietveldWJTaiJA randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilitiesJ Pineal Res200844576418078449
- PotockiLGlazeDTanDXParkSSKashorkCDShafferLGCircadian rhythm abnormalities of melatonin in Smith-Magenis syndromeJ Med Genet20003742843310851253
- De LeersnyderHInverted rhythm of melatonin secretion in Smith-Magenis syndrome: from symptoms to treatmentTrends Endocrinol Metab20061729129816890450
- De LeersnyderHClaustratBMunnichAVerloesACircadian rhythm disorder in a rare disease: Smith-Magenis syndromeMol Cell Endocrinol2006252889116723183
- CarpizoRMartínezAMediavillaDGonzálezMAbadASánchez-BarcelóEJSmith-Magenis syndrome: a case report of improved sleep after treatment with β1-adrenergic antagonists and melatoninJ Pediatr200614940941116939758
- JanJEEspezelHAppletonREThe treatment of sleep disorders with melatoninDev Med Child Neurol199436971078132132
- McArthurAJBuddenSSSleep dysfunction in Rett syndrome: a trial of exogenous melatonin treatmentDev Med Child Neurol1998401861929566656
- IshizakiASugamaMTakeuchiN[Usefulness of melatonin for developmental sleep and emotional/behavior disorders–studies of melatonin trial on 50 patients with developmental disorders.] [in Japanese]No To Hattatsu19993142843710487068
- AndersenIMKaczmarskaJMcGrewSGMalowBAMelatonin for insomnia in children with autism spectrum disordersJ Child Neurol20082348248518182647
- GiannottiFCortesiFCerquigliniABernabeiPAn open-label study of controlled-release melatonin in treatment of sleep disorders in children with autismJ Autism Dev Disord20063674175216897403
- DolbergOTHirschmannSGrunhausLMelatonin for the treatment of sleep disturbances in major depressive disorderAm J Psychiatry1998155111911219699707
- DaltonEJRotondiDLevitanRDKennedySHBrownGMUse of slow-release melatonin in treatment-resistant depressionJ Psychiatry Neurosci200025485210721684
- ShiloLDaganYSmorjikYWeinbergUDolevSKomptelBEffect of melatonin on sleep quality of COPD intensive care patients: a pilot studyChronobiol Int200017717610672435
- ShamirELaudonMBarakYAnisYRotenbergVElizurAMelatonin improves sleep quality of patients with chronic schizophreniaJ Clin Psychiatry20006137337710847313
- ShamirERotenbergVSLaudonMZisapelNElizurAFirst-night effect of melatonin treatment in patients with chronic schizophreniaJ Clin Psychopharmacol20002069169411106143
- SerfatyMKennell-WebbSWarnerJBlizardRRavenPDouble blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementiaInt J Geriatr Psychiatry2002171120112712461760
- BorjaNLDanielKLRamelteon for the treatment of insomniaClin Ther2006281540155517157111
- NeubauerDNA review of ramelteon in the treatment of sleep disordersNeuropsychiatr Dis Treat20084697918728808
- RothTSeidenDSainatiSWang-WeigandSZhangJZeePEffects of ramelteon on patient-reported sleep latency in older adults with chronic insomniaSleep Med2006731231816709464
- ErmanMSeidenDZammitGSainatiSZhangJAn efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomniaSleep Med20067172416309958
- RothTSeidenDWang-WeigandSZhangJA 2-night, 3-period, crossover study on ramelteon’s efficacy and safety in older adults with chronic insomniaCurr Med Res Opin2007231005101417519067
- ZammitGErmanMWang-WeigandSSainatiSZhangJRothTEvaluation of the efficacy and safety of ramelteon in subjects with chronic insomniaJ Clin Sleep Med2007349550417803013
- KrygerMWang-WeigandSRothTSafety of ramelteon in individuals with mild to moderate obstructive sleep apneaSleep Breath20071115916417294232
- OtmaniSDemazièresAStanerCJacobNNirTZisapelNEffects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteersHum Psychopharmacol20082369370518763235
- GarfinkelDZisapelNWainsteinJLaudonMFacilitation of benzodiazepine discontinuation by melatonin: a new clinical approachArch Intern Med19991592456246010665894
- WeishauptJHBartelsCPölkingEDietrichJRohdeGPoeggelerBReduced oxidative damage in ALS by high-dose enteral melatonin treatmentJ Pineal Res20064131332317014688
- SilmanREMelatonin: a contraceptive for the ninetiesEur J Obstet Gynecol Reprod Biol199349398365512
- García-PattersonAPuig-DomingoMWebbSMThirty years of human pineal research: do we know its clinical relevance?J Pineal Res199620168648556
- BubenikGABlaskDEBrownGMMaestroniGJPangSFReiterRJProspects of the clinical utilization of melatoninBiol Signals Recept199871952199730580
- SilmanRMelatonin and the human gonadotrophin-releasing hormone pulse generatorJ Endocrinol19911287111999677
- WaldhauserFBoepplePASchemperMMansfieldMJCrowleyWFJrSerum melatonin in central precocious puberty is lower than in age-matched prepubertal childrenJ Clin Endocrinol Metab1991737937961909703
- WalkerABEnglishJArendtJMacFarlaneIAHypogonadotrophic hypogonadism and primary amenorrhoea associated with increased melatonin secretion from a cystic pineal lesionClin Endocrinol (Oxf)1996453533568949574
- LuboshitzkyRHererPShen-OrrZUrinary 6-sulfatoxymelatonin excretion in hyperandrogenic women: the effect of cyproterone acetate-ethinyl estradiol treatmentExp Clin Endocrinol Diabetes200411210210715031776
- RohrUDHeroldJMelatonin deficiencies in womenMaturitas200241Suppl 1S85S10411955797
- LuboshitzkyRLeviMShen-OrrZBlumenfeldZHererPLaviePLong-term melatonin administration does not alter pituitary-gonadal hormone secretion in normal menHum Reprod200015606510611189
- LuboshitzkyRShen-OrrZNaveRLaviSLaviePMelatonin administration alters semen quality in healthy menJ Androl20022357257812065466
- PalmLBlennowGWetterbergLLong-term melatonin treatment in blind children and young adults with circadian sleep-wake disturbancesDev Med Child Neurol1997393193259236698
- JanJEFreemanRDFastDKMelatonin treatment of sleep-wake cycle disorders in children and adolescentsDev Med Child Neurol19994149150010454235
- DodgeNNWilsonGAMelatonin for treatment of sleep disorders in children with developmental disabilitiesJ Child Neurol20011658158411510929
- Takeda Pharmaceuticals America, IncRozerem™ (ramelteon) tablets. 05-1118;L-RAM-00010. 2005
- WillisGLParkinson’s disease as a neuroendocrine disorder of circadian function: dopamine-melatonin imbalance and the visual system in the genesis and progression of the degenerative processRev Neurosci20081924531619145986
- WillisGLThe role of ML-23 and other melatonin analogues in the treatment and management of Parkinson’s diseaseDrug News Perspect20051843744416362083
- OsadchukAMOsadchukMAKvetnoIM[Irritated bowel syndrome: clinico-morphological types.] [Article in Russian]Klin Med (Mosk)200785465017523405
- HardelandRPandi-PerumalSRCardinaliDPMolecules in focus – MelatoninInt J Biochem Cell Biol20063831331616219483
- FavaMAsnisGShrivastavaRLydiardRBBastaniBSheehanDImproved insomnia symptoms and daily functioning in patients with comorbid major depressive disorder and insomnia following zolpidem extended-release 12.5 mg and escitalopram co-treatmentSleep200831A324
- KrygerMRothTWang-WeigandSZhangJThe effects of ramelteon on respiration during sleep in subjects with moderate to severe chronic obstructive pulmonary diseaseSleep Breath200913798418584227
- RothTHypnotic use for insomnia management in chronic obstructive pulmonary diseaseSleep Med200910192518693067
- NunesDMMotaRMMachadoMOPereiraEDde BruinVMde BruinPFEffect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary diseaseBraz J Med Biol Res20084192693119030713