Abstract
The presence of suicidal manifestations (thoughts and behavior) was studied in a cohort of 30 patients with mild to moderate depression during a 6-week treatment with the serotonin-norepinephrine reuptake inhibitor, milnacipran. At baseline mild suicidal thoughts were present in 46.7% of patients, the mean Hamilton Depression Rating Score (HDRS17) was 23.9 ± 1.8 and the mean suicidality score on the Beck Scale for Suicidal Ideation (BSS) was 4.9 ± 4.9. Suicidal thoughts decreased progressively throughout the study in parallel with other depressive symptoms. At no time during treatment was there any indication of an increased suicidal risk. Notably, the items retardation and psychic anxiety on the HDRS17 decreased in parallel. This may possibly explain the lack of any “activation syndrome”, which is occasionally observed at the early stages of therapy with some antidepressants and may be linked to a temporary increase in suicidal ideation. To our knowledge this is the first detailed report of suicidality during treatment with milnacipran.
Keywords:
Introduction
There is a clear association between suicide and depression. Mood disorders, principally major depressive disorder and bipolar disorder, are associated with about 60% of all suicides.Citation1–Citation6 An often-quoted figure is that about 15% of patients with major depression will eventually die by suicide.Citation7,Citation8 This estimation is, however, derived largely from studies of severely depressed inpatients. More recently a more conservative lifetime suicide risk in the general population of depressed patients of about 6% has been calculated.Citation9 This discrepancy has been erroneously interpreted by many primary care physicians and certain psychiatrists as evidence that overt suicidal behavior occurs only in severe depression. This notion can lead to inappropriate management of less severely depressed patients.
The more recent clinical literature clearly supports the idea that suicidality (thoughts and behavior) is associated with any type of depressive disorder and any degree of severity.Citation10,Citation11 Antidepressant therapy, which is effective in managing the symptoms of major depressive disorder, is expected to reduce or prevent suicidal behavior associated with depression.Citation12,Citation13 This hypothesis has been questioned, however, by large multi-study analyses that have suggested that, at least in certain patients groups, antidepressants may enhance or even induce suicidal ideation.Citation14,Citation15 In 2004 the US Food and Drug Administration warned of the possible enhancement of suicidal ideation in pediatric and adult patients receiving modern antidepressants especially at the beginning of therapy and at dose increase. This interpretation is controversial and the results of other meta-analysesCitation16,Citation17 have not supported the enhancement of suicidal ideation in adult patients with short-term antidepressant treatment compared to placebo. The latest analyses indicated that patients receiving SSRIs and tricyclic antidepressants (TCAs) do not show significantly more suicidality than patients receiving placebo.Citation6,Citation18 Indeed several studies have shown that suicide rates have decreased since the introduction of modern antidepressants supporting the hypothesis that the use of antidepressants may prevent suicide.Citation19,Citation20
In the light of this controversy, the effect of an antidepressant, especially a recently introduced one, on suicidal ideation clearly remains an important question. Thus, in addition to assessing the general efficacy and tolerability of milnacipran (Ixel®), a serotonin and norepinephrine reuptake inhibitor (SNRI)Citation21 recently introduced into Russia, the primary aim of the present investigation was to observe the effects of the drug on the occurrence and intensity of suicidal thoughts and behavior in patients with mild to moderate depressive disorders and to assess whether suicidality decreased in parallel with other depressive symptoms.
Methods
The investigation was a 6-week open label study in patients enrolled from an outpatient clinic (Psychiatric Hospital N12) in Moscow. Inclusion criteria were: age 16 to 65 years, a diagnosis of mild to moderate depression which could be either a single depressive episode, recurrent depressive disorder, bipolar disorder, dysthymia, cyclothymia, continuous depressive reaction within an adaptation disorder or other mood disorder. Exclusion criteria included severe depression with psychotic manifestations; schizophrenia and schizoaffective disorder; abuse of alcohol and other psychoactive agents; pronounced manifestations of psycho-organic syndrome (or chronic brain disorder), including paroxysmal disorders; severe or decompensated somatic pathology requiring continuous polypharmacotherapy; pregnancy and lactation; participation in clinical trials within 30 days prior to start-up of the present study; and contradictions to milnacipran treatment. All diagnoses were based on ICD-10 criteria.
The study was approved by the local ethics committee and was carried out in accordance with the Declaration of Helsinki. All patients gave their written informed consent.
In addition to a standard psychiatric and general clinical examination (including urinalysis, hematology and biochemistry, electrocardiogram), the 17-item Hamilton Depression Rating Scale (HDRS17), Clinical Global Impression (CGI), Beck Depression Inventory (BDI), Beck Scale for Suicidal Ideation (BSS)Citation22 and the Udvalg for Kliniske Undersøgelser Side Effect Rating scale (UKU-side-effects) were used.
Milnacipran was administered initially, for 2 to 3 days, at 25 to 50 mg/day depending on the severity of depressive symptoms. If the drug was well tolerated the dose was increased to 100 mg/day (50 mg twice daily).
The statistical significance of changes from baseline during the study were determined using paired Student’s t-test and Wilcoxon nonparametric test using the statistical and analytical software package STATISTICA. The significance level was set as P < 0.05
Results
Thirty patients were enrolled in the study. Demographic characteristics of the patients are shown in . Women comprised 86.6% of the cohort and the mean age of patients was 41.4 ± 9.5 (all values are given as mean ± standard deviation). In general the educational level was high with 60% of patients having a higher (university) education and 33.3% a secondary education. Only 40% of patients were married.
Table 1 Patients’ clinical demographics
The majority of patients (60%) had a diagnosis of a single depressive episode. Most patients (86.6%) at baseline were considered to be moderately depressed. The baseline HDRS17 was 23.9 ± 1.8 and BDI was 37.7 ± 3.9. The apathetic form of depression was the most frequent while anxious, asthenic and hysteric forms were also common. The hypochondriac form was only found in 2 patients.
Two patients withdrew from the study, one at the end of the first week of treatment because of adverse events (nausea) and the other at the end of the 4th week of treatment due to lack of efficacy of the treatment.
Antidepressant efficacy
The efficacy of the antidepressant treatment was demonstrated by the steady reduction of the mean HDRS17 score throughout the study (). Statistically significant differences compared to baseline occurred from the first week of therapy. At endpoint (after 6 weeks treatment) mean HDRS17 was 9.5 ± 4.4 and 60% of patients were treatment responders (reduction of baseline HDRS17 of at least 50%). BDI scores showed a similar progressive reduction with a significant difference (P < 0.05) from baseline occurring from the third week of treatment. The mean BDI score at endpoint was 21.4 ± 6.1 (data not shown).
Figure 1 Reduction of mean global HDRS17 scores during the study.
Abbreviations: B, baseline; W1 to W6, number of weeks of treatment.
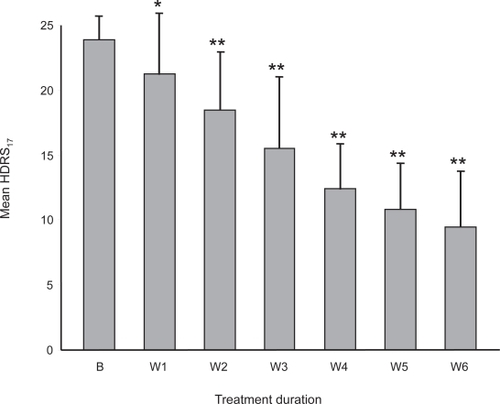
The mean baseline score on the CGI (Severity) scale was 3.9 ± 0.4 indicating a moderate severity. This value decreased progressively with a significant difference (P < 0.05) from baseline occurring after 3 weeks of treatment. At endpoint the mean CGI (Severity) score was 2.1 ± 0.3, indicating borderline depression (data not shown).
Suicidality
At baseline mild suicidal ideation was found in 14 patients (46.7%). No current or previous suicidal attempts, planning or preparation were noted in any patient.
A total of 57 suicidal manifestations were found during the study (). The most common suicidal manifestations were thoughts of “inanity of existence”, thoughts that death could resolve existing sufferings, suicidal thoughts and unwillingness to live.
Table 2 Suicidality (thoughts and behavior) reported during the study
More rarely, different signs of suicidality were reported: visualization of own suicide, death or funeral, as well as (supraliminal) death instinct. Only in a few patients did these suicidal ideations develop into either ideational compulsions (compulsive thoughts and concepts of death and funerals) or suicidal fantasies. Suicidal compulsive thoughts and concepts developed in patients with asthenic and hypochondriac depression, and suicidal fantasies in patients with hysteric depression. No other relationships, including those between suicidal phenomena and patients personality characteristics were detected.
The entry criteria of this study allowed the recruitment of patients with a single depressive episode, recurrent depressive disorder, chronic depression, bipolar disorder, dysthymia or cyclothymia. Unfortunately subanalysis of the different types of depression was not possible due to the small numbers of each subtype.
Mean baseline BSS score was 4.9 ± 4.9, indicating a mild level of suicidality in this group of patients. Suicidality decreased rapidly with a significant reduction observed from the second week of treatment (). After 4 weeks of treatment, mean BSS scores were close to zero indicating an absence of any suicidal activity in treated patients. None of the patients showed any increase in BSS score during milnacipran treatment indicating an absence of any “activation syndrome”, sometimes observed at the early stages of therapy with certain antidepressants.Citation10
Table 3 Evolution of mean global Beck Scale for Suicidal Ideation scores during the study
Analysis of the “suicidal thoughts/attempts” item of the HDRS17 at baseline showed a score of 1 in 10 patients (33.3%) and of 2 in one patient. The number of patients with scores 1 or 2 decreased progressively throughout the study. At endpoint only 3 patients had a score of 1, all others scoring zero.
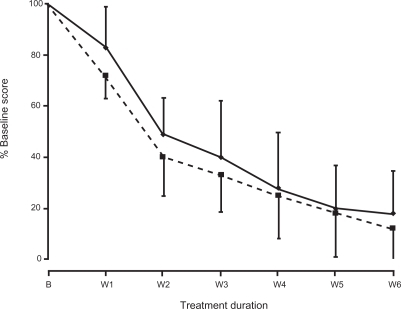
The HDRS17 items of “psychic anxiety” and “retardation” showed a similar progressive reduction throughout the study. The rate of decrease of the two items was approximately parallel (). At the end of the study anxiety was reduced by 82% and retardation by 88%. The anxiety/retardation ratio remained essentially constant during milnacipran therapy confirming the absence of any “activation syndrome”.Citation10
Figure 2 Reduction of mean “anxiety” and “retardation” scores during the study.
Abbreviations: B, baseline; W1 to W6, number of weeks of treatment; Solid line, “anxiety” item; broken line, “retardation” item.
Adverse events
Adverse events (AEs) with milnacipran therapy were scored with the UKU Side Effect Rating in all patients. A total of 48 AEs were recorded during the study (). No AE was scored with a severity rating greater than 2, indicating that they did not significantly affect the patients’ daily life. The most frequent AEs, which occurred in more than 5 patients, were nausea, anxiety, tachycardia and dizziness. The majority of AEs occurred within the first week of treatment with milnacipran and tended to resolve spontaneously from the beginning of the second week. Seventy percent of all AEs had resolved by the third week of treatment.
Table 4 Adverse events
Discussion
The cohort of the present study was a relatively classical mild to moderately depressed population although the proportion of women (86%) was somewhat higher than that commonly reported. At baseline nearly 50% of patients reported some form of suicidal ideation although none reported previous suicidal attempts, planning or preparation. Almost all of the suicidal manifestations observed during the study were limited to the cognitive sphere (thoughts, concepts). In addition, in the majority of cases, suicidal manifestations occurred only occasionally, and the suicidal fantasies, reported in only 2 patients, were transient (up to 1 to 2 hours per day over several days).
The wide prevalence of different suicidal manifestations in patients with mild to moderate depressive disorders shown here is important since, even in absence of a behavioral component they are considered to be a potent predictor of future suicidal behavior.Citation2,Citation13,Citation23
Regular patient assessment with HDRS17, CGI and BDI showed a progressive regression of depressive symptoms throughout the study. There were few AEs, all of them mild as assessed by the UKU Side Effect Rating. Only one patient withdrew because of side effects.
The progressive regression of suicidal thoughts during milnacipran therapy observed at weekly testing showed no enhancement of suicidal risk at early stages of antidepressant pharmacotherapy. On the contrary, the administration of milnacipran led to a progressive and almost total reduction of all suicidal manifestations. The drug’s effects on important aspects of the clinical profile of depression such as anxiety and retardation appeared to be well balanced. This may have contributed to the lack of “activation syndrome” which has been associated increased suicide risk.Citation10 Even the occurrence of AEs such as anxiety during milnacipran therapy did not negatively affect the general suicidality of patients.
This study suffers from several weaknesses, namely the small cohort studied, its open nature and the relatively short observation period (6 weeks). To our knowledge this is the first study to have analyzed suicidality and depressive symptoms in parallel during the treatment with milnacipran. A meta-analysis of suicidal behaviors and ideation in clinical trials in depression with another SNRI, duloxetine, has also demonstrated the absence of increased risk of suicidal behaviors or ideation during treatment with the SNRI compared with placebo.Citation24
From this small open study there is a clear suggestion that mild suicidal thoughts can be relatively common even in mild to moderately depressed patients. During treatment with milnacipran suicidal thoughts regressed progressively in parallel with other depressive symptoms. At no time was there any noticeable increase in suicidal thoughts even in patients who reported anxiety as an adverse event. This study clearly warrants replication in a larger cohort which should be followed for a longer period of time.
Acknowledgments and disclosures
We thank Dr Mike Briley for his help in correcting the manuscript. After completion of the study and its analysis, Pierre Fabre Médicament, manufacturers of milnacipran, offered to support the publication of the study with an unconditional educational grant. They played no role in the interpretation of the data or in writing the manuscript.
References
- IsometsaETHenrikssonMMAroHMSuicide in major depressionAm J Psychiatry19941515305368147450
- BradvikLBerglundMSuidical ideation in severe depressionEur Arch Psychiatry Clin Neurosci200025013914310941988
- BradvikLBerglundMSuicide in severe depression related to treatment: depressive characteristics and rate of antidepressant overdoseEur Arch Psychiatry Clin Neurosci200425524525016133742
- BradvikLThe occurrence of suicide in severe depression related to the months of the year and the days of the weekEur Arch Psychiatry Clin Neurosci2002252283212056579
- GibbonsRDHurKBhaumikDKThe relationship between antidepressant medication use and rate of suicideArch Gen Psychiatry20056216517215699293
- MartinezCRietbrockSWiseLAntidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control studyBMJ200533038939615718538
- GuzeSBRobinsESuicide and primary affective disordersBr J Psychiatry19701174374385481206
- GoodwinFKJamisonKRManic-Depressive Illness: Bispolar Disorders and Recurrent Depression2nd EditionOxford University PressUSA20076572
- InskipHMHarrisECBarracoughBLifetime risk of suicide for affective disorder, alcoholism, and schizophreniaBr J Psychiatr19981723537
- CulpepperLDavidsonJRTDietrichAJSuicidality as a possible side effect of antidepressant treatmentJ Clin Psychiatry200467986
- SavitzJBCupidoCLRamesarRSTrends in suicidology: personality as an endophenotype for molecular genetic investigationsPLoS Med20063e10716646633
- BaldwinDBullockTMontgomeryD5-HT reuptake inhibitors, tricyclic antidepressants and suicidal behaviourInt Clin Psychopharmacol19916 Suppl 349551839632
- IsacssonGSuicide prevention – a medical breakthroughActa Psychiatr Scand200010211311710937783
- TeicherMHGlodCAColeJOAntidepressant drugs and the emergence of suicidal tendenciesDrug Saf199381862128452661
- FergussonDDoucetteSGlassKCAssociation between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trialsBMJ200533039640415718539
- StorosumJGvan ZwietenBJvan den BrinkWSuicide risk in placebo-controlled studies of major depressionAm J Psychiatry20011581271127511481162
- KhanAKhanSKoltsRSuicide rates in clinical trials of SSRIs, other antidepressants, and placebo: analysis of FDA reportsAm J Psychiatry200316079079212668373
- SimonGESavarinoJOperskalskiBSuicide risk during anti-depressant treatmentAm J Psychiatry2006163414716390887
- CastelpietraGMorsanuttoAPascolo-FabriciEAntidepressant use and suicide prevention: a prescription database study in the region Friuli Venezia Giulia, ItalyActa Psychiatr Scand2008In press.
- KalmarSSzantoKRihmerZAntidepressant prescription and suicide rates: effect of age and genderSuicide Life Threat Behav20083836337418724785
- StahlSMGradyMMMoretCSNRIs: their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressantsCNS Spectr20051073274716142213
- BeckATKovacsMWeissmanAAssessment of suicidal intention: the Scale for Suicide IdeationJ Consult Clin Psychol197947343352469082
- MöllerHJAntidepressants – do they decrease or increase suicidalityPharmacopsychiatry1992252492531494590
- AcharyaNRosenASPolzerJPDuloxetine: meta-analyses of suicidal behaviors and ideation in clinical trials for major depressive disorderJ Clin Psychopharmacol20062658759417110815