Abstract
Cyclosporine (CYA) is used to preventing ocular attacks in Behçet’s disease patients. Yet there are inter-individual variations in efficacy. In order to analyze the relationship between CYA fluctuation with treatment effectiveness and genetic factors, an association of area under the plasma concentration time at 0–4 hours (AUC0-4) values and polymorphism for multidrug resistance 1 (MDR1) and cytochrome3A5 (CYP3A5) genes was investigated. Genomic DNA was collected from 17 Japanese patients with Behçet’s disease. MDR1 polymorphisms were determined by direct sequencing from amplified products for promoter and two exons regions and CYP3A5 polymorphisms were analyzed using polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) method. AUC0-4 value was determined by the trapezoidal rule from the data of 5 times blood sampling at 0–4 hours. The haplotype 2 in the promoter region of MDR1 influenced significantly lower AUC0-4 values, implying absorption decline of CYA. The CYP3A5 polymorphisms had no direct influence on the effectiveness for CYA treatment. In the relation of CYA and AUC0-4 in the patients, 7 cases were grouped effective and 4 ineffective. Though there was no difference in dosage, the trough values for AUC0-4 were higher in the effective group compared to the ineffective group.
Introduction
The four main symptoms of Behçet’s disease are recurrent oral aphthosis, skin lesions such as erythema nodosum, uveoretinitis, and genital ulcers. 20,000 patients have been diagnosed with Behçet’s disease in Japan; however, including the latent, the actual number is estimated higher. It is thought to be a multifactorial disease strongly associated with a certain genetic background and triggered by the involvement of an environmental factor. Studies show strong association of the gene HLA-B51 with the disease as a genetic factor (CitationMizuki et al 2000). Behçet’s disease, also known as “Silk Road disease,” being common in Mongoloids along the Silk Road, and rare in Caucasians, is thought to have environmental exposures to something around the Silk Road as a factor. Cyclosporine (CYA) is effective in preventing ocular attacks in Behçet’s disease patients, usually administered at the dosage of 5.0 mg/kg per day. Yet there are inter-individual variations in efficacy (CitationFujino et al 1999). In blood level monitoring of CYA, the trough level is most commonly used for its convenience, although AUC is considered most efficacious. However in organ transplantation, with the development of cyclosporine micro emulsion preparation, a new monitoring parameter is being evaluated as a possible alternative to trough level monitoring. The inter-individual variations of CYA is the most significant at 1–3 hours after administration; furthermore, the T-cell calcineurin inhibitor effect of CYA correlates with the CYA blood level at 2 to 3 hours from administration, suggesting the clinical significance of AUC0-4 (CitationKeown 1999; CitationMahalati et al 1999; CitationJohnston et al 2000; CitationLevy et al 2002). On the other hand, CYA has been reported to be the substrate of P-glycoprotein (CitationSaeki et al 1993). MDR1 protein has been reported to functionally express P-glycoprotein widely in normal tissue such as gastrointestinal epithelial cells, renal proximal tubules, luminal capillary bile ducts of the liver, cerebral capillary endothelial cells, choroid plexus epithelial cells (CitationThiebaut et al 1987; CitationSugawara et al 1988). In the gastrointestinal tract, P-glycoprotein is expressed in the brush border side, and CYA, digoxin, saquinavir which are good substrates of P-glycoprotein, are discharged from the gastrointestinal epithelial cells into the lumen of the gastrointestinal tract, resulting in reduced drug absorption (CitationFromm 2000). The MDR1 gene which codes P-glycoprotein is reported to have more than 20 SNPs, including intron and promoter region (CitationHoffmeyer et al 2000; CitationTanabe et al 2001; CitationMarzolini et al 2004). Allele difference influences quantity in protein expression, suggesting the difference in drug absorption. MDR1 expression is especially small in homozygotes with cytosine to thymine mutation and homozygotes with guanine to thymine or guanine to adenine mutations in 2677th base of the exon 21, accelerating the absorption of CYA (CitationBalram et al 2003; CitationChowbay et al 2003), however another report that in a healthy subject, C3435T mutation in exon 26 has no effect in the drug conductor of CYA (CitationMin and Ellingrod 2002). Also, studies show the polymorphism of CYP3A5 affects the clearance of CYA significantly (CitationYates et al 2003; CitationMin et al 2004). Therefore in order to analyze the relationship between CYA interindividual fluctuation and genetic factors in Behçet’s disease patients, AUC0-4 values, polymorphisms for MDR1 and CYP3A5 retrieved, and relationship with treatment effectiveness was investigated.
Materials and methods
Subjects
The subjects were 17 patients who had been diagnosed Behçet’s disease, and had been administered CYA at either Yokohama City University Hospital Department of Ophthalmology or Hokkaido University Hospital Department of Ophthalmology (). Judgment of effectiveness was possible in 11 cases. When the number of attacks during six months after the initial administration was half or less the number of attacks during six months before the initial administration, the case was evaluated effective. All patients and control subjects agreed to a blood examination conducted according to the guidelines of the Declaration of Helsinki.
Table 1 Background characteristics of patients
CYA blood level
5 times blood sampling, at before, 1, 2, 3 and 4 hours after administration of CYA, and levels were measured using AxSYM-Cyclosporine- II · Dynapack (Abbott Japan). AUC0-4 value was determined by the trapezoidal rule from the 5 time blood sampling data at 0–4 hours.
MDR1 typing
In examination of MDR1 polymorphisms, variants in position 2677 of exon 21 (G2677A/T), variants in position 3435 of exon 26 (G3435T), and promoter region were tested. In examination of MDR1 gene promoter region polymorphisms, in accordance with reports by CitationTakane and colleagues (2004), PCR was carried out using the primer pair 5′-GGAGCAAAGAAATGGAATACAATA-3′, 5′TTCTCCCGTGAAGACCAAGTTC-3′ and in addition to the two previous 5′CTTATGAACATAGAGGAAATAGG-3′ and 5′-TGTAGCTCACGCCTGTAATCC-3′ were used as sequence primers in sequence reaction using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Using Applied Biosystems 3130 Genetic Analyzer or ABI® 377 Genetic Analyzer, base sequence of the amplified genes were determined to assume the haplotype of the promoter region.
G2677A/T and G3435T were typed in accordance with reports by CitationTanabe and colleagues (2002).
CYP3A5
CYP3A5 typing was carried out in accordance with report by CitationFukuen and colleagues (2002) for CYP3A5*1, CYP3A5*3, CYP3A5*6 using the PCR-RFLP method.
Statistical analysis
Effective and ineffective groups of CYA, AUC0-4 value, the correlation of AUC0-4 with each polymorphism were evaluated by Student’s t-test.
Results
AUC0-4 monitoring
As with transplantation patients, the AUC0-4 level showed higher correlation with C2 level (r2 = 0.80, p < 0.001) than trough level (r2 = 0.44, p = 0.035). The trough values were significantly higher, but AUC0-4 and C2 values were not for effective groups of CYA ().
Table 2 Cyclosporine dosage and pharmacokinetics parameters of each patient group
CYP3A5 polymorphisms
2 out of 17 subjects had homozygous CYP3A5*3/*3, and 15 had heterozygous CYP3A5*1/*3. AUC0-4 values were 2486 ± 952 ng · hr/ml in those with CYP3A5*3/*3, 1390 ± 349 ng · hr/ml in those with CYP3A5*1/*3. AUC0-4 values standardized by dosage values were 614 ± 233 ng · hr/ml and 354 ± 38 ng · hr/ml (p = 0.146). CYP3A5*1/*3 tended to have lower AUC0-4. However this was thought to have no direct influence on the effectiveness because of majority of patients being CYP3A5*3/*3 ().
Table 3 CYP3A5 genotype and the effectiveness in each patient
MDR1 polymorphisms
The polymorphisms of MDR1 promoter region were examined to find neither the deletion at −1423a and −1132a nor mutation at −824a and −755a in the 17 subjects. In conformity with reports by CitationTakane and colleagues (2004), haplotype was estimated from SNP types −1517a, −1459a, −1017a, −41a, −145, −129. Of the estimated haplotypes 9 cases were found to be 1/1, 3 cases 1/2, 2 cases 1/4, and 1 case each 1/5, 1/6, 2/4 (). Of the mutations in the 2677th base of exon 21 of MDR1, 8 cases were found to be G/G wild-type, 5 cases G/T heterozygote, 3 cases T/T mutant, and one case A/A mutant. Of the polymorphisms found in the 3435th base of exon 26, 7 cases were G/G wild-type, 7 cases G/T heterozygote, 3 cases T/T mutants ().
Table 4 Promoter haplotype of MDRI gene in 17 Japanese Behçet’s disease patients
Table 5 Dose normalized cyclosporine AUC(0-4) and MDR1 polymorphism in each patient
MDR1 polymorphisms and AUC0-4 values
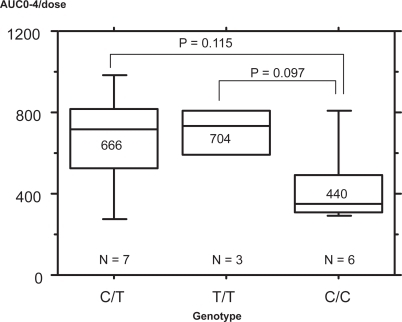
The effect of MDR1 polymorphisms on AUC0-4 standardized by dosage was examined. AUC0-4 values were 449 ± 195 ng · hr/ml in those with C/C wild-type in the G3435T, 666 ± 256 ng · hr/ml in those with C/T heterozygote, 704 ± 142 ng · hr/ml in those with T/T homozygote. C homozygotes tended to have lower AUC0-4 than other types (p = 0.101, p = 0.079) ().
Figure 1 Correlation of the MDR1 exon 26 SNP with dose normalized cyclosporine AUC0-4. AUC0-4 values were 449 ± 195 ng · hr/ml in those with C/C in the C3435T, 666 ± 256 ng · hr/ml in those with C/T, 704 ± 142 ng · hr/ml in those with T/T genotype. C homozygotes tended to have lower AUC0-4 than other types. (p = 0.101, p = 0.079).
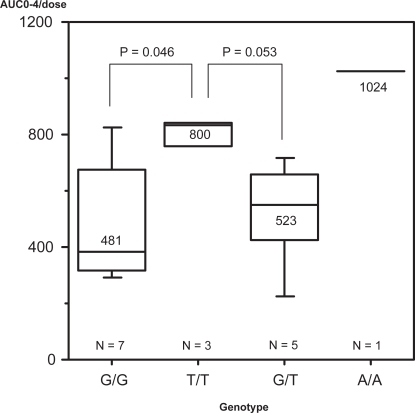
In terms of the G2677A/T, AUC0-4 values were 485 ± 206 ng · hr/ml in G/G homozygotes, 523 ± 189 ng · hr/ml in G/T heterozygotes, 856 ± 122 ng · hr/ml in T/T, A/A homozygotes. T/T and A/A which are mutant homozygotes had higher AUC0-4 than G/G which is a wild-type (). This does not disagree with previous reports.
Figure 2 Correlation of the MDR1 exon 21 SNP with dose normalized cyclosporine AUC(0-4). AUC(0-4) values were 485 ± 206 ng · hr/ml in G/G genotype, 523 ± 189 ng · hr/ml in G/T genotype, 856 ± 122 ng · hr/ml in T/T, A/A genotype, T/T and A/A which are mutant homozygotes had higher AUC0-4 than G/G genotype. (p = 0.008, 0.019).
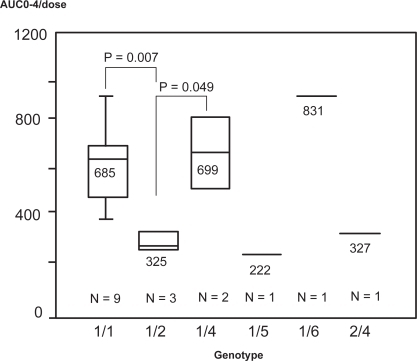
In terms of promoter region polymorphisms, types 1/1, 1/4, 1/6 had relatively high AUC0-4, while types 1/2, 1/5, 2/4 had relatively low AUC0-4. Heterozyogotes of haplotype 1/2 had significantly lower AUC0-4 values compared to types 1/1 and 1/4 (p = 0.007, 0.049) (). Also, all four subjects possessing haplotype 1/2 or 2/4 showed wild-type homozygotes at 3435th and 2677th.
Figure 3 Correlation of the MDR1 promoter region polymorphisms with dose normalized cyclosporine AUC0-4. Promoter types 1/1, 1/4, and 1/6 had high AUC0-4 levels, types 1/2, 1/5, 2/4 had low AUC0-4 levels, and heterozygote of haplotype 1/2, compared to 1/1 and 1/4 had significantly lower AUC0-4 levels. (p = 0.007 and 0.049).
Discussion
Although there was no significant difference between the two groups in dosage at 4.3 ± 1.3 mg/kg/day and 4.3 ± 1.4 mg/kg/day, trough levels, AUC0-4, C2 tended to be higher in those of the effective group than those of the ineffective group (). The fact that T-cell calcineurin inhibitor effect correlates well with the CYA blood levels 2–3 hours post-dose CYA indicates that AUC0-4 is clinically significant3–6. The correlation coefficient (r2) of AUC0-4 to trough and C2 were 0.44 (p = 0.035) and 0.80 (p < 0.001). C2 showed a higher positive correlation with AUC0-4, suggesting C2 to be a possible efficacy alternative to AUC0-4 in Behçet’s disease patients. However in this study, although the AUC0-4 of the effective group tended to be higher than AUC0-4 of the ineffective group, the significance of this difference is questionable. One patient with high AUC0-4 4231 ng · hr/ml later developed a side effect in the central nervous system presumably owing to CYA. Additional cases must be evaluated in order to reveal effective AUC0-4 and C2 levels.
It has been reported that certain CYP3A5 alleles express different CYA metabolic activity, consequently possibly effecting CYA blood levels greatly enough to alter the curative effect of CYA (CitationYates et al 2003; CitationMin et al 2004). In the present study when efficient group and inefficient group were investigated for CYP3A5 polymorphisms, one case in the efficient group had CYP3A5*1/*3 heterozygote, others in the efficient group had CYP3A5*3 homozygote with no protein expression. Low AUC0-4 values were shown in CYP3A5*1/*3 group. However it was hard to obtain genotypic influence of CYP3A5 to AUC0-4 values, because all 15 patients except for two were CYP3A5*3/*3.
No significant differentiation was shown between the two groups indicating the low possibility that CYP3A5 gene directly affects the efficacy of CYA.
Concerning the effect of MDR1 polymorphism, CitationHoffmeyer and colleagues (2000) have investigated Caucasian MDR1 polymorphisms in which the 3435th base mutate from C to T, revealing that the P-glycoprotein expression in the intestine of patients with CC genotype is less than half of those with TT genotype, that alimentary tract MDR1 expression is low and digoxin AUC high in TT subjects.
The clearance of CYA is significantly greater in patients with allele mutations to T than in patients with wild-type homozygotes (CitationYates et al 2003). On the other hand, it has been reported that MDR1 gene expression is limited and CYA absorption is accelerated in homozygote mutations from C to T and homozygote mutations of base 2677 of exon 21 (CitationHoffmeyer et al 2000; CitationTanabe et al 2001) and that mutation in the 3435th base of exon 26 in healthy subjects has no effect on the dynamics of CYA (CitationMarzolini et al 2004). Also, CitationAnglicheau and colleagues (2004) reported that effect vary between race, furthermore, SNPs T-129C, C1236T, G2677AT, and C3435T of MDR1 have no great impact on pharmacokinetics of CYA. In Asian heart transplantation patients, Chowbay investigated SNPs C1236T, G2677TA, and C3435T for effect on CYA pharmacokinetics, and suggested that not genotypes but haplotypes of each should be examined (CitationChowbay et al 2003). Concerning polymorphisms of the promoter region in Japanese and Caucasian subjects, there were greater MDR1 mRNA expressions in the placenta and liver of Japanese heterozygous haplotypes 1/2, 1/3 (CitationTakane et al 2004).
In the present study, MDR1 polymorphisms in Japanese Behçet’s disease patients were investigated to reveal that in genetic region SNP patients possessing alleles with replacement from C to T in base 3435 in exon 26 tended to have a high AUC(0-4) level but no significant difference. Also, MDR1 mutations in base 2677 of exon 21 which posses G (G/G, G/T) compared to those which do not posses G (A/A, A/T) had significantly higher AUC(0-4) levels. ()
Concerning promoter region polymorphisms, types 1/1, 1/4 and 1/6 had high AUC0-4 levels, types 1/2, 1/5, 2/4 had low AUC0-4 levels, and heterozygote of haplotype 1/2, compared to 1/1 and 1/4 had significantly lower AUC0-4 levels (p = 0.007, 0.049) (). Out of the five patients who showed low AUC0-4 levels, 4 patients had promoter region haplotypes which posses 2 and bases of 3435 and 2677 were wild-type homozygote. Thereupon, 10 C3435T homozygote subjects were investigated (). Out of 7 who possessed CC, 4 patients had promoter haplotype which posses 2 and 3 patients did not. The promoter haplotype of three out of three patients whose C3435T genotype was T/T did not possess 2. Out of those who do not possess promoter haplotype 2, the three CC patients showed AUC0-4 value of 615 ± 200 ng · hr/ml, and the three T/T patients showed AUC0-4 value of 704 ± 142 ng · hr/ml. It can be said that there was no significant difference between C3435T genotypes when there is no promoter haplotype 2 possession (p = 0.56). On the other hand, the four promoter haplotype 2 possessors whose C3435T genotype was CC showed AUC0-4 value of 326 ± 41 ng · hr/ml. This was significantly lower in comparison to the AUC0-4 values of promoter haplotype 2 nonpossessors whose C3435T genotype is CC and promoter haplotype 2 nonpossessors whose C3435T genotype is T/T (p = 0.034, 0.003).
Table 6 Dose normalized cyclosporine AUC0-4 and MDRI promoter haplotype polymorphism in C3435T homozygote patients
These findings suggested the necessity of investigation of MDR1 gene polymorphisms, including promoter region haplotypes.
Acknowledgements
This study was supported by grants as follows: Japanese Health and Labor Sciences Research and Grant-in-Aid for Scientific Research (KAKENHI).
References
- AnglicheauDThervetEEtienneI2004CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantationClin Pharmacol Ther754223315116055
- BalramCSharmaASivathasanC2003Frequency of C3435T single nucleotide MDR1 genetic polymorphism in an Asian population: phenotypic-genotypic correlatesBr J Clin Pharmacol56788312848778
- ChowbayBCumaraswamySCheungYB2003Genetic polymorphisms in MDR1 and CYP3A4 genes in Asians and the influence of MDR1 haplotypes on cyclosporin disposition in heart transplant recipientsPharmacogenetics13899512563178
- FrommMF2000P-glycoprotein: adfense mecanism limiting oral bioavailability and CNS accumulation of drugsInt J Clin Pharmacol Ther38697410706193
- FujinoYJokoSMasudaK1999Cyclosporine microemulsion preconcentrate treatment of patients with Behçet’s diseaseJpn J Ophthalmol433182610482480
- FukuenSFukudaTMauneH2002Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese populationPharmacogenetics12331412042671
- HoffmeyerSBurkOvon RichterO2000Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivoProc Natl Acad Sci USA973473810716719
- JohnstonADavidOJCooneyGF2000Pharmacokinetic validation of neoral absorption profilingTransplant Proc32SupplS536
- KeownPA1999Therapeutic strategies for optimal use of novel immunosuppressantsTransplant Proc311790210371954
- LevyGThervetELakeJ2002Consensus on Neoral C(2): Expert Review in Transplantation (CONCERT) Group. Patient management by Neoral C(2) monitoring: an international consensus statementTransplantation73SupplS121812023608
- MahalatiKBelitskyPSketrisI1999Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantationTransplantation68556210428267
- MarzoliniCPausEBuclinT2004Polymorphisms in human MDR1(P-glycoprotein): recent advances and clinical relevanceClin Pharmacol Ther75133314749689
- MinDIEllingrodVL2002C3435T mutation in exon 26 of the human MDR1 gene and cyclosporine pharmacokinetics in healthy subjectsTher Drug Monit24400412021632
- MinDIEllingrodVLMarshS2004CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjectsTher Drug Monit26524815385835
- MizukiNOtaMYabukiK2000Localization of the pathogenic gene of Behçet’s disease by microsatellite analysis of three different populationsInvest Ophthalmol Vis Sci413702811053265
- SaekiTUedaKTanigawaraY1993Human P-glycoprotein transports cyclosporin A and FK506J Biol Chem2686077807681059
- SugawaraIKataokaIMorishitaY1988Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16Cancer Res48192692894894
- TakaneHKobayashiDHirotaT2004Haplotype-oriented genetic analysis and functional assessment of promoter variants in the MDR1 (ABCB1) geneJ Pharmacol Exp Ther31111798715280437
- TanabeMIeiriINagataN2001Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 geneJ Pharmacol Exp Ther29711374311356939
- ThiebautFTsuruoTHamadaH1987Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissuesProc Natl Acad Sci USA84773582444983
- YatesCRZhangWSongP2003The effect of CYP3A5 and MDR1 polymorphic expression on cyclosporine oral disposition in renal transplant patientsJ Clin Pharmacol435556412817518