Abstract
Ziprasidone is an atypical antipsychotic with a unique receptor-binding profile. Currently, ziprasidone is approved by the US Food and Drug Administration for the acute treatment of psychosis in schizophrenia and mania in bipolar disorder. When compared to certain other atypical antipsychotics, ziprasidone appears to have a relatively benign side effect profile, especially as regards metabolic effects eg, weight gain, serum lipid elevations and glucose dysregulation. Taken together, these data suggest that ziprasidone may be a first line treatment for patients with bipolar mania. However, ziprasidone is a relatively new medication for which adverse events after long-term use and/or in vulnerable patient populations must be studied. Unstudied areas of particular importance include the efficacy and safety of ziprasidone in the treatment of bipolar depression and relapse prevention of mania as, well as in the subpopulations of pregnant women, the elderly and pediatric patients. The emergence of mania in patients taking ziprasidone is another topic for further study.
Introduction
There is a growing body of evidence for the use of atypical antipsychotics in the treatment of bipolar disorder both for acute mania and long-term therapy (CitationChengappa et al 2004; CitationVieta 2005; CitationVornik and Hirschfeld 2005). Concerns that arise with the use of antipsychotics in bipolar patients revolve around whether clinicians are exposing patients to unnecessary risks, especially for those patients without psychosis for whom other effective treatments are available. With the use of any antipsychotic medication, the development of extrapyramidal movement side effects, particularly tardive dyskinisia, is a concern. Patients receiving typical antipsychotics carry a risk of developing tardive dyskinisia of approximately 4%–5% per year (CitationAPA 1992). The rate of akathisia among young patients treated with conventional antipsychotic drugs is up to 90% and decreases as the age of the patients increases (CitationWirshing 2001). Even among 65 year olds, the rate of akathisia with typical antipsychotic medication treatment is 15% (CitationWirshing 2001). Evidence suggests that patients with mood disorders are more susceptible to the extrapyramidal side effects (EPS) of antipsychotics than patients with schizophrenia (CitationKane and Smith 1982; CitationMukherjee et al 1986; CitationNasrallah et al 1988). While the risk of developing EPS appears lower with atypical antipsychotics, little long-term treatment data have been collected to clarify the extent of the risk (CitationPierre 2005). The recently published Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study revealed that the rates of EPS in patients with schizophrenia were not significantly different between patients treated with the typical antipsychotic perphenazine and the various atypical antipsychotic drugs, though patients with a history of tardive dyskinisia were not randomized to the perphenazine arm of the study (CitationLieberman et al 2005).
Potential metabolic side effects must also be weighed when considering the use of antipsychotic medications. Weight gain, increase in plasma lipid concentrations and the development of type II diabetes have been associated with certain atypical antipsychotic medications (CitationAnanth et al 2004a; CitationNewcomer 2005). The risk of development of the full metabolic syndrome appears to be different among the members of the atypical antipsychotic drug class (CitationNewcomer 2005). Clozapine and olanzapine have been associated with the highest rates of weight gain, obesity, glucose dysregulation, diabetes and hyperlipidemia as well as life threatening diabetic ketoacidosis (CitationNewcomer 2005). Risperidone and quetiapine have been suggested to exhibit a moderate risk for these metabolic abnormalities, whereas ziprasidone and aripiprazole have been shown not only to have little or no effect on body weight, lipids or glucose (CitationNewcomer 2005) but often improve these indices when patients are switched from olanzapine and risperidone (CitationWeiden et al 2003).
Ziprasidone is an atypical antipsychotic of the benzisothiazolylpiperazine family approved by the FDA for use in the acute treatment of schizophrenia and schizoaffective disorder in February 2001. The available data, both basic pharmacology and clinical efficacy and tolerability have been comprehensively reviewed (CitationNemeroff et al 2005). Ziprasidone subsequently was approved by the FDA for the treatment of acute bipolar mania including mixed episodes in August 2004 (CitationNemeroff et al 2005). There is also some evidence that ziprasidone may be efficacious converting antidepressant non-responders to responders in patients with unipolar depression (CitationPapakostas et al 2004), in the treatment of agitation in patients with dementia (CitationCole et al 2005; CitationGreco et al 2005; CitationBerkowitz 2003), and in the treatment of delirium (CitationYoung and Lujan 2004; CitationLeso and Schwartz 2002). The pharmacologic profile of ziprasidone includes the following: antagonist activity at dopamine (DA) D2, serotonin 5-HT1D and 5-HT2C receptors, inverse agonist activity at serotonin 5-HT2A receptors and agonist activity at 5-HT1A receptors (CitationNemeroff et al 2005). Ziprasidone is the most potent antagonist of 5-HT2A receptors of the available atypical antipsychotic drugs (CitationSchmidt et al 2001). It is also a relatively potent inhibitor of serotonin (5-HT) and norepinephrine (NE) reuptake in vitro, unique among the atypical antipsychotics (CitationSchmidt et al 2001). Whether this effect occurs in vivo in light of ziprasidone’s tight protein binding in serum remains unknown, but it is of considerable interest because these properties are shared with clinically effective antidepressants. This review focuses on the efficacy and tolerability of ziprasidone in patients with bipolar disorder.
Pharmacokinetics and pharmacodynamics
Ziprasidone oral bioavailability is about 60% in healthy volunteers when taken with food, which increases absorption by more than 50%. Peak plasma concentrations occur in 3.7–4.7 hours (CitationMiceli et al 2000). The elimination half-life of oral ziprasidone given at doses of 80–120 mg/day is approximately 10 hours, thus it is recommended that ziprasidone be taken twice daily (CitationPatel and Keck 2006). This dosing schedule may increase the possibility of missed doses and breakthrough symptoms, though this has not been studied. Because ziprasidone half-life is longer at higher doses (CitationMiceli et al 2000), it has been proposed that once a day dosing may be appropriate at doses greater than 120 mg/day (CitationPatel and Keck 2006). The safety and efficacy of once daily dosing of ziprasidone has not been studied.
Efficacy
Monotherapy
Acute mania
Two 3-week double-blind, placebo-controlled trials have been conducted to determine the efficacy and tolerability of ziprasidone in patients with acute mania (see and ). CitationKeck et al (2003) reported the results of a multicenter (21 US and 3 Brazilian) trial of 210 patients with acute mania with a primary diagnosis of Bipolar I disorder, most recent episode manic or mixed as confirmed by the Structural Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P). Patients were required to have a Mania Rating Scale (MRS) (CitationEndicott and Spitzer 1978) score of ≥14 within 12 hours of the first dose of the double-blind medication. All psychotropic medications except lorazepam, temazepam and medications to manage movement disorders were discontinued. Patients received placebo single-blind during a washout period of 1–7 days before being randomly assigned to ziprasidone treatment or placebo groups in a 2:1 ratio. Ziprasidone was initiated at 40 mg bid on the first day, and then increased to 80 mg bid. Doses were adjusted to in the range of 80–160 mg per day during the remainder of the trial based on tolerability and efficacy. Raters blinded to the patients’ medications evaluated the patients using the Schedule for Affective Disorders and Schizophrenia, Change Version (SADS-C)(CitationEndicott and Spitzer 1978), which contains the MRS, Positive and Negative Syndrome Scale (PANSS) (CitationKay et al 1987), investigator-rated Clinical Global Impression (CGI) severity scale (CitationGuy 1976), investigator-rated CGI improvement scale, and Global Assessment of Functioning Scale (GAF) (CitationGuy 1976). A difference of 5 points on the MRS was defined a priori as the minimal clinically relevant difference in endpoint values. Primary efficacy analyses were the differences on MRS and CGI severity scale scores from baseline to endpoint in the ziprasidone group compared to the placebo group on an intent-to-treat basis. The mean dose of ziprasidone during days 8 to 14 of the trial was 139.1 mg/day and during days 15–21, it was 130.1 mg/day. By day 7, the ziprasidone-treated patients showed significant improvement on all of the symptom severity scales. The ziprasidone-treated patients showed a mean reduction of 12.4 points in their MRS scores, the placebo-treated patients, a decrease of only 7.8 points. This difference, while statistically significant, did not meet the minimally clinically relevant superiority standard established a priori. By day 2 the mean change on the MRS between the two groups was statistically significant (p < 0.003) and this difference was maintained until the end of the 3-week trial (p < 0.001). One-half of the ziprasidone-treated patients were responders (as defined by a ≥50 % improvement of MRS) versus only 35% of the placebo-treated group (p < 0.05). Statistically significant differences on CGI severity and improvement scales were observed by day 4 in the ziprasidone group. At endpoint, the mean CGI severity scores as compared to baseline were reduced by 1.3 in the ziprasidone-treated patients and 0.9 in the placebo-treated patients (p < 0.01). PANSS scores at endpoint were significantly reduced from baseline in the ziprasidone group compared to the placebo group (4.8 versus 2.0, p < 0.001). GAF scores were higher at endpoint in both groups, though greater in the ziprasidone-treated patients (15.3 versus 8.3, p < 0.005). Discontinuation rates were lower in the ziprasidone-treated group (46.4%) when compared to the placebo group (55.7%).
Table 1 Safety and Tolerability Trial I (CitationKeck et al 2003)
Table 2 Safety and Tolerability Trial II (CitationPotkin et al 2005)
Patient withdrawal secondary to adverse events was 6.4% in the ziprasidone-treated group and 4.3% in the placebo group. Adverse events occurred in 90.0% of the patients receiving ziprasidone and 77.1% of those receiving placebo. The vast majority, 96% and 99%, of these adverse events were rated as mild or moderate in severity in the ziprasidone and placebo groups, respectively. No serious adverse events occurred in either group. Events that occurred more often in the ziprasidone-treated group were somnolence (37.1% versus 12.9%), headache (21.4% versus 18.6%), dizziness (22.1% versus 10.0%), hypertonia (11.4% versus 2.9%), nausea (11.4% versus 10.0%) and akathisia (10.7% versus 5.7%). No changes in blood pressure, pulse rate or body weight were observed in either treatment group. Significant changes in laboratory values were found in less that 2% of patients in both groups. Pre- and post-treatment ECG data revealed a mean QTc interval prolongation of 11 msec in the ziprasidone-treated group. No patient had a QTc interval of greater than 500 msec.
A second and similar multisite treatment trial of adult inpatients with a primary diagnosis of Bipolar I disorder, most recent episode manic or mixed was reported by CitationPotkin et al (2005). This study utilized inclusion criteria and dosing schedules similar to those used in the CitationKeck et al (2003) study. The patients were evaluated using the Schedule for Affective Disorders and Schizophrenia, Change Bipolar Scale (SADS-CB) (CitationEndicott and Spitzer 1978) which included the extracted Hamilton Depression Rating Scale (HAM-D) (CitationEndicott et al 1981) and Montgomery Asberg Depression Rating Scale (MADRS) (CitationMontgomery and Asberg 1979) scores. The primary efficacy endpoint was mean MRS change from baseline to endpoint. A total of 206 patients were randomized. The mean ziprasidone dose was 126 mg/day. A statistically significant difference (p < 0.05) in mean MRS scores was noted on the second day of the study, as well as on day 21 (p ≤ 0.01). The percentage of patients classified as responders (≥50% improvement in symptom severity) was 46% in the ziprasidone group and 29% in the placebo group (p < 0.05). Mean CGI severity scale scores of the ziprasidone group showed significant improvement versus the placebo group at day 2 (p < 0.05) and this difference was maintained until day 21 (p < 0.001). Patients in the ziprasidone group experienced greater improvements from baseline to endpoint in PANSS Total and Positive Subscales scores compared to those in the placebo group (p ≤ 0.01). The magnitude of the GAF score improvement in the ziprasidone group was more than twice that observed in the placebo group (15.82 versus 7.59, p < 0.001). Discontinuation rates were lower in the ziprasidone-treated patients (39%) than in the placebo group (46%). In the ziprasidone group, 6.5% dropped out because of adverse events that were considered treatment-related. Treatment-related dropouts comprised 1.5% of the placebo group. Treatment-emergent side effects were reported in 78% of ziprasidone-treated and in 67% of placebo-treated patients. Events that occurred more often in the ziprasidone-treated group were somnolence (22.3% versus 6.1%), headache (12.2% versus 7.6%), extrapyramidal syndrome (10.8% versus 1.5%), dizziness (10.1% versus 1.5%), akathisia (9.4% versus 4.5%), tremor (7.9% versus 1.5%), nausea (6.5% versus 1.5%), and asthenia (5.0% versus 1.5%). Mean changes in blood pressure, pulse rate or body weight were not considered clinically significant in either group. Significant changes in laboratory values were found in less that 2% of patients in both groups. 21% of patients taking ziprasidone (versus 19% in the placebo group) developed elevations in triglycerides. However, the mean triglyceride level in the treatment group dropped by 1mg/dL over the course of the trial. Electrocardiogram (ECG) evaluations revealed a mean QTc interval prolongation of 10.1 msec in the ziprasidone-treated group versus 2.1 msec in the placebo group. No patient had a QTc interval of greater than 480 msec. Serious adverse events were reported in 4 patients in the ziprasidone group and 1 in the placebo group, though none of these events was considered to be related to the study medication. Taken together, these results indicate that ziprasidone is effective and safe in treating acute mania.
Mixed mania
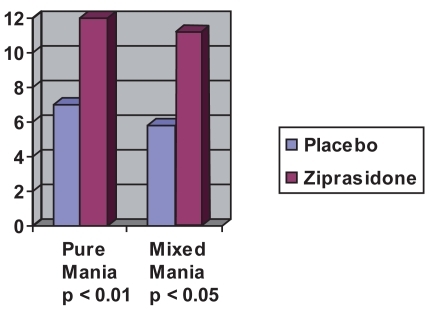
A pooled analysis of the 2 randomized, double-blind, placebo-controlled 21-day trials of ziprasidone in patients with acute bipolar mania described in detail above examined the response of patient subgroups, including those with mixed mania who were treated with ziprasidone (n = 101) or placebo (n = 50) (CitationPotkin et al 2004) (see ). The mean decrease in MRS scores for patients with mixed mania in the ziprasidone group was significantly greater than those in the placebo group (p < 0.01 at 14 days and p < 0.05 at 21 days).
Figure 1 Mean decrease in MRS pure mania vs mixed episodes after 21 days (estimated from CitationPotkin et al 2004).
Psychotic mania
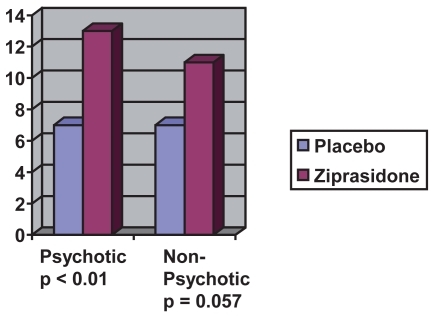
In the pooled analysis discussed above, patients with psychotic symptoms who were in the ziprasidone treatment group (n = 116) had nearly double the magnitude of decrease in their MRS scores by endpoint when compared to psychotic patients who received placebo (n = 52) (see ). This difference was statistically significant at day 4 (p < 0.01) and was maintained to endpoint (CitationPotkin et al 2004).
Figure 2 Decrease in MRS psychotic mania vs non-psychotic mania after 21 days (estimated from CitationPotkin et al 2004).
Depression as part of mixed or pure mania
Among patients with depressive symptoms in the CitationPotkin et al (2005) bipolar mania trial, treatment with ziprasidone was associated with a mean decrease of 2.43 points on the HAM-D compared to 1.37 points in the placebo-treated patients (CitationPotkin et al 2004; CitationPotkin et al 2005). Mean MADRS score reduction in patients who had baseline MADRS scores of ≥ 14 at baseline was 8.6 in the ziprasidone-treated patients (n = 52) and 4.5 (n = 26) in the placebo-treated group. Neither the difference in HAM-D or MADRS scores at endpoint, however, attained statistical significance. Another pooled analysis revealed that in patients with mixed mania and a baseline HAM-D score ≥14, ziprasidone produced greater improvement (p < 0.05) than placebo in HAM-D scores from day 2–21 (CitationKeck et al 2004).
Combination/augmentation studies
Although monotherapy with a traditional mood stabilizer has been the recommended initial treatment for bipolar disorder (CitationAPA 2002), there is a growing body of evidence that suggests that combination therapy with a mood stabilizer and an antipsychotic medication is more efficacious, especially in cases of severe mania (CitationBowden 2005). The Texas Medication Algorithm Project (TMAP) also developed guidelines for treatment of Bipolar Disorder (CitationSuppes and Dennehy 2001). The initial TMAP algorithm for mania/hypomania recommended the atypical antipsychotic, olanzapine, as an initial monotherapy option (Stage 1) along with lithium and divalproex as other options. Patients with psychotic mania who failed to fully respond to initial treatment were suggested to be then put on a combination of lithium or anticonvulsant plus an atypical antipsychotic, which could be olanzapine, risperidone, quetiapine or ziprasidone. In 2005, the algorithm was revised to include aripiprazole, risperidone, quetiapine, and ziprasidone as Stage 1a options along with lithium and divalproex (CitationSuppes et al 2005). Olanzapine, however, was categorized, along with carbamazepine, as a Stage 1b choice due to “safety and other concerns”.
Weisler et al reported that the addition of ziprasidone to lithium treatment reduced time to improvement (CitationWeisler et al 2003) (see ). This 21-day, double-blind trial randomized 205 patients with Bipolar I disorder who were taking lithium and had MRS ≥ 14 to ziprasidone or placebo. Dosage of lithium was adjusted to maintain a plasma lithium level of 0.8–1.2 mEq/L. Ziprasidone was initiated at a dose of 80 mg on day 1 and 160 mg on day 2, with dose adjustments in the range of 80–160 mg per day during days 3–21. Primary efficacy variables were improvement in MRS and CGI-severity scores. By day 4, significantly greater improvement was observed in the ziprasidone plus lithium treated patients compared to the placebo plus lithium treated patients in MRS (2.31 versus 1.59, p < 0.05) and CGI-severity (2.0 versus 0.12, p < 0.01). The significant differences in the improvements on these measures between the treatment groups disappeared by day 14, though numerically greater improvement was maintained through the end of the study. The PANSS scores of the ziprasidone/lithium group were significantly better than those of the lithium only group on day 21 (13.4 versus 8.45, p < 0.01). This led the authors to conclude that augmentation with ziprasidone helped provide rapid reduction of manic symptoms and greater improvement in overall psychopathology, although symptoms of mania were not significantly decreased by ziprasidone augmentation.
Table 3 Ziprasidone augmentation with lithium (CitationWeisler et al 2003)
A 52-week open-label extension revealed that 89 patients treated with ziprasidone and concomitant mood stabilizers and/or antidepressants had a mean MRS score at all study visits that was significantly (p < 0.01) improved from baseline (CitationWeisler et al 2004) (see ). The incidence of treatment-emergent side effects included: somnolence (55.1%), abnormal vision (23.6%), tremor (22.5), extrapyramidal syndrome (21.3%), dizziness (20.2%), headache (19.1%), nausea (19.1%), anxiety (16.9%), insomnia (16.9%), and akathisia (12.4%). Simpson-Angus and Barnes Akathisia scores changed minimally. No QTc intervals over 480 msec were recorded despite coadministration of ziprasidone with lithium. No increases in body weight or serum cholesterol levels were observed. A significant decrease in serum triglyceride levels was noted in the ziprasidone group (p = 0.01).
Table 4 Adjunctive ziprasidone with lithium 52-week open label extension (CitationWeisler et al 2004)
Plasma lithium levels are not altered by the addition of oral ziprasidone in healthy patients (CitationApseloff et al 2000). However adverse events have been reported during ziprasidone augmentation of lithium therapy. One report documented two schizoaffective patients who experienced abrupt rises in plasma lithium levels and symptoms of lithium toxicity within two days of the initiation of intramuscular ziprasidone treatment (CitationMiodownik et al 2005).
Comparison of ziprasidone with other atypical antipsychotics in acute mania
A recent meta-analysis concluded that each of the 5 newer agents (aripiprazole, olanzapine, quetiapine, risperidone and ziprasidone) are superior to placebo in the treatment of bipolar mania and that efficacy differences between the atypical antipsychotics, if they exist, are small (CitationPerlis 2006). Primary outcome measures were changes in the MRS or Young Mania Rating Scale (YMRS) (CitationYoung et al 1978) scores between baseline and endpoint (either day 21 or 28). Data from 12 placebo-controlled trials were evaluated, including 1881 drug-treated and 1233 placebo-treated patients. Included were at least two trials for each of the 5 atypical antipsychotic medications. No statistically significant differences were found among the medications. Two studies of each of 4 drugs (aripiprazole, olanzapine, risperidone and ziprasidone) used 50% reduction in MRS or YMRS as the criteria for response. The overall response rates for the atypicals was 53% as compared to 30% for placebo. Ziprasidone had the highest odds ratio for response relative to placebo among the medications studied, though the difference was not statistically significant. Six add-on therapy studies were also included in the meta-analysis. Although ziprasidone showed the smallest difference between the antipsychotic/mood stabilizer treatment versus mood stabilizer monotherapy, the difference between ziprasidone and other atypical medications was not statistically significant. Because of the minimal differences in treatment effects among published trials of atypical antipsychotic medications for acute mania, the authors recommended that treatment selection be based on factors such as maintenance efficacy, tolerability, safety and cost.
Maintenance studies
A 52-week, open-label extension of a 21-day placebo-controlled trial of ziprasidone in patients with acute mania revealed sustained improvement in manic and depressive symptoms (CitationKeck et al 2004) (see ). Of the 107 patients who entered the extension, 39 remained in the study for the entire year. Only 8.7% of patients discontinued due to treatment related adverse events. The mean duration of treatment was 105 days, with the mean ziprasidone dose 132 mg per day. Mean MRS and CGI-severity scores maintained statistically significant improvement over baseline scores throughout the one-year follow-up (p < 0.0001). There was a reduction in mean body weight over the 52-week period with an equal number (11%) of patients experiencing significant (≥7% of baseline weight) weight loss and gain. Patients experienced a small (1.59 mg/dL) rise in mean plasma cholesterol levels and a moderate (14.8 mg/dL) decrease in mean plasma triglyceride levels. There was little change from baseline to endpoint in scales measuring extrapyramidal symptoms.
Table 5 Ziprasidone 52-week open label extension (CitationKeck et al 2004)
IM ziprasidone studies
In 2002, ziprasidone became the first atypical antipsychotic available in intramuscular (IM) form for the treatment of acute agitation in psychotic patients. In a randomized double-blind 24 hour study which included patients with bipolar disorder or schizoaffective disorder, bipolar type, improvements in the PANSS Agitation subscale, Behavioral Activity Rating Scale (BARS) and CGI-severity scores were noted within 15 minutes of receiving the 20 mg dose (CitationDaniel et al 2001). In a subgroup analysis of 2 randomized, double-blind, fixed-dose, 24-hour studies of the efficacy of IM ziprasidone, patients with bipolar disorder or schizoaffective disorder, bipolar type showed improvements in the PANSS Agitation subscale, Behavioral Activity Rating Scale (BARS) and CGI-severity scores within 15 minutes of receiving the 20 mg dose (CitationDaniel et al 2004). The 10 mg dose was not as effective. There was an 80% responder rate (defined by a ≥2 point decrease in BARS 90 minutes after the first dose) with the 20 mg dose, a 58% responder rate with the 10 mg dose, and an 18.2% responder rate with the 2 mg control dose (p < 0.01). Adverse events were not different in patients receiving the 2 mg control dose compared to patients receiving the 10 mg and 20 mg dose. No dystonia or excessive sedation was reported in the 10 mg (n = 20) and 20 mg (n = 15) groups. One patient in the 10 mg group experienced akathisia. A naturalistic study conducted on a psychiatric emergency service (CitationPreval 2005) reported a decrease in the Behavioral Activity Rating Scale (BARS) (CitationSwift 2002) agitation scores using 20 mg IM ziprasidone in 110 patients with various diagnoses. Scores decreased significantly from baseline after 15 minutes (p < 0.5) and were maintained at 2 hours (p < 0.01). None of the patients given ziprasidone showed lasting side effects, though one patient had an acute dystonic reaction, which responded rapidly to diphenhydramine. ECGs obtained in 19 patients revealed no QTc longer than 460 msec.
Pediatric bipolar disorder
While there are no FDA-approved treatments for mania in children and adolescents, atypical antipsychotics have been used increasingly in this population (CitationKowatch and DelBello 2006). CitationBarnett (2004) reported on 4 patients (ages 7–16) who successfully were switched from other medications to ziprasidone to control hypomanic and depressive symptoms and in two cases, auditory hallucinations, in the absence of other psychotic symptoms. After switching to ziprasidone these patients experienced relief of symptoms and reported a euthymic and stable mood. Each of the patients were maintained on daily doses of ziprasidone ranging from 40–80 mg per day. One patient experienced akathisia at a dose of 20 mg tid, which completely abated after a dose reduction to 20 mg bid.
Safety and tolerability
Extrapyramidal symptoms
A flexible-dose trial in patients with schizophrenia compared the development of extrapyramidal side effects in patients taking ziprasidone (mean 116 mg/day) and haloperidol (mean 8.6 mg/day) (CitationHirsch et al 2002). Akathisia (14% versus 16%), hypertonia (2% versus 7%), tremor (6% versus 10%), and extrapyramidal syndrome (1% versus 5%) were all less common with ziprasidone than with haloperidol. Recent case reports have implicated ziprasidone in acute dystonic reactions in adults with schizophrenia and bipolar disorder (CitationMason et al 2005; CitationWeinstein et al 2006), as well as in a child with developmental delay and attention-deficit/hyperactivity disorder (CitationRamos et al 2003). There have also been several reports of tardive dyskinesia reemergence during ziprasidone treatment (CitationRosenquist et al 2002; CitationAnanth, Burgoyne et al 2004; CitationMendhekar 2005) and its emergence during ziprasidone treatment (CitationAnanth, Burgoyne et al 2004; CitationKeck et al 2004). A recent study suggests that the frequency of movement side effects of atypical antipsychotics may be much higher (over 50%) than those reported in clinical trials (CitationGhaemi et al 2006). Also of importance is that more than half of the patients enrolled in the ziprasidone trials were receiving benzodiazepines, anticholinergic or beta-blocking agents (CitationGentile 2007). Thus the EPS rates during ziprasidone treatment may be underestimated.
The rates of akathisia in the ziprasidone studies in acute mania have been reported at 10.7% and 9.4% (CitationKeck et al 2003; CitationPotkin et al 2005). In the augmentation trial with lithium, 9% of the ziprasidone-treated group reported akathisia, versus 0% in the lithium-only group (CitationWeisler et al 2003). Akathisia rates with conventional antipsychotics have been estimated from 20% to 75% (CitationAPA 2000). Other atypical antipsychotics have also been associated with akathisia during monotherapy trials of bipolar patients, including aripiprazole (6.5%–11.4%), olanzapine (0%–9.6%) and quetiapine (3.6%–5.9%) (CitationGentile 2007). In short term bipolar mania trials, aripiprazole treatment was associated with akathisia in 15% of treated patients (CitationBristol-Myers Squibb Company 2006). The rate of akathisia with risperidone in short-term bipolar mania trials was 16% (CitationJanssen 2007). Antidepressant medications have been associated with akathisia (CitationGill et al 1997). The incidence of fluoxetine-induced akathisia is estimated between 9.8% and 25% (CitationLipinski et al 1989). The pathophysiology of akathisia is thought to be primarily due to a decrease in dopamine activity in the ventral tegmental area, a potential consequence of both the dopamine blockade by antipsychotics and of the enhanced serotinergic and noradrenergic neurotransmission by antidepressants (CitationCatalano et al 2005). The antipsychotic and antidepressant effects of ziprasidone may both contribute to the development of akathisia in patients taking ziprasidone.
Neuroleptic malignant syndrome
Because patients treated with antipsychotic drugs are at risk for the development of neuroleptic malignant syndrome (NMS) (CitationKeck et al 1989; CitationCaroff and Mann 1993), physicians treating bipolar patients with atypical antipsychotic medications must be aware of this possibility. A review of NMS cases associated with the use of atypical antipsychotics revealed 68 cases in the literature, though no ziprasidone-induced NMS cases were identified (CitationAnath et al 2004b). Since then, there have been a few cases of NMS associated with ziprasidone treatment (CitationMurty et al 2002; CitationYang and McNeely 2002; CitationLiebold et al 2004; CitationGray 2004; CitationOzen et al 2007), as well as one case of NMS associated with the combination of lithium and ziprasidone (CitationBorovicka et al 2006).
Weight gain
While evidence mounts that atypical antipsychotics as a class may have deleterious effects on patients’ weight, ziprasidone may have an advantage by promoting less weight gain (CitationMcIntyre and Konarski 2005; CitationMarken and Pies 2006). However the data on weight changes with ziprasidone are not conclusive. In short term studies of ziprasidone treatment have shown the risk of weight gain ≥7% of body weight to be as high as 10% (CitationSprague 2004). In a meta-analysis calculating the effects of antipsychotic medication on weight, 10 weeks of ziprasidone treatment was associated with a mean increase in weight of 0.04 kg (CitationAllison et al 1999). Placebo was associated with a reduction in weight of 0.39 kg. Other atypical antipsychotic were associated with weight gain after 10 weeks of treatment, including clozapine (4.45 kg), olanzapine (4.15 kg), and risperidone (2.10 kg). Studies in patients with schizophrenia treated with ziprasidone for at least 1 year have shown no effect on weight, though conclusions are limited by the small sample size (less than 400 total patients) (CitationGentile 2006). In patients with bipolar disorder, the data on weight change are less substantial.
Cardiac adverse effects
Since the initial clinical trials, ziprasidone has been known to prolong the QTc interval of some patients. As discussed above, in the two pivotal clinical trials demonstrating the efficacy of ziprasidone in the treatment of mania, mean QTc lengthening was reported at 11 msec (CitationKeck et al 2003) and 10.1 msec (CitationPotkin et al 2005). A report studying the effects of six antipsychotics (thioridazine, haloperidol, risperidone, olanzapine, quetiapine and ziprasidone) on QTc interval showed that none of the atypical antipsychotics studied increased the QTc by more than 16 msec and that their use does not appear to be associated with an increased risk of cardiac events (CitationHarrigan 2004). The mean QTc interval lengthening was 15.9 msec for subjects taking ziprasidone. There is one case report of a patient who experienced QTc prolongation during treatment with ziprasidone associated with an asymptomatic episode of torsades de pointes (CitationHeinrich 2006). During the treatment of this patient, on two occasions, discontinuation of ziprasidone was associated with a shortening of the QTc. As the authors note, the patient had multiple problems and was treated with other medications, including lithium, which has been associated with arrhythmias and QT alterations. A case series examined the effect of ziprasidone on the ECGs of 15 patients receiving above the recommended daily dose of ziprasidone (CitationLevy et al 2004). The mean baseline QTc of these patients (including two patients already taking 160 mg/day of ziprasidone) was 415 msec. After the patients had received at least one week of their maximum ziprasidone dose (240–320 mg/day), the mean QTc had increased to 416 msec. The maximum post treatment QTc interval was 452 msec. The authors concluded that if the study could be replicated in a double-blind placebo controlled trial, high dose ziprasidone potentially could safely be used in certain populations. Nonetheless, ziprasidone is contraindicated in patients with a history of prolonged QT, recent myocardial infarction, or uncompensated heart failure, and in patients taking medications known to prolong the QT interval (CitationPfizer 2007).
Adverse effects–comparison with other antipsychotics
Recent reviews on the adverse effect profiles of atypical antipsychotics indicate that ziprasidone has advantages over several of the other medications in its class, especially in the area of metabolic side effects (CitationMcIntyre and Konarski 2005; CitationMarken and Pies 2006). The review by Marken and Pies compared the risk of adverse events with the atypical antipsychotics and haloperidol in 8 categories. Ziprasidone was classified, along with haloperidol and aripiprazole, as having the lowest risk for development of diabetes and worsening of lipid profiles. Ziprasidone and aripiprazole had the lowest risk for weight gain. The risk was rated as minor or lower with ziprasidone in the following other categories: sedation/somnolence, EPS, anticholinergic effects, orthostasis and prolactin elevation. The review by McIntyre and Konarski similarly rated ziprasidone and aripiprazole as having the lowest risk of all the atypical antipsychotics in 6 of 8 categories studied: weight gain, dyslipidemia, glucose dysregulation, myocarditis/cardiomyopathy (along with olanzapine, risperidone and quetiapine), somnolence/sedation and prolactin (along with clozapine and quetiapine). Aripiprazole also had the lowest risk of developing QTc prolongation. Quetiapine and clozapine were rated as having the lowest risk of developing extrapyramidal symptoms.
Ziprasidone-induced mania
Manic symptom induction has been reported with atypical antipsychotics, including ziprasidone. A review of atypical antipsychotic induced mania or hypomania revealed 34 reports and 53 cases from 1994–2005 (CitationMichalopoulou and Lykouras 2006). Of these reports there were 22 cases associated with risperidone, 14 with olanzapine, 11 with ziprasidone, 5 with quetiapine, 1 with amisulpride and none with clozapine or aripiprazole. Although the authors were unable to report the rate of medication-induced mania, it is noteworthy that at the time of the review, ziprasidone had been on the market during only 5 of the years that were studied by the authors. Clozapine and risperidone had been available during all 12 years studied, while olanzapine (10 years) and quetiapine (8 years) had been available for several years more than ziprasidone. The primary diagnoses of the patients in the 11 cases of ziprasidone-induced mania/hypomania were as follows: 3 patients had Bipolar I disorder, one patient had Bipolar II disorder, four had unipolar depression, two had schizophrenia and one had schizoaffective disorder, bipolar type. The dosages prescribed to the patients were in the 20–160 mg per day range with a mean dose of 76 mg per day. The average onset from the start of treatment with ziprasidone to the induction of manic/hypomanic symptoms was 4.3 days (range 10 hours to 10 days), which was somewhat lower than the time-until-onset of symptoms with the other antipsychotics reported. Discontinuation of ziprasidone in 7 of the 11 cases resulted in successful management of mania/hypomania. In the four remaining cases, the symptoms resolved when the ziprasidone dose was lowered. Possible mechanisms of mania induction were discussed, including the 5HT-2A affinity of atypical antipsychotics. As mentioned above, ziprasidone is known to have the highest affinity for 5HT-2A receptors among the antipsychotics. The inhibition of serotonin and norepinephrine reuptake by ziprasidone, has been hypothesized as contributing to the induction of mania (CitationBaldassano et al 2003).
Special populations
Pregnancy
Ziprasidone is categorized by the FDA and American Academy of Pediatrics (AAP), along with the other atypical antipsychotics (except for clozapine, which is a category B) as Pregnancy Category C (human fetal teratogenicity cannot be ruled out) (Pfizer 2005) A recent prospective study examined the effects of atypical antipsychotic medication taken by pregnant women (CitationMcKenna et al 2005). The study included 151 women treated with atypical antipsychotics in three countries. An increase in major malformations was not found, but an increased rate of low birth weight (10% versus 2%, p < 0.05) was observed in babies of mothers treated with atypical antipsychotics. No mothers in this study were treated with ziprasidone. Little data have been gathered on the effects of ziprasidone treatment during pregnancy (CitationErnst and Goldberg 2002).
Patients with liver disease
Ziprasidone is metabolized by the CYP 3A4 isoenzyme, but no clinically significant alterations in metabolism occur in mild-to-moderate hepatic impairment (CitationEverson et al 2000).
Ziprasidone in overdose
As many as 1 in 5 patients with bipolar disorder will die from suicide (CitationIsometsa et al 1994). Nearly 50% of bipolar patients will attempt to take their own lives (CitationSimpson and Jamison 1999). Because suicide attempts are common in patients with bipolar disorder, clinicians must be aware of the potential lethality of medications dispensed to their patients. Four cases of ziprasidone overdoses including two in combination with benzodiazepines were reported from the Pfizer-Spain database without cardiac events or abnormal QTc recordings (CitationGomez-Criado et al 2005). Another case of a 57-year old woman who ingested an overdose of 4440 mg of ziprasidone recorded a maximum QTc of 457.20 msec 12 hours after the ingestion, but QTc remained under 440 msec after 48 hours (CitationPrieto et al 2005). Cases of ingestions as high as 12,800 mg have been reported without significant EEG changes (CitationArbuck 2005).
Conclusion
In summary, ziprasidone appears to be a safe and effective option to treat bipolar disorder, especially acute mania. Ziprasidone does appear to have minimal effects on metabolic parameters, although the database of the long-term effects of ziprasidone on weight and serum lipids is limited. Initial concerns over the potential adverse cardiac effects of ziprasidone appear unwarranted. However additional investigation is needed to identify populations at risk for cardiac complications with ziprasidone. Clarification is also needed to determine the risk of EPS, especially akathisia in patients taking ziprasidone. Also needed are further studies in bipolar depression and maintenance therapy to prevent the recurrence of mood episodes. As a relatively new medication, the use of ziprasidone in special populations such as pregnant patients, children or the elderly has not been well studied. The potential of ziprasidone to cause treatment–emergent mania warrants investigation. Establishing the safety and efficacy of once-daily dosing of ziprasidone would also be helpful for clinicians.
Acknowledgments
Supported by NIMH MH-39415 and RR-000039. In the last 3 years, Dr. Nemeroff consulted to, served on the Speakers’ Bureau and/or Board of Directors, has been a grant recipient, and/or owned equity in one or more of the following: Abbott Laboratories, Acadia Pharmaceuticals, AFSP, APIRE, AstraZeneca, BMC-JR LLC, Bristol-Myers-Squibb, CeN-eRx, Corcept, Cypress Biosciences, Cyberonics, Eli Lilly, Forest Laboratories, George West Mental Health Foundation, GlaxoSmithKline, i3 DLN, Janssen Pharmaceutica, Lundbeck, NARSAD, NIMH, NFMH, NoveDel Pharma, Otsuka, Pfizer Pharmaceuticals, Quintiles, Reevax, UCB Pharma, Wyeth-Ayerst.
References
- AllisonDBMentoreJLHeoM1999Antipsychotic-induced weight gain: a comprehensive research synthesisAmerican Journal of Psychiatry15616869610553730
- American Psychiatric Association1992Tardive dyskinesia: a task force report of the American Psychiatric AssociationWashington, DC
- American Psychiatric Association2000Diagnostic and statistical manual of mental disorders4th ed, text revisionWashington, DC
- American Psychiatric Association2002Practice guideline for the treatment of patients with bipolar disorder revisionAmerican Journal of Psychiatry159Suppl150
- AnanthJBurgoyneKSNizD2004Tardive dyskinesia in 2 patients treated with ziprasidoneJournal of Psychiatry and Neuroscience29467915644988
- AnanthJParameswaranSGunatilakeS2004aSide effects of atypical antipsychotic drugsCurrent Pharmaceutical Design1022192915281897
- AnanthJParameswaranSGunatilakeS2004bNeuroleptic malignant syndrome and atypical antipsychotic drugsJournal of Clinical Psychiatry654647015119907
- ApseloffGMulletDWilnerKD2000The effects of ziprasidone on steady-state lithium levels and renal clearance of lithiumBritish Journal of Clinical Pharmacology49Suppl 161S64S10771456
- ArbuckDM200512,800-mg ziprasidone overdose without significant ECG changesGeneral Hospital Psychiatry27222315882771
- BaldassanoCFBallasCDattoSM2003Ziprasidone-associated mania: a case series and review of the mechanismBipolar Disorders572512656943
- BarnettMS2004Ziprasidone monotherapy in pediatric bipolar disorderJournal of Child and Adolescent, Psychopharmacology14471715650505
- BerkowitzA2003Ziprasidone for dementia in elderly patients: case reviewJournal of Psychiatric Practice94697315985971
- BorovickaMCBondLCGaughanKM2006Ziprasidone- and lithium-induced neuroleptic malignant syndromeAnnals of Pharmacotherapy401394216352776
- BowdenCL2005Atypical antipsychotic augmentation of mood stabilizer therapy in bipolar disorderJournal of Clinical Psychiatry3121915762830
- Bristol-Myers Squibb Company2006Abilify prescribing information
- CaroffSNMannSC1993Neuroleptic malignant syndromeMedical Clinics of North America771852028093494
- CatalanoGGraceJWCatalanoMC2005Acute akathisia associated with quetiapine usePsychosomatics4629130116000672
- ChengappaKNSuppesTBerkM2004Treatment of bipolar mania with atypical antipsychoticsExpert Review of Neurotherapeutics4 6 Suppl 2S172516279862
- ColeSASaleemRSheaWP2005Ziprasidone for agitation or psychosis in dementia: four casesInternational Journal of Psychiatry in Medicine3591815977947
- DanielDGBrookeSWarringtonLE2004IM ziprasidone in agitated patients with bipolar diagnosesPresented at National Institute of Mental Health New Clinical Drug Evaluation Unit, 44th Annual MeetingPheonix, AZ
- DanielDGPotkinSGReevesKR2001Intramuscular (IM) ziprasidone 20 mg is effective in reducing acute agitation associated with psychosis: a double-blind, randomized trialPsychopharmacology1551283411401000
- EndicottJCohenJNeeJ1981Hamilton Depression Rating Scale. Extracted from regular and change versions of the schedule for affective disorders and schizophreniaArchives of General Psychiatry38981037458574
- EndicottJSpitzerRL1978A diagnostic interview: the schedule for affective disorders and schizophreniaArchives of General Psychiatry3583744678037
- ErnstCLGoldbergJF2002The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropicsJournal of Clinical Psychiatry4425511913676
- EversonGLasseterKCAndersonKE2000The pharmacokinetics of ziprasidone in subjects with normal and impaired hepatic functionBritish Journal of Clinical Pharmacology49Suppl 121S26S10771450
- GentileS2007Extrapyramidal adverse events associated with atypical antipsychotic treatment of bipolar disorderJournal of Clinical Psychopharmacology271354517224710
- GentileS2006Long-term treatment with atypical antipsychotics and the risk of weight gain: a literature analysisDrug Safety2943031916569080
- GhaemiSNHsuDJRosenquistKJ2006Extrapyramidal side effects with atypical neuroleptics in bipolar disorderProgress in Neuro Psychopharmacology and Biological Psychiatry302091316412546
- GillHSDeVaneCLRischSC1997Extrapyramidal symptoms associated with cyclic antidepressant treatment: a review of the literature and consolidating hypothesesJournal of Clinical Psychopharmacology17377899315989
- Gomez-CriadoMSBernardoMFlorezT2005Ziprasidone overdose: cases recorded in the database of Pfizer-Spain and literature reviewPharmacotherapy251660516232029
- GrayNS2004Ziprasidone-related neuroleptic malignant syndrome in a patient with Parkinson’s disease: a diagnostic challengeHuman Psychopharmacology19205715079855
- GrecoKETuneLEBrownFW2005A retrospective study of the safety of intramuscular ziprasidone in agitated elderly patientsJournal of Clinical Psychiatry66928916013910
- GuyW1976Early clinical drug evaluation manualWashington, DCUnited States Department of Health, Education, and Welfare
- HarriganEPMiceliJJAnzianoR2004A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibitionJ Clin Psychopharmacol2462914709949
- HeinrichTWBibloLASchneiderJ2006Torsades de pointes associated with ziprasidonePsychosomatics47264816684946
- HirschSRKisslingWBaumlJ2002A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophreniaJournal of Clinical Psychiatry635162312088164
- IsometsaETHenrikssonMMAroHM1994Suicide in bipolar disorder in FinlandAmerican Journal of Psychiatry151102048010358
- Janssen2007Risperdal prescribing information
- KaneJMSmithJM1982Tardive dyskinesia: prevalence and risk factors, 1959 to 1979Archives of General Psychiatry39473816121548
- KaySRFiszbeinAOplerLA1987The positive and negative syndrome scale (PANSS) for schizophreniaSchizophrenia Bulletin13261763616518
- KeckMEMullerMBBinderEB2004Ziprasidone-related tardive dyskinesiaAmerican Journal of Psychiatry161175614702272
- KeckPEJrPopeHGJrCohenBM1989Risk factors for neuroleptic malignant syndrome. A case-control studyArchives of General Psychiatry4691482572206
- KeckPEJrVersianiMPotkinS2003Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trialAmerican Journal of Psychiatry160741812668364
- KeckPEJrWarringtonLPotkinS2004Efficacy and safety of ziprasidone in bipolar disorder: short- and long-term data157th annual meeting of the American Psychiatric AssociationNew York, NY
- KowatchRADelBelloMP2006Pediatric bipolar disorder: emerging diagnostic and treatment approachesChild and Adolescent Psychiatric Clinics of North America157310816321726
- LeiboldJPatelVHasanRA2004Neuroleptic malignant syndrome associated with ziprasidone in an adolescentClinical Therapeutics261105815336475
- LiebermanJAStroupTSMcEvoyJP2005Effectiveness of antipsychotic drugs in patients with chronic schizophreniaNew England Journal of Medicine35312092316172203
- LevyWORobichaux-KeeneNRNunezC2004No significant QTc interval changes with high-dose ziprasidone: a case seriesJournal of Psychiatric Practice102273215552544
- LipinskiJFJrMallyaGZimmermanPPopeHGJr1989Fluoxetine-induced akathisia: clinical and theoretical implicationsJournal of Clinical Psychiatry50339422549018
- LesoLSchwartzTL2002Ziprasidone treatment of deliriumPsychosomatics4361211927760
- MarkenPAPiesRW2006Emerging treatments for bipolar disorder: safety and adverse effect profilesAnnals of Pharmacotherapy402768516403851
- MasonMNJohnsonCEPiaseckiM2005Ziprasidone-induced acute dystoniaAmerican Journal of Psychiatry162625615741487
- McIntyreRSKonarskiJZ2005Tolerability profiles of atypical antipsychotics in the treatment of bipolar disorderJournal of Clinical Psychiatry3283615762832
- McKennaKKorenGTetelbaumM2005Pregnancy outcome of women using typical antipsychotic drugs: a prospective comparative studyJournal of Clinical Psychiatry66444915816786
- MendhekarDN2005Ziprasidone-induced tardive dyskinesiaCanadian Journal of Psychiatry Revue Canadienne de Psychiatrie50567816262114
- MiceliJJWilnerKDHansenRA2000Single- and multiple-dose pharmacokinetics of ziprasidone under non-fasting conditions in healthy male volunteersBritish Journal of Clinical Pharmacology49Suppl 15S13S10771448
- MichalopoulouPGLykourasL2006Manic/hypomanic symptoms induced by atypical antipsychotics: a review of the reported casesProgress in Neuro Psychopharmacology and Biological Psychiatry305496416442194
- MiodownikCHausmannMFrolovaK2005Lithium intoxication associated with intramuscular ziprasidone in schizoaffective patientsClinical Neuropharmacology28295716340388
- MontgomerySAAsbergM1979A new depression scale designed to be sensitive to changeBritish Journal of Psychiatry1343829444788
- MukherjeeSRosenAMCaracciG1986Persistent tardive dyskinesia in bipolar patientsArchives of General Psychiatry4334262869742
- MurtyRGMistrySGChackoRC2002Neuroleptic Malignant Syndrome with ziprasidoneJournal of Clinical Psychopharmacology22624612454565
- NasrallahHAChurchillCMHamdan-AllanGA1988Higher frequency of neuroleptic-induced dystonia in mania than in schizophreniaAmerican Journal of Psychiatry145145562903686
- NemeroffCBLiebermanJAWeidenPJ2005From clinical research to clinical practice: a 4-year review of ziprasidoneCNS Spectrums10s120
- NewcomerJW2005Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature reviewCNS Drugs19Supp 119315998156
- OzenMEYumruMSavasHA2007Neuroleptic malignant syndrome induced by ziprasidone on the second day of treatmentWorld Journal of Biological Psychiatry842417366349
- PapakostasGIPetersenTJNierenbergAA2004Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorderJournal of Clinical Psychiatry652172115003076
- PatelNCKeckPEJr2006Ziprasidone: efficacy and safety in patients with bipolar disorderExpert Review of Neurotherapeutics611293816893341
- PerlisRHWelgeJAVornikLA2006Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trialsJournal of Clinical Psychiatry675091616669715
- Pfizer2007Geodon prescribing information
- PierreJM2005Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and managementDrug Safety2819120815733025
- PotkinSGKeckPEJrSegalS2005Ziprasidone in acute bipolar mania: a 21-day randomized, double-blind, placebo-controlled replication trialJournal of Clinical Psychopharmacology253011016012271
- PotkinSGKeckPEGillerE2004Ziprasidone in bipolar mania: efficacy across patient subgroupsPresented at the 157th annual meeting of the American Psychiatric AssociationNew York, NY
- PrevalHKlotzSGSouthardR2005Rapid-acting IM ziprasidone in a psychiatric emergency service: a naturalistic studyGeneral Hospital Psychiatry27140415763126
- PrietoTBenabarreABernardoM2005The highest intentional ziprasidone overdose was not fatalActa Psychiatrica Scandinavica112798015952953
- RamosAEShytleRDSilverAA2003Ziprasidone-induced oculogyric crisisJournal of the American Academy of Child and Adolescent Psychiatry421013412964566
- RosenquistKJWalkerSSGhaemiSN2002Tardive dyskinesia and ziprasidoneAmerican Journal of Psychiatry159143612153846
- SchmidtAWLebelLAHowardHRJr2001Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profileEuropean Journal of Pharmacology42519720111513838
- SimpsonSGJamisonKR1999The risk of suicide in patients with bipolar disordersJournal of Clinical Psychiatry253610073388
- SpragueDALoewenPSRaymondCB2004Selection of atypical antipsychotics for the management of schizophreniaAnnals of Pharmacotherapy382313914742771
- SuppesTDennehyEB2001Bipolar disorder algorithmsTIMA procedural manualDallas2001
- SuppesTDennehyEBHirschfeldRM2005The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorderJournal of Clinical Psychiatry668708616013903
- SwiftRHHarriganEPCappelleriJC2002Validation of the behavioral activity rating scale (BARS): a novel measure of activity in agitated patientsJournal of Psychiatric Research36879511777497
- VietaE2005Bipolar mixed states and their treatmentExpert Review of Neurotherapeutics563815853475
- VornikLAHirschfeldRM2005Bipolar disorder: quality of life and the impact of atypical antipsychoticsAmerican Journal of Managed Care119 SupplS2758016232010
- WeidenPJDanielDGSimpsonG2003Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidoneJournal of Clinical Psychopharmacology2359560014624190
- WeinsteinSKAdlerCMStrakowskiSM2006Ziprasidone-induced acute dystonic reactions in patients with bipolar disorderJournal of Clinical Psychiatry67327816566635
- WeislerRDunnJEnglishP2003Ziprasidone in adjunctive treatment of acute bipolar mania: randomized, double-blind, placebo-controlled trial55th Institute on Psychiatric Services MeetingBoston, MA
- WeislerRWarringtonLDunnJ2004Adjunctive ziprasidone in bipolar mania: short- and long-term data157th annual meeting of the American Psychiatric AssociationNew York, NY
- WirshingWC2001Movement disorders associated with neuroleptic treatmentJournal of Clinical Psychiatry2115811584982
- YangSHMcNeelyMJ2002Rhabdomyolysis, pancreatitis, and hyperglycemia with ziprasidoneAmerican Journal of Psychiatry159143512153844
- YoungCCLujanE2004Intravenous ziprasidone for treatment of delirium in the intensive care unitAnesthesiology1013794515329607
- YoungRCBiggsJTZieglerVE1978A rating scale for mania: reliability, validity and sensitivityBritish Journal of Psychiatry13342935728692