Abstract
Vigabatrin (VGB) is an antiepileptic drug that was designed to inhibit GABA-transaminase, and increase levels of γ-amino-butyric acid (GABA), a major inhibitory neurotransmitter in the brain. VGB has demonstrated efficacy as an adjunctive antiepileptic drug for refractory complex partial seizures (CPS) and for infantile spasms (IS). This review focuses on its use for complex partial seizures. Although VGB is well tolerated, there have been significant safety concerns about intramyelinic edema and visual field defects. VGB is associated with a risk of developing bilateral concentric visual field defects. Therefore, the use of VGB for complex partial seizures should be limited to those patients with seizures refractory to other treatments. Patients must have baseline and follow-up monitoring of visual fields, early assessment of its efficacy, and ongoing evaluation of the benefits and risks of VGB therapy.
Introduction
The discovery of γ-aminobutyric acid (GABA) as the first major inhibitory neurotransmitter, and a program exploring the use of enzyme inhibition as a therapeutic tool provided the basis for the conception of vigabatrin (VGB). VGB was first approved in the United Kingdom in 1989, and is used in over 50 countries as an adjunctive therapy of adult patients with refractory complex partial seizures (CPS), and as a treatment for patients with infantile spasms (IS). This review focuses on its use for CPS. VGB has not been available in the United States, due to concerns about its safety. After further analysis of data regarding adverse effects and safety, clinical monitoring guidelines are being developed to reduce the potential risks associated with its use in patients with these severe epileptic conditions.
Pharmacology
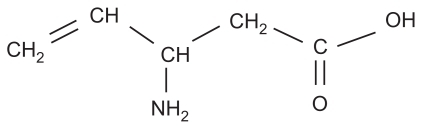
The VGB molecule, a structural analog of GABA, was designed to have enzyme-activated highly specific activity as an irreversible inhibitor of GABA-transaminase. Vigabatrin (4-amino-5-hexenoic acid, or γ-vinyl GABA) was first synthesized in 1977 as a selective irreversible inhibitor of gamma-aminobutyric acid transaminase (GABA-T).Citation1 By inhibiting GABA-T, the enzyme responsible for the catabolism of GABA, VGB increases whole brain levels of GABA, leading to a reduction in seizure activity.
The molecular structure of VGB is shown in . VGB is a racemic compound and its [S]-enantiomer is pharmacologically active.Citation2,Citation3 When administered orally, VGB is rapidly and near-completely absorbed and has dose-proportional and linear pharmacokinetics. VGB is completely absorbed following oral administration and can be given without regard to meals.Citation4 After administration, peak VGB concentration occurs within 2 hours.Citation5 It is widely distributed with a volume of distribution steady-state of 1.1 L/kg and does not bind to plasma proteins. VGB is not metabolized and is eliminated unchanged by renal excretion.Citation6
The T1/2 of VGB is approximately 5 to 7 hours; however plasma levels are not correlated with clinical effect.Citation7 A study of patients with epilepsy given single oral dose of VGB found that peak levels were achieved in less than 1 hour in the serum, and 6 hours in the CSF. GABA levels remained elevated in the CSF for over 1 week.Citation8 The duration of the clinical effect of VGB is thought to be dependent on the rate of GABA-T resynthesis rather than on the plasma concentration of VGB. The rate of recovery of GABA-T is 5 days.Citation1
In vitro metabolism studies show that there is a low potential for drug-drug interactions with VGB due to enzyme induction of CYP2B6 or CYP3A4.Citation9 However, in some clinical studies, VGB was associated with modest decreases in plasma phenytoin levels.Citation10,Citation11 The cause of these findings is unclear, as VGB is not protein bound, and is not metabolized.Citation12 A mild increase in carbamazepine (CBZ) levels in patients receiving adjunctive VGB has also been reported.Citation13 These findings suggest that levels of concomitant antiepileptic drugs should be monitored, but dose adjustment is usually unnecessary.
Vigabatrin and infantile spasms
Although VGB plays an important role in the treatment of IS, a comprehensive review of its efficacy for spasms is beyond the scope of this article. In brief, investigators have reported cessation of infantile spasms in 16% to 76% of patients with IS.Citation14,Citation15 Studies have been complicated by methodological issues, including the ethics of administering a placebo, and the challenge of accurately documenting outcomes in a condition that causes numerous brief spasms daily. Evidence suggests that hormonal treatment achieves spasm resolution more quickly and in more infants than VGB treatment.Citation16 However, VGB may be the treatment of choice for IS due to tuberous sclerosis.Citation16 A meta-analysis of studies assessing the efficacy of VGB for IS found that 95% of patients with tuberous sclerosis achieved freedom from spasms, while the rate was 54% for patients without tuberous sclerosis.Citation17 A 2009 long-term follow-up study comparing VGB and ACTH treatment in 28 patients with idiopathic West Syndrome found no significant difference in short-term seizure response (80 and 88%, respectively), although ACTH was associated with better long-term cognitive outcome.Citation18
Efficacy of vigabatrin for refractory complex partial seizures
A number of well designed trials have found VGB effective as adjunctive therapy in patients with refractory complex partial seizures. Responder rates (≥50% seizure reduction) have varied according to study design and VGB dose. A review of double-blind, placebo-controlled studies of adjunctive therapy with newer antiepileptic drugs reported that the overall responder rate for VGB 3 g daily was 44.2%, and the placebo responder rate was 13.8%.Citation19
In the 1980s, several double-blind, placebo-controlled crossover design studies of VGB as adjunctive treatment for partial seizures were performed, demonstrating responder rates ranging from 33% to 67% with doses of 2 to 3 g daily.Citation11,Citation20–Citation23 The variability of responder rates is likely due several factors: the small number of patients analyzed, and the inclusion, in some studies, of generalized seizures.Citation11,Citation23 A larger, similarly designed 1996 Australian study of 97 patients with uncontrolled CPS found a responder rate of 42%.Citation24
A single-blind, placebo-controlled, multicenter trial of VGB was carried out in 101 patients with epilepsy, most of whom had refractory partial seizures. The inclusion of patients with other types of epilepsy may have contributed to the 11% drop-out rate, which was primarily due to increased seizure frequency. Among those completing the trial, the median number of monthly seizures decreased from 16 during the placebo phase to 5 during the final 8 weeks of treatment. A greater than 50% reduction in seizure frequency (compared to placebo) was observed in 60 patients.Citation25
A Canadian multicenter, double-blind, placebo-controlled parallel group trial of VGB in patients with refractory complex partial seizures and/or partial seizures with secondary generalization included 111 patients. The responder rate was 48% for VGB in doses up to 4 g daily, and 26% for placebo.Citation26
Two large trials of VGB for refractory complex partial seizures have been carried out in the US. The studies enrolled patients 18 to 60 years old, with an average 22-year history of epilepsy. Patients were taking 1 or 2 concomitant antiepileptic drugs (AEDs) at the time of entry into the studies. In both trials, a parallel group design was used, and the primary endpoint for efficacy was change in median monthly seizure frequency, compared to baseline, during the final 8 weeks of the study.
The study by French et al analyzed 182 patients taking either adjunctive VGB 3 g daily or placebo. VGB significantly decreased baseline monthly seizure frequency (−3.0) compared with placebo (−0.8), and 5.4% of the VGB patients became seizure free, while none of the placebo-treated patients achieved seizure freedom. The responder rate (50% reduction from baseline seizure frequency) was 43% for VGB and 19% for placebo. Statistically significant seizure reduction occurred early, after 2 weeks of VGB therapy, and was maintained during the 16-week treatment phase.Citation27
A second US placebo-controlled, randomized, double-blind, multicenter study examined the efficacy and safety of 3 daily doses of VGB (1, 3, or 6 g) as add-on therapy in 174 patients with previously uncontrolled complex partial seizures. The responder rates were 7% for placebo and 24%, 51%, and 54% for patients taking daily VGB doses of 1, 3, and 6 g, respectively. Seizure freedom occurred in 9.5 % of those taking 3 g daily, 12.2% of those taking 6 g daily, and none of those taking 1 g daily or placebo. As in the earlier study, seizure reduction was evident after 2 weeks of therapy (at the 3 and 6 g daily doses), and was maintained during the remainder of the study. There was no statistically significant difference in efficacy between the 3 and 6 g regimens.Citation28
Adjunctive VGB therapy is effective for children with partial seizures. A prospective study including 178 patients with refractory partial seizures, aged 1 week to 19 years, found a 70% responder rate, and a 30% seizure freedom rate. Those with tuberous sclerosis had a particularly robust responder rate of 85%.Citation29 These responder rates are higher than the rates reported for adults. The authors attribute the high efficacy rate to the fact that infants made up 22% of their study population, and infants responded better than older children to VGB. However, the inclusion of single-blind and open-label cohorts may also have introduced bias in favor of VGB treatment. A recent retrospective study reported a 34% responder rate in 59 infants and children with partial seizures, including 17% who became seizure free on VGB.Citation30
To summarize, VGB has demonstrated significant efficacy as adjunctive therapy for patients with poorly controlled partial epilepsy, refractory to other antiepileptic drugs. Differences in inclusion criteria (especially type of epilepsy), VGB dosing, study population size and characteristics, and study design contribute to the variability of the reported response rates (approximately 40% to 60%). A meta-analysis of key clinical trials for 5 newer AEDs found that VGB had the most favorable improvement rates (responder rate minus placebo response) at recommended doses.Citation19 Thus, adjunctive treatment with VGB is an appropriate consideration for patients who have failed treatment with available AEDs, have poor quality of life due to frequent complex partial seizures, and will comply with monitoring of for adverse effects. The potential for seizure reduction must be weighed against safety risks, which will be reviewed below.
Vigabatrin monotherapy studies
Several studies have evaluated vigabatrin as monotherapy, compared with carbamazepine (CBZ). The results suggest that VGB is the less effective, but better tolerated, of the 2 drugs. An open-label, randomized controlled study evaluated the efficacy, safety and cognitive effects of initial VGB monotherapy compared to initial CBZ monotherapy in patients with newly diagnosed epilepsy. A total of 100 patients, aged 15 to 64 years, with partial seizures and/or generalized tonic-clonic seizures were randomized to receive either VGB (mean dose 50 mg/kg), or CBZ (titrated to plasma concentrations of 35 μmol/L) for 1 year. The primary outcome measure was the proportion of patients continuing successful treatment, and was 60% for both drugs, but this number reflects a broad definition of treatment success, which included “acceptable seizure control” in addition to seizure freedom. It is debatable whether the definition of acceptable seizure control (1 to 4 partial seizures and no more than 1 generalized seizure during a treatment period) is appropriate for patients with newly diagnosed epilepsy. Seizure freedom rates significantly differed between the groups: 52% for the CBZ group, and 32% for the VGB group. Although VGB had to be discontinued in some patients due to lack of efficacy, none discontinued due to adverse effects. In contrast, 24% of the CBZ group discontinued treatment due to adverse effects, primarily rash.Citation31
Similar findings of relatively lower efficacy but better tolerability of VGB occurred in a randomized crossover study comparing CBZ and VGB in 51 patients with newly diagnosed partial epilepsy. In this study, patients started on either 200 mg daily of CBZ or 1 g daily of VGB, and the doses were increased weekly until seizures ceased or intolerable side effects occurred. Seizure-free rates were 56% and 46% for CBZ and VGB respectively. Those patients with persistent seizures or intolerable side effects entered the cross-over phase, and received the other drug. Of the crossover patients, 43% receiving CBZ and 45% receiving VGB achieved seizure freedom. Considering both phases together, 51% of CBZ patients and 46% of VGB patients had seizure freedom and acceptable tolerance. Differences in efficacy were not statistically significant. Side effects were more frequent in the CBZ group (41%) compared with the VGB group (22%), but this difference was not statistically significant. The most frequent complaint was drowsiness, and fewer side effects occurred with VGB.Citation32
The largest monotherapy study involved 459 patients, aged 12 to 65 years, with previously untreated newly diagnosed partial seizures, randomized to monotherapy with either CBZ 600 mg daily or VGB 2 g daily. Patients had had at least 2 seizures in the previous year, including simple or complex partial seizures, with or without secondary generalization. The primary outcome was time to withdrawal from drug, a measure that encompasses efficacy and tolerability, and the CBZ and VGB groups did not significantly differ on this measure. Secondary outcomes included additional efficacy and tolerability parameters. Efficacy outcomes favored CBZ, with a significant difference in time to first seizure. At 1 year, 58% of CBZ-treated patients and 38% of VGB-treated patients remained seizure-free. VGB was better tolerated than CBZ, with significantly fewer VG patients withdrawing due to adverse effects (19% VGB vs 27% CBZ at 1 year). Patients reported drowsiness, fatigue, headache, and dizziness with each of the drugs. VGB was more frequently associated with weight gain and psychiatric symptoms, most commonly depression.Citation33
The results of these monotherapy trials consistently demonstrate that, in terms of efficacy, VGB is inferior to CBZ in patients with newly diagnosed partial onset epilepsy. VGB is not recommended for use as monotherapy in this clinical setting.
Seizure type
Limited data suggest that VGB is more effective for CPS than for generalized seizures. In general, few patients with primary generalized epilepsy or symptomatic generalized epilepsies were included in studies, and analysis has been hampered by variability in the diagnostic labels used by various authors. A review of 487 patients treated with VGB from published clinical trials included 52 patients with generalized seizures, including tonic-clonic, juvenile myoclonic epilepsy, Lennox-Gastaut syndrome, Ramsay-Hunt syndrome, absence, myoclonic seizures, symptomatic or secondary generalized epilepsies, and generalized seizures of unspecified type. The responder rate (at least a 50% decrease in seizure frequency) was 21% for these patients, while 46% were unchanged, and 25% were worse. The authors note that those with secondary generalized epilepsy had the worst response. In comparison, the VGB responder rate for patients from the same studies with CPS, with or without secondary generalization, was 49%.Citation34
Long-term follow-up
Several studies examining long-term follow-up have found that those patients whose seizures improved during initial treatment with VGB continued to show significant benefit 1–5 years later.Citation35,Citation36 Two small case series following VGB-treated refractory epilepsy patients for 6 to 10 years suggest that epilepsy may improve with continued VGB treatment, with some patients becoming seizure free over time.Citation37,Citation38
Tolerability of vigabatrin
In general, VGB is well tolerated, with side effects that are frequently seen in the setting of AED therapy. When data were pooled from controlled trials in epilepsy patients, excluding those with IS, there were 588 patients on VGB and 373 taking placebo. The most frequent side effects were fatigue (VGB 22.3%, placebo 15.3%), dizziness (VGB 18.9%, placebo 15.6%), somnolence (VGB 16.3%, placebo 9.9%), and increased weight (VGB 11.1%, placebo 7.2%).Citation6 Among these patients, 15% of those treated with VGB discontinued their participation in a study due to an adverse event, compared with 4.6% of the patients receiving placebo. The most common symptoms leading to discontinuation in the VGB treatment group were depression (VGB 1.7%, placebo 0.5%), convulsion (VGB 1.2%, placebo 0.5%), disturbance in attention (VGB 1.0%, placebo 0.5%), head-ache (VGB 1%, placebo 0.5%), and agitation (VGB 1.0%, placebo 0%).Citation6
Weight gain occurs more frequently in epilepsy patients treated with VGB, compared with placebo.Citation33,Citation35,Citation37 In a monotherapy study, 11% of VGB patients gained weight, compared with 5% of those treated with CBZ.Citation33 The degree of weight gain is variable, averaging 3.7 kg ± 0.2 kg in a long-term study of adjunctive treatment with VGB.Citation39 Another long-term follow-up study of 25 VGB responders found increases of 5–16% of initial body weight in 10 of the patients. Weight gain in these patients tended to occur after 3 to 6 months on VGB, and plateaued within several months.Citation40
Cognitive and psychiatric side effects
Cognitive side effects are an important concern of patients with refractory CPS, and VGB has little effect on cognitive function.Citation31,Citation41–Citation43 Although participants in one study reported sedation early in the course of treatment, the VGB responders showed significant improvement in composite scales of psychomotor function, memory, and self-rating.Citation42 A battery of 8 standardized cognitive tests found a significant difference between the VGB and placebo groups only on the Digit Cancellation Test. Scores on this test decreased with increasing dose of VGB.Citation44 A randomized, placebo-controlled, parallel-group study of VGB adjunctive therapy in 45 patients found a small but statistically significant reduction in motor speed, and a modest impairment of performance on a visual memory task.Citation45
The potential for psychiatric side effects with VGB treatment has been under scrutiny since an early report of 14 cases of psychosis among 210 patients treated with VGB.Citation46 Further studies have found a lower incidence of these symptoms. A 1996 literature review of controlled trials reported an incidence of severe abnormal behavior in 3.4% of adults treated with VGB.Citation47 Pooled data from controlled studies of epilepsy patients, excluding those with IS, indicate that 7.8% of those on VGB had depression, and 5.4% reported confusional state, compared with 4.6% and 1.6%, respectively, in placebo-treated patients.Citation6 A meta-analysis of data from placebo-controlled trials of adjunctive therapy with VGB found that the incidence of psychotic events with VGB was 2.5%, vs 0.3% with placebo.Citation48 In smaller monotherapy trials, psychosis was not reported.Citation31,Citation32 In a larger monotherapy trial, 25% of patients taking VGB had psychiatric side effects, described as agitation, depression, insomnia, or “other,” compared with 15% of those taking CBZ.Citation33
VGB is one of several antiepileptic drugs associated with depression.Citation49 French et al reported depression in 12% of patients titrated to 3 g VGB daily, compared with 3% of patients receiving placebo.Citation27 These findings are comparable to the results of a meta-analysis, which showed an overall incidence of depression of 12.1% in patients treated adjunctively with VGB, compared to 3.5% with placebo.Citation48 The incidence of depression is lower, about 6%, with VGB monotherapy.Citation33 Depression in the setting of VGB treatment is typically mild. In general, psychiatric side effects decrease with dose reduction or slow taper of VGB, and typically reverse with discontinuation of VGB.Citation14,Citation48
Safety issues
In addition to the side effects reported by patients, there are two safety issues that have been extensively investigated: intramyelinic edema (IME) and visual field defects.
Intramyelinic edema
IME was initially reported in rodents and dogs treated with VGB.Citation50 In rats, myelin microvacuolation leading to IME was localized to the hypothalamus, fornix columns, and cerebellar white matter, while in dogs it was found in the hypothalamus, fornix columns, optic tract and chiasm.Citation51 In another study, VGB caused dose- and time-dependent microvacuolation within white matter tracts of the cerebellum, reticular formation and thalamus in rodents, and the fornix and anterior commissure in dogs.Citation52 On electron microscopy, the microvacuolation was caused by separation of the outer lamellar sheaths of myelinated fibers and has been termed IME. The edema developed over a period of several weeks, after which a relative plateau was reached. It was reversible in both rats and dogs 12 to 16 weeks after stopping VGB. Dogs did not have any residual pathology after recovery, but rodents retained swollen axons and foci of microscopic mineralization within the cerebellum after recovery. Monkeys were studied, but did not demonstrate any conclusive pathological changes.Citation52 Because the inactive R-enantiomer of VGB is not associated with IME, while the active S form is, IME is thought to be related to higher levels of brain GABA.Citation6,Citation52
Human trials of VGB were suspended in 1983 in the US due to the recognition of IME in rodents and dogs. Evoked potential studies and MRI proved to be sensitive non-invasive techniques to diagnose IME in these animals, and to confirm its absence in humans and other primates. Clinical trials were allowed to resume in 1990 after review of additional data.Citation6 Autopsy and surgical brain specimens failed to show evidence of IME in children or adults with complex partial seizures.Citation53 MRI was also normal in humans treated with VGB in doses from 1 to 6 g per day for 3 months to 12 years. A review of data from 350,000 patient-years of VGB exposure found no definite case of VGB-induced IME.Citation54
The assumption that IME is a species-specific adverse effect of VGB not affecting humans was called into question in 2006, when MRI signal changes consistent with IME were reported in infants with IS, treated with VGB.Citation55 A follow-up study reported MRI T2 hyperintensities in the basal ganglia, thalami, anterior commissure and corpus callosum in 7 out of 22 patients, ranging in age from 3 months to 18 years, all of whom were treated for IS.Citation55 In all of the patients, the T2 hyperintensities resolved with discontinuation of the medication.
Subsequent studies have confirmed MRI signal changes associated with VGB in infants.Citation56,Citation57 Wheless et al retrospectively reviewed MRI findings in VGB-treated patients, including 205 infants with IS, and a group of 668 patients (children over the age of 2 years, and adults) with refractory CPS.Citation56 A statistically significant increase of pre-specified MRI abnormalities occurred only in the infants with IS treated with VGB. The prevalence of MRI abnormalities was 22% in the VGB-treated infants, compared with 4% for VGB-naïve infants, while no statistically significant difference occurred in those treated for CPS.Citation56 VGB-associated MRI changes in infants may not be limited to those with IS; they have also been described in a small number of infants with focal epilepsy and epileptic encephalopathy.Citation57
MRI abnormalities associated with VGB in infants have typical characteristics. They are best seen on T2-weighted, FLAIR and diffusion-weighted images, and occur predominantly in the basal ganglia, thalamus, brainstem, or cerebellum.Citation56 They tend to peak after 3 to 6 months of exposure to VGB and most resolve, even with continued use of VGB.Citation56,Citation57 Although VGB is associated with a risk of MRI abnormalities in infants, there is no evidence for MRI changes due to VGB in children or adults with refractory CPS.Citation56 Investigators have hypothesized that developmental changes in myelination in infants, or an underlying metabolic condition, may predispose infants, but not older individuals, to VGB-induced MRI changes.Citation56
Visual field defect
The most significant and unique VGB-specific side effect is a peripheral visual field defect, occurring in one third or more of patients treated with VGB.Citation58 Rare sporadic visual field defects were reported during VGB development. In 1997, Eke et al published 3 case reports describing severe persistent peripheral visual field loss in patients who had been on VGB for over 2 years.Citation59 The patients presented with “tunnel vision” and one presented after noticing increased frequency of bumping into objects after 2 to 3 years on VGB. Eke’s report was followed by several case series documenting visual field defects in the setting of VGB adjunctive therapy. A troubling finding was that visual field defects frequently were asymptomatic. Patients did not recognize that their visual fields were impaired. Even patients with severe visual impairments sometimes attributed the difficulties that they experienced to clumsiness or drowsiness, and the nature of the problem was not clarified until visual field testing was performed.Citation58 These difficulties in reporting and diagnosing visual field defects led to delayed recognition of this problem as an adverse effect of VGB, and under-estimation of the risk in early studies. The median time to onset of the first observation of bilateral concentric peripheral field constriction in patients with CPS was over 4 years.Citation6
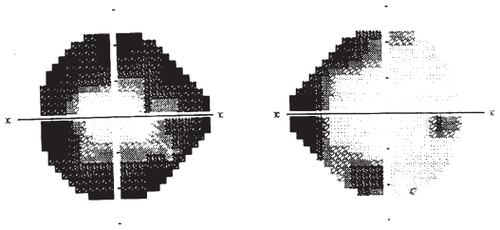
The peripheral visual field defect typically begins as a bilateral nasal defect, and progresses to bilateral concentric field constriction (see ). Initially, the visual defect is asymptomatic and is detected only by static or kinetic perimetry or electroretinogram (ERG). The earliest detectable visual field deficit is usually peripheral field depressions that later increase centripetally and rarely progress centrally.Citation60 It is hypothesized that patients do not notice the decreased peripheral vision loss early in the course of treatment because the initial peripheral vision loss occurs nasally and the binocularity of human vision compensates for nasal visual field loss. Symptomatic patients tend to have bilateral concentric field constriction, with involvement of their temporal visual fields, which are more likely to affect daily function since there is no visual field overlap temporally.Citation6 VGB exposure has not been shown to cause significant problems with central visual acuity.
Figure 2 Humphrey visual field map of a patient treated with vigabatrin (VGB). While the visual field defect in patients treated with VGB is typically similar in both eyes, in this patient, the right eye has restriction of the nasal field, while the left eye is more severely affected, demonstrating concentric restriction.
Pathophysiology of VGB-associated visual field defect
Although the pathophysiology of VGB-induced visual field defects is not entirely clear, animal studies have characterized retinal changes. Albino rats demonstrate dose-dependent concentration of VGB in the outer retina and also demonstrate retinal degeneration.Citation61 The retinal changes occur in the periphery and involve the outer nuclear layer, with displacement of the nuclei into the rod layer. VGB itself accumulates in the retina in significantly higher concentrations relative to other tissues, and is associated with accumulation of GABA in the retina.Citation62 In the setting of VGB treatment, animal studies demonstrate decreased activity of glutamic acid decarboxylase and GABA transaminase activity, and accumulation of GABA in the retina. Wang et al hypothesize that VGB may be associated with impaired glutamate release, based on abnormal retinal synaptic plasticity seen on examination of retinal tissue of albino mice treated with VGB.Citation63
A recent study suggests that light exposure and taurine deficiency may contribute to retinal damage and peripheral field defects in the setting of VGB treatment.Citation64 In this study, VGB-treated rats exposed to cycles of 12 hours of light and 12 hours of darkness exhibited more pronounced retinal lesions than those kept in darkness alone. It was also noted that taurine levels were 67% lower in VGB-treated animals compared with control animals, and taurine levels correlated with ERG amplitudes. Although dietary taurine supplementation did not reverse existing retinal changes, it reduced the development of retinal damage. The study suggests that, in rats, VGB induces a deficiency in taurine, resulting in retinal phototoxicity.Citation64 To assess the potential relevance of these findings to humans, the authors retrospectively reviewed data for 6 VGB-treated infants with infantile spasms. Five of the infants had low or undetectable taurine levels, including one who had a normal level prior to VGB treatment.Citation64
Risk factors for the development of visual field defects
Visual field defects occur in a significant number of patients treated with VGB, but studies of the prevalence and risk factors for VGB-associated visual field defects have reported widely varying results due to a variety of factors. These include the retrospective nature of the studies, the small numbers of patients studied, the lack of patient symptoms, the long latency until the condition was recognized and characterized by clinicians, and the variety of visual testing techniques used.Citation65 Reviewing 11 studies, Kalviainen et alCitation58 found an overall prevalence of bilateral concentric visual field defects in 32% of 528 patients treated with VGB. In 22 studies reviewed by Kinirons et alCitation66 peripheral visual field constriction occurred in 19% to 92% of adults with CPS treated with VGB, and up to 31% of infants with infantile spasms. In most of these studies, VGB was used as an adjunctive antiepileptic drug. VGB-associated visual field deficit has also been seen in patients treated with monotherapy. A study of newly diagnosed epilepsy patients who had been randomized to monotherapy with either VGB or CBZ, found that 41% of the 32 patients receiving VGB had visual field constriction, while none of the 18 patients receiving CBZ was affected.Citation67
It is unclear why some patients develop peripheral visual field defects on VGB and others do not. Studies suggest that males have twice the risk of developing a visual field defect from VGB compared to females.Citation58,Citation68–Citation72 Smoking has also been reported as a risk factor.Citation6,Citation58 However, a cohort study of 93 patients did not identify increased risk due to these factors.Citation73
There are contradictory reports regarding the risk of visual field defect and its relationship to cumulative exposure to VGB, maximum dose, and duration of dose. Several studies did not find evidence that these parameters correlated with the development of visual field defects.Citation58,Citation68,Citation73 However, a cohort study of the cumulative incidence of visual field defects and cumulative VGB dose in 291 patients found that the cumulative incidence of visual field defects rapidly increased within the first 2 kg of VGB intake, and stabilized after a total of 3 kg of VGB.Citation69 Conway et alCitation74 suggest that maximum daily VGB dose is a predictor for development of peripheral visual field defects, and not cumulative or duration of dose.Citation74 Other studies have also suggested that degree or duration of VGB exposure correlate with the development of peripheral visual field defects.Citation60,Citation75–Citation80
Visual field defects in children
Although some studies have suggested that children treated with VGB are less likely then adults to develop visual field defects, a recent study found only a small difference between children 8 to 12 years of age and older study participants. The proportion of patients with visual field defects was 29% for those 8 to 12 years of age, and 33% for those over age 12. This difference was not statistically significant, possibly due to the small size of the comparison groups.Citation65 The risk of VGB-associated visual field abnormalities may be lower in children who were treated with VGB in infancy, compared to those who received it later in childhood. Mild visual field loss was found in 1 of 16 children, aged 6 to 12 years, who had been treated with VGB during infancy for IS.Citation81
Time course of visual field defect
Analyses of the time course of peripheral visual field defects with VGB suggest that it develops gradually. Ovation Pharmaceuticals reports that, in patients with CPS on VGB, the earliest documented peripheral visual defect occurred after 9 months of treatment in adults, and after 11 months in children.Citation6 There is a case report of a visual field defect developing within 6 months of VGB treatment.Citation82 After 5 years of VGB treatment, the risk of development of peripheral visual field defect decreases sharply.Citation73
Although varying methods of testing and the variability of patient responses make it difficult to combine study results, on average the progression of the visual field defect is <2 degrees per year from the temporal visual field and <1 degree per year in the nasal field.Citation6 Once the peripheral visual fields are affected, the defect is usually irreversible but does not worsen over time.Citation70,Citation83 In a study of 60 adults with CPS treated with VGB for up to 14 years, 40% had visual field defects. At follow-up, after an average of 15 months, there was no significant progression in patients who continued to take VGB and no recovery in those who had discontinued it. Ovation Pharmaceuticals reported 1 patient who had progression of his visual field defect 4 years after discontinuing VGB.Citation6
Diagnostic testing of visual fields
Several options are available to test for visual field defects. The only method appropriate for testing infants, young children, and adults unable to cooperate with visual field testing is the ERG, during which an electrode is placed on the eye to monitor the response of the retina to flashes of light. Although the ERG is not a direct test of visual fields, wide-field and multifocal ERG techniques are highly sensitive at detecting VGB-associated retinal pathology.Citation84–Citation86 Perimetry techniques require patient cooperation. Kinetic perimetry, of which the most commonly performed is Goldmann perimetry, consists of an examiner moving stimuli through the patient’s peripheral visual field and mapping defects on a reference grid. Static perimetry uses automated visual field analyzers, such as the Humphrey visual field analyzer, to determine the threshold intensity that the patient can perceive at specific locations in the visual field. The patient’s reaction is then measured and mapped to display the visual field. Routine visual evoked potentials (VEPs) are not useful for assessing retinal changes, but field-specific VEPs have identified VGB-induced retinal defects in children over 2 years of age.Citation87
A “gold standard” test for identifying VGB-associated visual field defects has not been established. Thus the true sensitivities and specificities of various techniques in this clinical setting cannot be calculated. In one study, objective outer limit testing (a “bedside” method performed by an examiner with a flashlight) detected 83% of the visual field defects identified by Goldmann perimetry, while manual kinetic perimetry detected 93%, and high pass resolution perimetry (a computer-based central field test) had a sensitivity of 72%.Citation60 In general, while ERG is the most specific test, both static and kinetic perimetry are believed to have adequate sensitivity to monitor peripheral vision with VGB.Citation14 As our knowledge of the pathophsyiology of the VGB field defect progresses, it is likely that the optimal strategy for visual testing will evolve.
A promising technique for identification of VGB-associated visual abnormalities involves imaging of the retinal nerve fiber layer. The characteristic pattern consists of thinning of the nasal quadrant but sparing of the temporal quadrant. This finding may precede visual field loss. A recent study that measured the thickness of the retinal nerve fiber layer using ocular coherence tomography found this pattern in all of the 11 patients with confirmed VGB-associated visual field deficits, as well as 4 of 15 VGB-treated patients who had normal fields. These findings suggest that nasal retinal nerve fiber layer attenuation is a promising biomarker for VGB toxicity, and may be valuable indicator for consideration of VGB withdrawal.Citation88
Recommendations for visual field monitoring
All patients with refractory CPS who are considering treatment with VGB should have a baseline visual field examination. VGB should not be used in those with restricted visual fields at baseline. VGB-treated adults should have a follow-up visual field examination every 6 months. Infants should be tested at 3-month intervals for the first 18 months of treatment, and then every 6 months. It has been well documented that response of CPS to VGB is evident by the 12th week of therapy, earlier than the reported onset of visual field defects.Citation14 If substantial improvement in CPS has not been achieved by 12 weeks of VGB therapy, then the drug should be stopped in order to minimize the risk of developing a peripheral visual field defect. If VGB treatment is successful in treating refractory CPS, the risks and benefits at that point should be re-evaluated with the patient. Data suggest that after 5 years of VGB exposure, the risk of developing a peripheral visual field defect stabilizes and therefore less intensive monitoring may suffice at that point.Citation6
Conclusions and recommendations
Approximately 30% of patients with epilepsy have seizures that continue to occur despite pharmacologic treatment.Citation89 Uncontrolled seizures can severely impair a patient’s quality of life, and may lead to seizure-related injuries or even sudden unexplained death in epilepsy.Citation90,Citation91 Improvement in seizure control in this population can positively affect prognosis and patient well-being.
The major benefit of VGB is that it has demonstrated efficacy in some patients whose seizures have been resistant to other drugs. A comparison of key clinical trials of newer antiepileptic drugs found a favorable efficacy and side effect profile for VGB in the adjunctive treatment of CPS.Citation19 It has few cognitive side effects, and is generally well tolerated by patients.
These potential benefits must be balanced with the significant risk of developing a visual field defect, which develops in about one third of patients taking VGB. Because of this risk, VGB should be considered only as adjunctive therapy for those patients whose CPS have not responded to other treatments, and who are not appropriate candidates for other therapies, such as epilepsy surgery.Citation92 Since the onset of visual field defects is usually asymptomatic, visual fields and/or ERG must be checked at baseline, and every 3–6 months during treatment, to monitor for the development of defects. General recommendations for patient selection and monitoring are listed in . A cautious strategy of targeted patient selection and careful monitoring for visual field defects should optimize the risk-benefit ratio of VGB in the clinical setting.
Table 1 Recommendations for use of vigabatrin (VGB) in adults with complex partial seizures
Disclosures
Dr Waterhouse has received research support from UCB Pharma, Supernus, and GlaxoSmithKline.
References
- JungMFLippertBMetcalfBWBöhlenPSchechterPJgamma-Vinyl GABA (4-amino-hex 5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in miceJ Neurochem197729797802591956
- SheeanGSchrammTAndersonDSEadieMJVigabatrin – plasma enantiomer concentrations and clinical effectsClin Exp Neurol1992291071161343855
- GramLLarssonOMJohnsenASchousboeAExperimental studies of the influence of vigabatrin on the GABA systemBr J Clin Pharmacol198927Suppl 113S17S2757904
- HokeJFChiEMAntonyKKKulmalaHKWalkerBJEffect of food on the bioavailability of vigabatrin tabletsEpilepsia199132Suppl 37
- HaegeleKDHuebertNDEbelMTellGPSchechterPJPharacokinetics of vigabatrin: implications of creatinine clearanceClin Pharmacol Ther1988445585653180638
- Ovation Pharmaceuticals, Inc.Sabril (vigabatrin) tablet and powder for oral solution for adjunctive treatment of refractory complex partial seizures in adults (NDA 20-427) and for monotherapy treatment of infantile spasms (NDA 22-006)Advisory Committee Briefing DocumentJan–Aug2009 URL: www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4396b1-020Ovation.pdfAccessed April 14, 2009
- ElwesRDBinnieCDClinical pharmacokinetics of newer antiepileptic drugs. Lamotrigine, vigabatrin, gabapentin and oxcarbazepineClin Pharmacokinet1996304034158792055
- MenachemEBPerssonLISchechterPJEffects of single doses of vigabatrin on CSF concentrations of GABA, homocarnosine, homovanillic acid and 5-hydroxyindoleacetic acid in patients with complex partial epilepsyEpilepsy Res19882961013143561
- EasterwoodLIn vitro assessment of the induction potential of vigabatrin in primary cultures of human hepatocytesProject Report 3210-0628-1800/OVNC-90142007
- BrowneTRMattsonRHPenryJKVigatabrin for refractory complex partial seizures: multicenter single-blind study with long-term follow-upNeurology1987371841893808298
- RimmerEMRichensADouble-blind study of gamma-vinyl-GABA in patients with refractory epilepsyLancet198411891906141335
- RimmerEMRichensAInteraction between vigabatrin and phenytoinBr J Clin Pharmacol19892727S33S2757906
- JedrzejcazkJDlawichowskaEOwczarekKMajikowskiJEffect of vigabatrin addition on carbamazepine blood serum levels in patients with epilepsyEpilepsy Res20003911512010759299
- WillmoreLJAbelsonMBBen-MenachemEPellockJMShieldsWDVigabatrin: 2008 UpdateEpilepsia20095016317319230067
- ParisiPBombardieriRCuratoloPCurrent role of vigabatrin in infantile spasmsEur J Pediatr Neurol200711331336
- HancockECOsborneJPEdwardsSWTreatment of infantile spasmsCochrane Database of Syst Rev20084CD00177018843624
- HancockEOsborneJPVigabatrin in the treatment of infantile spasms in tuberous sclerosis: Literature reviewJ Child Neurol199914717410073425
- Cohen-SadanSKramerUBen-ZeevBMulticenter long-term follow-up of children with idiopathic West Syndrome: ACTH versus vigabatrinEur J Neurol20091648248719348622
- CramerJAFisherRBen-MenachemEFrenchJMattsonRHNew anti-epileptic drugs: comparison of key trialsEpilepsia19904059060010386528
- GramLKlosterskovPDamMgamma-Vinyl GABA: a double-blind placebo-controlled trial in partial epilepsyAnn Neurol1985172622663922282
- LoiseauPHardenbergJPPestreMGuyotMSchechterPJTellGPDouble-blind, placebo-controlled study of vigabatrin (gamma-vinyl GABA) in drug-resistant epilepsyEpilepsia1986271151203514204
- TartaraAManniRGalimbertiCAHardenbergJOrwinJPeruccaEVigabatrin in the treatment of epilepsy: a double-blind, placebo-controlled studyEpilepsia1986277177233536469
- TassinariCAMichelucciRAmbrosettoGSalviFDouble-blind study of vigabatrin in the treatment of drug-resistant epilepsyArch Neurol1987449079102887152
- BeranRGBerkovicSFBuchananNA double-blind, placebo-controlled crossover study of vigabatrin 2 g/day and 3 g/day in uncontrolled partial seizuresSeizure199652592658952010
- The Italian Study Group on VigabatrinSingle-blind, placebo-controlled multicenter trial of vigabatrin in the treatment of epilepsyItal J Neurol Sci1992137417471483856
- BruniJGubermanAVachonLDesforgesCThe Canadian Vigabatrin Study GroupVigabatrin as add-on therapy for adult complex partial seizures: a double-blind, placebo-controlled multicentre studySeizure2000922423210777431
- FrenchJAMosierMWalkerSSommervilleKSussmanNthe Vigabatrin Protocol 024 Investigative Cohort. A double-blind, placebo-controlled study of vigabatrin three g/day in patients with uncontrolled complex partial seizuresNeurology19964654618559421
- DeanCMosierMPenryKDose-Response Study of Vigabatrin as add-on therapy in patients with uncontrolled complex partial seizuresEpilepsia19994074829924905
- NabboutRCChironCMumfordJDumasCDulacOVigabatrin in partial seizures in childrenJ Child Neurol1997121721779130090
- CamposanoSEMajorPHalpernEThieleEAVigabatrin in the treatment of childhood epilepsy: a retrospective chart review of efficacy and safety profileEpilepsia2008491186119118479386
- KalviainenFAikiaMSaukkonenAMMervaalaERiekkinenPJSr.Vigabatrin vs carbamazepine monotherapy in patients withnewly diagnosed epilepsy. A randomized, controlled studyArch Neurol1995529899967575227
- TanganelliPRegestaGVigabatrin vs carbamazepine monotherapy in newly diagnosed focal epilepsy: a randomized response conditioned cross-over studyEpilepsy Res1996252572628956924
- ChadwickDthe Vigabatrin European Monotherapy Study GroupSafety and efficacy of vigabatrin and carbamazepine in newly diagnosed epilepsy: a multicentre randomized double-blind study. Vigabatrin European Monotherapy Study GroupLancet1999354131910406359
- MichelucciRTassinariCAResponse to vigabatrin in relation to seizure typeBr J Clin Pharmacol198927Suppl 119S24S2667604
- RemyCBeaumontDEfficacy and safety of vigabatrin in the long-term treatment of refractory epilepsyBr J Clin Pharmacol198927Suppl 1125S129S2757903
- SiveniusJYlinenAMurrosKMumfordJPRiekkinenPJVigabatrin in drug-resistant partial epilepsy: a 5-year follow-up studyNeurology1991415625651901397
- TartaraAManniRGalimbertiCASix-year follow-up study on the efficacy and safety of vigabatrin in patients with epilepsyActa Neurol Scand1992862472511414241
- YlinenASalmenperaTMumfordJPRiekkinenPJLong-term treatment with vigabatrin – 10 years of clinical experienceSeizure1999618118310356378
- GubermanABruniJLong-term openmulticentre, add-on trial of vigabatrin in adult resistant partial epilepsy. The Canadian Vigabatrin Study GroupSeizure2000211211810845734
- TartaraAManniRGalimbertiCAMumfordJPIudiceAPeruccaEVigabatrin in the treatment of epilepsy: a long-term follow-up studyJ Neurol Neurosurg Psychiatry1989524674712661725
- McGuireAMDuncanJSTrimbleMREffects of Vigabatrin on cognitive function and mood when used as add-on therapy in patients with intractable epilepsyEpilepsia1992331281341733746
- GillhamRABlacklawJMcKeePJBrodieMJEffect of vigabatrin on sedation and cognitive function in patients with refractory epilepsyJ Neurol Neurosurg Psychiatry199356127112758270925
- ProvincialiLBartoliniMMariFDel PesceMCeravoloMGInfluence of vigabatrin on cognitive performances and behaviour in patients with drug-resistant epilepsyActa Neurol Scand19969412188874587
- DodrillCBArnettJLSommervilleKWSussmanNMEffects of differing dosages of vigabatrin (Sabril) on cognitive abilities and quality of life in epilepsyEpilepsia1995361641737821274
- GrunewaldRAThompsonPJCorcoranRCordenZJacksonGDDuncanJSEffects of Vigabatrin on partial seizures and cognitive functionJ Neurol Neurosurg Psychiatry199457105710638089668
- SanderJWHartYMTrimbleMRShorvonSDVigabatrin and psychosisJ Neurol Neurosurg Psychiatry1991544354391865207
- FerrieCDRobinsonROPanayiotopoulosCPPsychotic and severe behavioural reactions with vigabatrin: a reviewActa Neurol Scand199693188825264
- LevinsonDFDevinskyOPsychiatric adverse events during vigabatrin therapyNeurology1999531503151110534259
- MulaMSanderJWNegative effects of antiepileptic drugs on mood in patients with epilepspyDrug Safety20073055556717604407
- ButlerWHThe neuropathology of vigabatrinEpilepsia198930Suppl 3S15172767014
- GrahamDNeuropathology of vigabatrinBr J Clin Pharmacol198927Suppl 143S45S2757908
- GibsonJPYarringtonJTLoudyDEGerbigCGHurstGHNewberneJWChronic toxicity studies with vigabatrinToxicol Pathol1990182252382399411
- CannonDJButlerWHMumfordJPLewisPJNeuropathologic findings in patients receiving long-term vigabatrin therapy for chronic intractable epilepsyJ Child Neurol1991Suppl 2S17241940119
- CohenJAFisherRSBrigellMGPeysterRGSzeGThe potential for vigabatrin-induced intramyelinic edema in humansEpilepsia20004114815710691111
- PearlPLVezinaLGSanetoRPCerebral MRI abnormalities associated with vigabatrin therapyEpilepsia200950218419418783433
- WhelessJWCarmantLBebinMMagnetic resonance imaging abnormalities associated with vigabatrin in patients with epilepsyEpilepsia20095019520519054414
- MilhMVilleneuveNChaponFTransient magnetic resonance imaging hyperintensity in basal ganglia and brainstem of epileptic infants treated with vigabatrinJ Child Neurol200924330531519258289
- KalviainenRNousiainenIVisual field defects with vigabatrin: epidemiology and therapeutic implicationsCNS Drugs20011521723011463129
- EkeTTalbotJFLawdonMCSevere persistent visual field constriction associated with vigabatrinBMJ19973141801819022432
- MalmgrenKBen-MenachemEFrisénLVigabatrin Visual Toxicity: Evolution and dose dependenceEpilepsia20014260961511380567
- ButlerWHFordGPNewberneJWA study of the effects of vigabatrin on the central nervous system and retina of Sprague-Dawley and Lister-Hooded ratsToxicol Pathol1987151431483616399
- SillsGJPatsalosPNButlerEForrestGRatnarajNBrodieMJVisual field constriction: accumulation of vigabatrin but not tiagabine in the retinaNeurology20015719620011468302
- WangQPJammoulFDubocATreatment of epilepsy: the GABA-transaminase inhibitor, vigabatrin, induces neuronal plasticity in the mouse retinaEur J Neurosci2008272177218718412635
- JammoulFWangQNabboutRTaurine deficiency is a cause of vigabatrin-induced retinal phototoxicityAnn Neurol2009659810719194884
- WildJMAhnHSBaulacMVigabatrin and epilepsy: Lessons learnedEpilepsia2007481318132717635558
- KinironsPCavalleriGLSinghRA pharmacogenetic exploration of vigabatrin-induced visual field constrictionEpilepsy Res20067014415216675198
- KalviainenRNousiainenIMantyjarviMVigabatrin, a gabaergic drug, causes concentric visual field defectsNeurology19995392292610496247
- WildJMMartinezCReinshagenGHardingGFCharacteristics of a unique visual field defect attributed to vigabatrinEpilepsia1999401784179410612345
- Committee for proprietary medicinal productsOpinion following an article 12 referral. VigabatrinThe European Agency for the Evaluation of Medicinal products Committee for Proprietary Medicinal Products (CPMP) – report/1357/99, URL http://www.emea.europa.eu/pdfs/human/phv/135799ENB.pdf
- HardusPVerduinWMPostmaGStilmaJSBerendschotTTvan VeelenCWConcentric contraction of the visual field in patients with temporal lobe epilepsy and its association with the use of vigabatrin medicationEpilepsia20004158158710802764
- HardusPVerduinWMPostmaGStilmaJSBerendschotTTvan VeelenCWLong term changes in the visual fields of patients with temporal lobe epilepsy using vigabatrinBr J Ophthalmol20008478879010873996
- HardusPVerduinWMEngelsmanMVisual field loss associated with vigabatrin: quantification and relation to dosageEpilepsia20014226226711240600
- KinironsPCavalleriGLO’RourkeDVigabatrin retinopathy in an Irish cohort: lack of correlation with doseEpilepsia20064731131716499754
- ConwayMCubbidgeRPHoskingSLVisual field severity indices demonstrate dose-dependent visual loss from vigabatrin therapyEpilepsia20084910811618184224
- LawdenMCEkeTDeggCHardingGFWildJMVisual field defects associated with vigabatrin therapyJ Neurol Neurosurg Psychiatry19996771672210567485
- HardingGFWildJMRobertsonKAElectro-oculography, electroretinography, visual evoked potentials, and multifocal electroretinography in patients with vigabatrin-attributed visual field constrictionEpilepsia2000411420143111077455
- ManuchehriKGoodmanSSiviterLNightingaleSA controlled study of vigabatrin and visual abnormalitiesBr J Ophthalmol20008449950510781514
- ToggweilerSWieserHGConcentric visual field restriction under vigabatrin therapy: extent depends on the duration of drug intakeSeizure20011042042311700995
- JensenHSjoOUldallPGramLVigabatrin and retinal changesDoc Ophthalmol200210417118011999624
- SchmidtTRutherKJokielBPfeifferSTiel-WilckKSchmitzBIs visual field constriction in epilepsy patients treated with vigabatrin reversibleJ Neurol20022491066107112195456
- GailyEJonssonHLappiMVisual fields at school age in children treated with Vigabatrin in infancyEpilepsia20095020621619215279
- KiratliHTurkcuogluPRapid development of visual field defects associated with vigabatrin therapyEye2001Pt 567267411702991
- NousiainenIMantyjarviMKalviainenRNo reversion in vigabatrin-associated visual field defectsNeurology2001571916191711723291
- HardingGFWildJMRobertsonKARietbrockSMartinezCSeparating the retinal electrophysiologic effects of vigabatrin: treatment versus field lossNeurology20005534735210932265
- McDonaghJStephenLJDolanFMPeripheral retinal dysfunction in patients taking vigabatrinNeurology2003611690169414694031
- PonjavicVAndreassonSMultifocal ERG and full-field ERG in patients on long-term vigabatrin medicationDoc Ophthalmologica20011026372
- HardingGFSpencerELWildJMConwayMBohn RL Field-specific visual-evoked potentials; identifying field defects in vigabatrin-treated childrenNeurology2002581261126511971096
- LawthomCSmithPEMWildJMNasal retinal nerve fiber layer attenuation: A biomarker for vigabatrin toxicityOphthalmology200911656557119168223
- BrodieMJKwanPStaged approach to epilepsy managementNeurology2002588 Suppl 5S2811971127
- SperlingMRThe consequences of uncontrolled epilepsyCNS Spectr200499810114999166
- TomsonTWalczakTSillanpaaMSanderJWSudden unexpected death in epilepsy; a review of incidence and risk factorsEpilepsia200546Suppl 11546116393182
- WhelessJWRamsayRECollinsSDVigabatrinNeurotherapeutics2007416317217199033