Abstract
Objective:
To estimate first-year treatment costs among new initiators of topical prostaglandin analogs in a managed care population.
Research design and methods:
A model was developed to estimate first-year medical costs. Model inputs were based on weighted results from three previous studies. Treatment patterns were derived from a claims database analysis. Published studies were used to estimate visit-related resource use. Costs were obtained from standard sources.
Results:
Across studies, 27,809 patients met study criteria, 44.2% of whom remained on their index therapy for 12 months. Adjunctive therapy was needed in 22.5%, 18.5%, and 11.9% of bimatoprost, latanoprost, and benzalkonium chloride (BAK)-free travoprost patients, respectively. Median days to initiating adjunctive therapy were 64, 67, and 127 for bimatoprost, latanoprost, and BAK-free travoprost patients. Estimated first-year medical costs were $1,945, $1,803, and $1,730 for patients initiating therapy with bimatoprost, latanoprost, and BAK-free travoprost. Findings were consistent through sensitivity analysis.
Conclusions:
A BAK-free prostaglandin analog may permit longer duration of monotherapy and be associated with lower first-year treatment costs. Use of a claims database and the selection of new initiators of prostaglandin analogs limit the ability to project findings to all glaucoma patients.
Introduction
Across studies of treatments for primary open-angle glaucoma (POAG), it is clear that there are many challenges to achieving and maintaining maximum reduction of intraocular pressure (IOP) and delaying disease progression. One challenge is increasingly complex regimens, known to be problematic for adherence in glaucoma treatment and across all chronic diseases.Citation1–Citation3 Proper instillation of eyedrops also cannot be assumed.Citation2,Citation4,Citation5 Another is the presence of adverse events, such as the development of ocular surface disease (OSD),Citation3 that can affect adherence.Citation6 In fact, approximately half of glaucoma patients experience some signs and/or symptoms of OSD in at least one eye.Citation7,Citation8
Given these impediments to adherence, the logical treatment should be a monotherapy with once daily dosing and a low likelihood of adverse events. However, even highly effective glaucoma therapies may not be sufficient to achieve adequate IOP reduction.Citation9–Citation11 Prostaglandin analogs, which are recommended as first-line therapy for POAG by the American Academy of Ophthalmology,Citation12 often require the use of adjunctive therapies, increasing exposure to preservatives commonly found in ophthalmic solutions. This increased exposure may decrease the success of filtration surgery if needed to treat more severe disease.Citation13 A key question is whether there are differences among these recommended therapies, and a series of studies suggests that there may be one differentiating factor.
Of the three topical prostaglandin analogs currently approved and available in the United States, only travoprost is available without the preservative BAK. This newer product, BAK-free travoprost, is comparable in concentration to the conventional travoprost (including BAK) and has demonstrated equivalent IOP-lowering efficacy compared to travoprost with BAK.Citation14 Furthermore, additional studies suggest that transitioning patients from a BAK-containing prostaglandin analog to BAK-free travoprost may improve ocular surface health, as indicated by signs and symptoms of OSD.Citation15–Citation21 Three studies explored the impact on use and type of adjunctive therapy and annual costs for patients initiating glaucoma therapy with prostaglandin analogs.Citation22–Citation24 Each study used the same inclusion criteria and identified patients with a prescription for a topical prostaglandin analog during a six-month period who had no glaucoma therapy claims in the six months prior and had at least 12 months of data available after the initial prostaglandin analog claim. Thus the analyses are comparable, although costs were updated to current values at the time each study was conducted.
The first study in the seriesCitation24 compared treatment patterns and estimated annual costs of patients newly initiating therapy with one of three prostaglandin analogs: bimatoprost, latanoprost, or the BAK-free travoprost. Significantly fewer patients using BAK-free travoprost required adjunctive therapy compared to the other treatments, and the duration of monotherapy was twice as long for BAK-free travoprost (109 days) compared with the shortest duration of monotherapy identified (bimatoprost, 53 days). In addition, costs were approximately 11% lower for patients initiating therapy with BAK-free travoprost ($1,160) compared with the comparator with the highest first-year costs (bimatoprost, $1,294). The second study in the seriesCitation23 also found BAK-free travoprost associated with rates of adjunctive therapy use of less than half of comparators (8.9% vs 16.5% for latanoprost and 20.7% for bimatoprost) and duration of monotherapy significantly lower than comparators (158.5 days vs 69.5 days for bimatoprost and 67.0 days for latanoprost). The differential in costs remained approximately 11%, with first-year costs for BAK-free travoprost estimated to be $1,307 compared with the highest cost of $1,457 for bimatoprost. Finally, the third study in the seriesCitation22 found that adjunctive intraocular pressure lowering therapy was needed in 23.6%, 18.5%, and 13.3% of bimatoprost, latanoprost, and BAK-free travoprost patients, respectively. Median numbers of days to the first prescription filled for adjunctive therapy (if required) were 72.5, 74.0, and 125.0 for patients initiating on bimatoprost, latanoprost, and BAK-free travoprost. Total estimated first-year costs were $1,973, $1,807, and $1,739 for patients initiating therapy with bimatoprost, latanoprost, and BAK-free travoprost.
The present study pooled data across all three studies, looking at 18 months of data on FDA-approved prostaglandin analogs.
Methods
We used three primary sources of input into the economic model. We first conducted multiple retrospective cohort studies, over time, using a prescription benefits database.Citation22–Citation24 Data from these analyses were pooled and were used to identify the population of patients receiving prostaglandin analogs and to explore patterns of use of adjunctive therapies. Second, we reviewed published studies identified through a literature review to estimate the components of a typical outpatient visit (initial evaluation, diagnostics tests, and follow-up care). Finally, we consulted standard cost sources to provide costs for each resource (initial treatments, adjunctive therapies, and visits) identified.
Database analysis: study population and use of adjunctive therapies
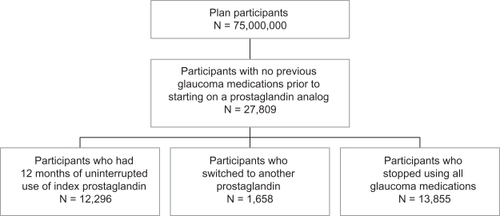
The patients described in the retrospective cohort analysis were receiving prescription benefits and were included in a prescription claims database of a large pharmaceutical benefits manager (PBM). This PBM serves more than 75 million plan participants across the United States. All data were de-identified in accordance with Protected Health Information standards under the Health Information Portability and Accountability Act so that no individually identifiable information was included in the study database. Therefore, review by an institutional review board was not required.
The study cohort included patients who first initiated therapy with one of three prostaglandin analog products (bimatoprost, latanoprost, or BAK-free travoprost) between November 1, 2006 and April 30, 2008. To qualify for each of the previous three studies, patients had to have more than one prescription claim of their index prostaglandin analog and six months of prior claims data in which there were no glaucoma therapy claims of any class. Glaucoma therapy claims were defined by therapeutic class plus generic code number (GCN) codes. GCNs are a system of unique numbers assigned by drug pricing service First DataBank to medications according to strength, formulation, route of administration, and size. The study population was further defined by requiring patients to have at least 12 months of uninterrupted prescription refills of the index prostaglandin analog following the initial prescription. This was operationalized as not having prescriptions for the other prostaglandin analogs during the year and having at least one prescription for the index prostaglandin analog in the fourth quarter of the follow-up period. Patients were required to be continuously enrolled during the 18-month period (six months prior to the first prostaglandin claim and the 12 months of follow-up), according to the PBM’s enrollment files. Patients who did not meet these requirements (sufficient prior claims data, uninterrupted use, and use of the index prostaglandin analog during the fourth quarter of observation) were excluded from the study database. We considered that patients added an adjunctive medication, defined by therapeutic class plus GCN codes, if there was a sequential and subsequent refill of an adjunctive agent in the presence of continued refills for the index agent.
Literature review: resource use and cost inputs
The American Academy of Ophthalmology Preferred Practice Patterns for glaucoma suggest that follow-up care should be based on achievement of target IOP and the amount of disease progression,Citation12 neither of which was available in the prescription database used for this study. Therefore, the base case analysis uses resource rates from a survey-based studyCitation25 while sensitivity analyses explore visits as recommended by other studies and guidelines.Citation12,Citation26 Since all patients in the model are assumed to undergo the same procedures and tests at their visits regardless of the prostaglandin analog they receive, the relatively small differences across studies in terms of the components of each visit have minimal impact on study findings.
At the initial visit, patients are assumed to have a level four (comprehensive) evaluation (CPT 92004). Follow-up visits are assumed to be level two (intermediate) visits (CPT 92012), with three follow-up visits during the year (likely but not necessarily at 30 and 90 days following initiation of the index therapy, and at 12 months). Two additional follow-up visits are assumed to be associated with the initiation and monitoring of adjunctive medication among patients for whom it is prescribed. Thus patients who stay on monotherapy are assumed to have one comprehensive visit and three intermediate visits while those who require adjunctive therapy had one comprehensive visit and five intermediate visits. As the model only considered one year of treatment costs, it was not essential when in the year these visits occurred (ie, discounting based on timing was not necessary); only the total number and type were relevant. presents the procedures and diagnostic tests that comprise each visit as well as the costs used for each.
Table 1 Unit costs
We used average wholesale price (AWP) for 2009 as the basis of prescription costs.Citation27 The range of published AWPs for the prostaglandin analogs was fairly narrow ($80.53 to $82.67).Citation27 For the prostaglandin analogs, the midpoint cost for the agents is used in the model because AWP is the best publicly available estimate for the analysis. Further, the number of prescriptions for prostaglandin analogs was expressed as 2.5 mL size bottle equivalents, as this is the most common size found in the claims database and represents approximately a 30–45 day supply when used per label in both eyes. For example, a 5 mL bottle was treated as two 2.5 mL bottles. The AWPs for adjunctive therapies were also reviewed in the Red Book.Citation27 For the base case of the model, branded products were used, but sensitivity analyses explored lower costs. Days of use per bottle were based on data from these studies. The number of bottles required was rounded to the nearest tenth. Medical charges were estimated using the 75th percentile of physicians’ fees from a published benchmark.Citation28 All the costs used in the model are presented in .
Analysis
The analysis identified patients by the prostaglandin prescription they first filled (ie, the “index medication”) during the study period. Gender was compared using a chi-square test and age was compared using analysis of variance (ANOVA) with Bonferroni’s correction for multiple comparisons. The number of refills per year of each prostaglandin was calculated to use as an input to the economic model. Median and mean number of days to initiation of adjunctive therapy were calculated for each cohort, with the distribution of number of days examined to determine which measure to use in the model (ie, mean if the days were normally distributed, median if they were not). The median numbers of days until patients added adjunctive therapies were compared using the Wilcoxon rank sum test; mean times were compared using ANOVA. Statistical comparisons were conducted in SAS (v9.2; SAS Institute, Inc., Cary, NC).
Sensitivity analyses modified clinical and cost input parameters and explored the impact of assumptions on model findings. Two clinical inputs were varied. First, the proportion of patients remaining on monotherapy for the entire year was changed to the lowest and highest values among the initial treatments, that is, the proportion of patients remaining on monotherapy was changed to 77.5% (the base case value for bimatoprost) and then to 88.1% (the base case value for BAK-free travoprost) for all treatments. Second, the days to initiating adjunctive therapy was varied in two ways: a) median number of days to the addition of adjunctive medication was changed to the lowest and highest values among the treatments and b) the mean number of days was substituted for the median. A number of cost-related variables were also subjected to sensitivity analyses. The costs of treatments were varied by assuming that the least and most expensive adjunctive therapies were the only therapies used. Sensitivity analysis was also conducted on the cost of visits, using resource use estimates from other published sources.Citation12,Citation26 A minimum value for frequency of visits and resource use was estimated from the American Academy of Ophthalmology Preferred Practice PatternsCitation12 and assumed that patients were meeting IOP targets; the maximum value assumed that patients were not meeting IOP targets and that there was progression of disease. In addition, the 50th percentile of physicians’ fees was substituted for the 75th percentile.
Results
provides a flow chart of the patient selection process for the retrospective cohort analysis. Of more than 75 million plan participants, 27,809 initiated treatment with a prostaglandin analog. Approximately 6% of these patients switched from their index prostaglandin analog to another glaucoma medication and about 50% of the patients stopped taking all glaucoma medications within the 12-month period. Of those switching, the primary alternative therapies were another prostaglandin analog, beta-blocker, or fixed combination therapy. The analysis examined only those newly initiating patients who remained on their initial prostaglandin analog therapy for one year, which represents approximately 44% of the glaucoma patients newly initiating with prostaglandin analogs identified from the database.
Demographic characteristics of the newly initiating patients are presented in . More than half (55.4%) of the patients were women (P < 0.01). Mean age ranged from 63 to 65 years and was significantly different across groups (P < 0.0001, with BAK-free travoprost significantly different by less than two years from the other prostaglandin analogs).
Table 2 Demographic characteristics
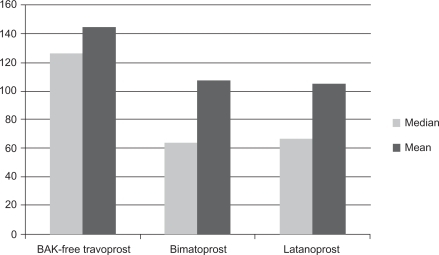
The mean number of bottles per year of each prostaglandin (expressed as 2.5 mL bottle equivalents) was calculated, based on weighting findings from previous studies (8.7 for BAK-free travoprost, 9.0 for latanoprost, and 10.2 for bimatoprost), and was used to calculate costs for prostaglandin analogs. In the bimatoprost, latanoprost, and BAK-free travoprost treatment groups, 22.5%, 18.5%, and 11.9%, respectively, initiated adjunctive therapy during the course of the year at significantly different proportions (P < 0.0001, see ). Patients on BAK-free travoprost were able to continue without adjunctive therapy longer than patients treated with other prostaglandins. shows that the median numbers of days until patients added adjunctive therapies were 127 days for patients initiating therapy with BAK-free travoprost, 64 days for bimatoprost, and 67 days for latanoprost (P = 0.0004, Wilcoxon rank sum test). The mean numbers of days to initiating adjunctive therapy were 144.9 days in the BAK-free travoprost group, 107.5 for patients receiving bimatoprost, and 105.4 for patients receiving latanoprost. Mean time to initiating adjunctive therapy was also significantly different across the cohorts (P = 0.0002, ANOVA).
Table 3 Treatment patterns during follow-up period
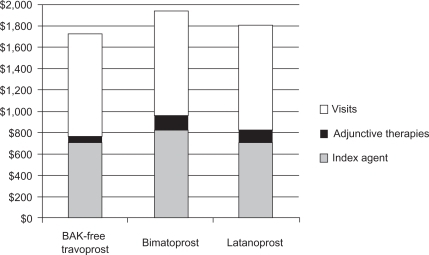
presents the primary findings from the analysis. Estimated annual costs for patients initiating therapy with BAK-free travoprost were lowest ($1,730; $964 medical and $766 pharmacy), with increasing annual costs for latanoprost ($1,803; $979 medical and $824 pharmacy), and bimatoprost ($1,945; $989 medical and $956 pharmacy). For all agents, visits comprised at least 50% of the total annual cost (50.8% for bimatoprost to 55.7% for BAK-free travoprost).
Results of sensitivity analyses are shown in . Findings remained consistent across univariate sensitivity analyses, with BAK-free travoprost having the lowest annual cost and bimatoprost having the highest cost in all scenarios. In the base case, the range of one-year costs across index medications was 12%; in univariate sensitivity analyses, it ranged from 8% to 16%. The single most influential assumption was that all adjunctive therapy received was the most costly agent available. Bivariate sensitivity analyses resulted in differences in the range of 3% (highest rate of continued monotherapy and visit components based on AAO targets not met)Citation12 to 18% across index medications (most costly adjunctive therapy and visit components based on Quigley estimates).Citation26 In all cases, BAK-free travoprost remained the least costly option and bimatoprost the most costly option.
Table 4 Univariate sensitivity analyses
Discussion
This analysis adds to a growing body of literature finding consistent differences in treatment patterns and costs for glaucoma patients initiating treatment with prostaglandin analogs with varying rates of adjunctive therapy use. Over a period of 18 months after initial availability of BAK-free travoprost, patients initiating glaucoma treatment with this medication remained on monotherapy in greater proportions and for a longer duration compared to other prostaglandin analogs.
The early studies in this series included fewer patients as it takes time for patients to be transitioned to a new formulation of an existing product. Although significant findings were detected in each study, there are benefits to pooling the data. Small variations are likely washed out, and the variance of continuous variables is narrower. For example, while the standard deviations of the mean time to adjunctive therapy remained large, they were considerably tighter in the pooled analysis. Using pooled results, with a larger population as the base for the model input parameters, may also suggest that the results are more generalizable.
The use of a large claims database offers an important strength in terms of sample size. However, there are also disadvantages to the use of a pharmaceutical claims database without additional clinical input in terms of characterizing patients and/or prescribers.Citation29 Patients are not randomized to treatment, although age and gender of the population reflected that of the general glaucoma populationCitation30 and varied little across treatment groups. The reasons for adding adjunctive therapy, switching, or discontinuing therapies cannot be surmised from a claims database alone. In addition, claims databases report on prescriptions filled, but cannot be used as a definitive statement of adherence to therapy.Citation31–Citation34 Also affecting adherence and cost estimates is that there is no way to account for product samples, which one study identified as being received by 20% of patients.Citation29 We assumed that the use of samples would be similar across products studied in a database of this size. The population of prescribers may not accurately reflect national patterns, which could misrepresent prescribing patterns. There are no data on the number of prescribers whose patients appear in the database, but as the data were from a large PBM, it is likely that there is a wide distribution of prescribers and practices. Finally, claims databases may contain coding biases or errors, although there is no reason to believe that these errors would be different across index treatments; in addition, the claims in this database were reviewed and adjudicated before the database was prepared for this analysis.
Beyond the limitations of a pharmaceutical claims database, other assumptions may also have affected our study findings. The study attempted to identify only patients who were new to therapy, although even a criterion as simple as this is difficult to implement.Citation29 Also, based on the study methodology, patients could have had poor adherence but still have been included; patients in the cohort analysis were required to have a minimum of two prescriptions for the same prostaglandin analog filled during the follow-up period, but no attempt was made to assess medication possession ratio or to evaluate persistence with therapy over time. The subset of patients identified through this analysis may not be representative of glaucoma patients in other aspects, although the nature of a prescription claims database does not allow detailed exploration. For example, we could not detect other comorbid conditions, although it may have been possible to make some assumptions based on a thorough evaluation of all prescription medications taken by each patient. All assumptions about costs and resource utilization were explored through sensitivity analysis.
As with any economic model, findings are dependent on the strength of the assumptions. We have made every attempt to be transparent about the source of the input parameters, to use data from public and/or peer-reviewed sources, and we conducted thorough sensitivity analyses on the impact of these parameters. There will always remain uncertainty inherent in models, but the difficulty of obtaining complete data on individual patients is often insurmountable. For example, data vendors tend to provide standardized costs rather than actual costs and even complete electronic health records cannot consistently answer why patients discontinue treatment. Many studies report high rates of discontinuation with glaucoma treatment but provide no definitive answers,Citation35–Citation37 even when the interesting question of restarts during the observation period is considered.Citation38
These findings showed that prostaglandin analogs with a greater proportion of patients remaining on monotherapy and a longer time to initiation of adjunctive agents had lower first-year direct medical costs from a third party payer’s perspective. Additionally, while our resource use estimates assumed that follow-up visits to evaluate adjunctive therapies would be coded as intermediate visits, up-coding (ie, coding these visits as comprehensive) could occur and this would further differentiate products by rates of adjunctive therapy use. Accordingly, costs from the patient’s perspective (eg, co-pays for visits and prescriptions) would also be lower with longer duration of monotherapy. Interestingly, there were no remarkable differences in the distribution of types of adjunctive therapies across treatment groups. Further study is needed to identify to what extent tolerability, specifically the absence of BAK, contributes to use of adjunctive medications either to supplement glaucoma treatment or to treat resultant co-morbidities such as dry eye or ocular surface disease.
There are important follow-up questions to these results. Why are there differences in rates of adjunctive therapy? Are there also differences in disease progression or the rate of surgical interventions? What other patient characteristics might affect use of adjunctive therapy, adherence with therapy, or rates of disease progression? Unfortunately, the use of pharmaceutical databases unaided by medical claims information leaves these questions unanswered. We can speculate that patients who do not experience OSD may be more likely to adhere to their prostaglandin treatment for longer periods of time, as has been shown with other studies of BAK-free travoprost,Citation14,Citation16,Citation39 but other variables may be relevant. We are currently undertaking an analysis of a comprehensive health insurance claims database over a two-year time horizon in an effort to explore some of these questions.
Disclosures
Author JKS is an employee of Exponent, which received funding from Alcon to conduct this study. Author DWC is an employee of Alcon Research Ltd.
References
- YeawJBennerJSWaltJGSianSSmithDBComparing adherence and persistence across 6 chronic medication classesJ Manag Care Pharm200915972874019954264
- RobinALNovackGDCovertDWCrockettRSMarcicTSAdherence in glaucoma: objective measurements of once-daily and adjunctive medication useAm J Ophthalmol2007144453354017686450
- RobinALCovertDDoes adjunctive glaucoma therapy affect adherence to the initial primary therapy?Ophthalmology2005112586386815878067
- StoneJLRobinALNovackGDCovertDWCagleGDAn objective evaluation of eyedrop instillation in patients with glaucomaArch Ophthalmol2009127673273619506189
- OkekeCOQuigleyHAJampelHDAdherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid studyOphthalmology2009116219119919084273
- FriedmanDSHahnSRGelbLDoctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency StudyOphthalmology200811581320132718321582
- LeungEWMedeirosFAWeinrebRNPrevalence of ocular surface disease in glaucoma patientsJ Glaucoma200817535035518703943
- FechtnerRDGodfreyDGBudenzDStewartJAStewartWCJasekMCPrevalence of ocular surface complaints in glaucoma patients using topical intraocular pressure-lowering medicationsCornea201049 [Epub ahead of print]. doi:10.1097/ICO.0b013e3181c325b2.
- SitAJWeinrebRNCrowstonJGKripkeDFLiuJHSustained effect of travoprost on diurnal and nocturnal intraocular pressureAm J Ophthalmol200614161131113316765686
- CostagliolaCDel PreteAVerolinoMEffect of 0.005% latanoprost once daily on intraocular pressure in glaucomatous patients not adequately controlled by beta-blockers twice daily: a 3-year follow-up. Experience and incidence of side effects in a prospective study on 76 patientsGraefes Arch Clin Exp Ophthalmol2002240537938612073061
- NetlandPALandryTSullivanEKTravoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertensionAm J Ophthalmol2001132447248411589866
- American Academy of Ophthalmology Glaucoma Panel, Preferred Practice Patterns CommitteePrimary Open-Angle Glaucoma. Limited RevisionSan Francisco, CAAmerican Academy of Ophthalmology2003
- BroadwayDCGriersonIO’BrienCHitchingsRAAdverse effects of topical antiglaucoma medication. II. The outcome of filtration surgeryArch Ophthalmol199411211144614547980134
- LewisRAKatzGJWeissMJTravoprost 0.004% with and without benzalkonium chloride: a comparison of safety and efficacyJ Glaucoma20071619810317224758
- BaudouinCDetrimental effect of preservatives in eyedrops: implications for the treatment of glaucomaActa Ophthalmol200886771672618537937
- HenryJCPeaceJHStewartJAStewartWCEfficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapyClin Ophthalmol2008261362119668762
- KahookMYNoeckerRJComparison of corneal and conjunctival changes after dosing of travoprost preserved with sof Zia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tearsCornea200827333934318362664
- HorsleyMBKahookMYEffects of prostaglandin analog therapy on the ocular surface of glaucoma patientsClin Ophthalmol2009329129519668581
- ChaSHLeeJSOumBSKimCDCorneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitroClin Experiment Ophthalmol200432218018415068436
- WhitsonJTCavanaghHDLakshmanNPetrollWMAssessment of corneal epithelial integrity after acute exposure to ocular hypotensive agents preserved with and without benzalkonium chlorideAdv Ther200623566367117142200
- YeeRWNorcomEGZhaoXCComparison of the relative toxicity of travoprost 0.004% without benzalkonium chloride and latanoprost 0.005% in an immortalized human cornea epithelial cell culture systemAdv Ther200623451151917050493
- SchmierJKCovertDWRobinALFirst-year treatment costs among new initators of topical prostaglandin analogsClin Opthalmol20093637644
- SchmierJKCovertDWRobinALFirst-year treatment costs among new initiators of topical prostaglandin analogsClin Ophthalmol2009363764419997567
- SchmierJKCovertDWRobinALFirst-year treatment patterns among new initiators of topical prostaglandin analogsCurr Med Res Opin200925485185819231912
- FremontAMLeePPMangioneCMPatterns of care for open-angle glaucoma in managed careArch Ophthalmol2003121677778312796247
- QuigleyHAFriedmanDSHahnSREvaluation of practice patterns for the care of open-angle glaucoma compared with claims data: the Glaucoma Adherence and Persistency StudyOphthalmology200711491599160617572498
- Thomson HealthcareRed Book Drug TopicsMontvale, NJThomson Healthcare2009
- Physicians’ Fee Reference: Comprehensive Fee ReportNew Haven, CTYale Wasserman D.M.D. Medical Publishers2009
- FriedmanDSQuigleyHAGelbLUsing pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS)Invest Ophthalmol Vis Sci200748115052505717962457
- FriedmanDSWolfsRCO’ColmainBJPrevalence of open-angle glaucoma among adults in the United StatesArch Ophthalmol2004122453253815078671
- BrownMMBrownGCSpaethGLImproper topical self-administration of ocular medication among patients with glaucomaCan J Ophthalmol1984191256608974
- AshburnFSJrGoldbergIKassMACompliance with ocular therapySurv Ophthalmol1980242372486987764
- KholdebarinRCampbellRJJinYPBuysYMMulticenter study of compliance and drop administration in glaucomaCan J Ophthalmol20084345446118711461
- NorellSEGranstromPASelf-medication with pilocarpine among outpatients in a glaucoma clinicBr J Ophthalmol1980641371417362816
- VanelliMPedanALiuNHoarJMessierDKiarsisKThe role of patient inexperience in medication discontinuation: a retrospective analysis of medication nonpersistence in seven chronic illnessesClin Ther200931112628265220110007
- NordstromBLFriedmanDSMozaffariEQuigleyHAWalkerAMPersistence and adherence with topical glaucoma therapyAm J Ophthalmol2005140459860616226511
- ShayaFTMullinsCDWongWChoJDiscontinuation rates of topical glaucoma medications in a managed care populationAm J Manag Care20028Suppl 10S271S27712188170
- SchwartzGFPlattRReardonGMychaskiwMAAccounting for restart rates in evaluating persistence with ocular hypotensivesOphthalmology2007114464865217398318
- GrossRLPeaceJHSmithSEDuration of IOP reduction with travoprost BAK-free solutionJ Glaucoma200817321722218414108