Abstract
In a clinical trial, we have previously shown that a telephone intervention can significantly increase participation in dilated fundus examination (DFE) screening among low-income adults with diabetes. Here the costs and cost-effectiveness ratio of this intervention are calculated. Intervention effectiveness was estimated as the difference in DFE utilization between the telephone intervention and print groups from the clinical trial multiplied by the size of the telephone intervention group. A micro-costing approach was used. Personnel time was aggregated from logs kept during the clinical trial of the intervention. Wage rates were taken from a commercial compensation database. Telephone charges were estimated based on prevailing fees. The cost-effectiveness ratio was calculated as the ratio of total costs of the intervention to the number of DFEs gained by the intervention. A sensitivity analysis estimated the cost-effectiveness of a more limited telephone intervention. A probabilistic sensitivity analysis using bootstrap samples from the clinical trial results quantified the uncertainties in resource utilization and intervention effectiveness. Net intervention costs were US$18,676.06, with an associated gain of 43.7 DFEs and 16.4 new diagnoses of diabetic retinopathy. The cost-effectiveness ratio is US$427.37 per DFE gained. A restricted intervention limiting the number of calls to 5, as opposed to 7, would achieve the same results, but would cost approximately 17% less. In the probabilistic sensitivity analysis, the 5th and 95th percentiles of the cost-effectiveness ratio were US$304.05 and US$692.52 per DFE gained, respectively. Our telephone intervention is more expensive than simple mail or telephone reminders used in other settings to promote preventive care; it is, however, also considerably more effective, and is effective in a low-income minority population at greater risk for diabetes complications. The costs are dominated by labor costs, and may be substantially defrayed, without loss of effectiveness, by restricting the number of telephone calls to 5 per patient.
Introduction
The American Diabetes Association recommends that people with diabetes be screened regularly with dilated fundus examination (DFE) to detect diabetic retinopathy (DR) (CitationAiello et al 1998; CitationAmerican Diabetes Association 2004). Background and early proliferative retinopathy can be readily treated with laser photocoagulation, and such treatment reduces the incidence of sight-destroying retinal hemorrhages and other severe complications of diabetic retinopathy (CitationAiello et al 1998).
Notwithstanding the evidence that DFE screening can save sight, and the widespread acceptance of this by physicians, adherence to this recommendation is poor, estimated variously at 49% (CitationBrechner et al 1993), 33% (CitationBeckles et al 2007), and 23% (CitationTaylor et al 2007) in different settings. Even when a physician recommends a DFE, typically the patient must then arrange for and keep an appointment for the procedure, and sometimes must identify a suitable provider. In addition, patients may be unaware of the nature of, need for, and sensitivity of a DFE for identifying ophthalmic disease at an early stage when it can be treated most effectively. Patients may not understand the implications of their diagnosis of diabetes, or may underestimate their personal risk of developing diabetic retinopathy. Thus, the less than optimal screening rates can be attributed to patient, provider and system challenges (CitationZhang et al 2007). This paper describes the costs of a patient-centered intervention.
We have previously described a randomized controlled trial of a telephone-based intervention to increase adherence with DFE screening recommendations in a population of predominantly low income minority adults with diabetes in Bronx, NY (CitationWalker et al 2008). The telephone intervention produced a 74% increase in retinopathy screening compared with a standard print intervention (CitationWalker et al 2008). Here we outline the costs associated with implementing this intervention and estimate its cost-effectiveness.
Methods
The telephone intervention itself has been described elsewhere (CitationWalker et al 2008). Briefly, adult patients with diabetes from 3 primary care settings in Bronx, NY, most of them poor and from minority groups, who had not had a DFE within the preceding 12 months were randomly assigned to the print or telephone intervention. The print group received a mailed pamphlet with information about retinal disease in diabetes and its prevention through DFE. The colorful, low-literacy pamphlet is similar to literature mailed by many health maintenance organizations to their enrollees. Those in the telephone intervention group received up to 7 telephone calls from trained bilingual health educators over the 6-month intervention period. During the telephone calls, time was devoted to establishing rapport, educating about diabetes eye complications, and the rationale for routine DFEs, and problem solving regarding the logistics of arranging for their DFE. The interventionists would not, however, make the appointments for the patients, as the goal of the intervention was to activate and empower patients who were in health care systems to have this screening exam. Once the patient reported having obtained a DFE, no further calls were made.
The perspective of this analysis is that of a provider of health care to a population of patients with diabetes. We have excluded from the analysis any costs that were associated specifically with the research aspects of the trial (as opposed to implementation of the intervention). And in estimating costs we have applied figures representative of current levels in the US, as opposed to the actual costs incurred in our specific setting.
Intervention logs were kept of all attempted and completed calls, including the time of initiation and termination. To assess costs, we tallied the number of calls to each patient, their durations, and the number of attempted calls that were not completed (such as no answer, busy signal, wrong number, not at home). We then associated several types of costs with each call.
The minimal qualifications for our interventionists/health educators were a bachelor’s degree in a social science or health education and excellent communication skills. The costs of labor for the health educators during the phone calls was accounted at an average salary of US$36,500 per year, based on US median earnings for this job category identified from a commercial compensation database (CitationPayScale, Inc. 2008). At the start of the program, each interventionist received approximately 20 hours of training about diabetes, retinopathy, counseling for behavior change, and the resources available in our setting. We estimated that all calls, whether completed or not, required 5 minutes of preparation time (eg, to locate and review the records). For completed calls, an additional 5 minutes were required afterward for making notes and re-filing the chart. The health educators received 1 hour of supervision for every 20 hours of intervention work from a nurse certified diabetes educator. We assessed training and supervision time at the hourly rate of the health educators plus the salary of the supervisor. We attributed an annual salary of US$70,000 to the supervisor, a slight increment above the median US$59,140 reported for a registered nurse credentialed as a certified diabetes educator (CitationPayScale, Inc. 2008), in recognition of the wage premium typically accorded supervisory work. In addition to base salary, all labor costs incurred an additional 28% charge for fringe benefits. Telephony charges were accounted at US$0.05 for each call (completed or attempted), plus an additional US$0.10 for each minute’s duration of a completed call.
Because an intervention of this nature would normally be implemented by assigning the telephone intervention responsibility to existing personnel whose skills are similar to those of health educators, we have not included costs for office space or recruitment in our analysis. We also assume that the magnitude of telephony generated would not require capital investment in additional equipment in the typical setting. Similarly, although our intervention maintained separate records, in a clinical setting, record keeping would ordinarily be done in the regular clinical chart, so we have not counted any costs for paper, filing cabinets, or other related supplies.
The effectiveness of the telephone intervention, the number of DFEs generated by the intervention, was calculated as the difference in probabilities of DFE in the telephone and print groups, multiplied by the number of people in the telephone group. A cost-effectiveness ratio was calculated as the cost of the intervention divided by the number of DFEs gained. Because the time-frame of the intervention and its sequelae was only 6 months, no discounting was applied to costs or effects.
Probabilistic sensitivity analysis was performed by generating 1,000 bootstrap samples from the clinical trial data set of individual patient records, thus capturing the uncertainty in the effectiveness of the intervention and the uncertainty in the number and duration of calls (CitationHunink et al 1998). We also calculated the cost-effectiveness ratio for a limited version of the intervention that terminates after the fifth call.
All calculations were carried out using Stata version 10 MP (Stata Corp., College Station, TX, USA).
Results
Study sample
Three hundred and five patients were randomized to the telephone intervention, and 298 to the print group. The mean age of all participants was 56.6 years, and 61% were women. Forty-five percent self-identified as black, and 17% as white; the remainder were other or did not choose a racial identification. Twenty-three percent chose to use Spanish during the study, and 42.5% self-identified as Hispanic. Forty-two percent were married or living with a partner. The median education level was a high school diploma, median household income fell in the US$15,000–30,000 range. Overall access to health care in this study group was good, as they were recruited from the panels of primary care treatment centers: 90.1% had some form of health insurance. The intervention groups did not differ significantly on any demographic or socioeconomic variable.
Median duration of diabetes at randomization was 7 years, interquartile range 3–11 years. Fully 10% of participants had been diagnosed with diabetes 20 or more years earlier. Baseline ophthalmic health information was not available in this study. Self-reported prior laser photocoagulation therapy, blindness in both eyes, proliferative retinopathy, and other serious vision problems were exclusion criteria for the study.
Base case
Labor inputs
Providing the telephone intervention to the 305 intervention group participants required a total of 4,147 attempted calls, plus 930 calls resulting in contact with the patients, having a total duration of 8,212 minutes. Health educator pay for these calls therefore totaled US$14,890.83 (including fringe benefits). In addition, 52 hours of training and supervision time, costing US$3,535.63, were required.
Telephony charges
Telephony charges of US$871.80 were incurred for 930 completed calls totaling 8,212 minutes, and 4,147 unsuccessful call attempts.
Total costs, effectiveness, and cost-effectiveness ratio
The total costs were therefore US$19,298.26. Of the 305 telephone group participants 103 (33.8%) ultimately underwent DFE within 6 months of randomization, compared with 57 (19.5%) of 293 controls. The intervention thus resulted in a gain of 43.7 DFEs, which were associated with an additional 3 incident diagnoses of macular edema and 16.4 incident diagnoses of diabetic retinopathy While most of these cases were background or mild nonproliferative retinopathy, they do include 1 case of severe nonproliferative, and 2 cases of proliferative disease. At these visits, 1 macular and 3 pan-retinal laser photocoagulations were recommended. An additional 4 patients were advised to have further evaluation by fluorescein angiography. No surgical treatments were prescribed. The print intervention cost US$2.04 per participant for the brochure, envelope, postage, and mailing labor. Thus the incremental cost of the telephone intervention above the print intervention was US$18,676.06. Therefore the incremental cost-effectiveness ratio is estimated at US$427.37 per DFE gained.
Sensitivity analysis
Limited intervention sensitivity analysis
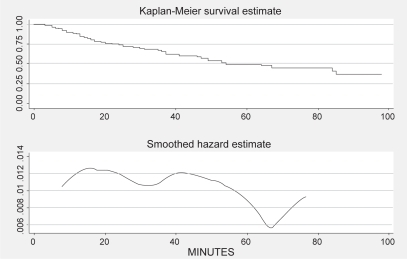
Most of the participants who received a DFE did so fairly early during the intervention period. If we view the total time spent on telephone calls leading up to the DFE as a “survival” time (with DFE as the end event, and subjects with no DFE censored at the end of their last call), we can examine the impact of additional phone contact using survival analysis. shows the Kaplan-Meier survival function estimator, and a smoothed hazard function for our study. It is evident that the probability of obtaining a DFE if the participant had not already done so in the first 60 minutes of total telephone contact time is quite small.
It is probably impractical to limit the intervention to 60 minutes of telephone contact. But it would be possible to terminate the intervention after a smaller number of calls. Nearly all participants who obtained a DFE did so after 4 or fewer calls; all did so by the fifth call. No additional DFEs took place after the sixth or seventh calls. Had we stopped our intervention after 5 calls, we would still have gained the same number of DFE events, but we would have avoided 1,221 call attempts, and 50 completed calls lasting a total of 391 minutes. Thus a total of US$3265.30 in labor, supervision, and telephony costs could have been saved with no loss in effectiveness. The cost-effectiveness ratio would have been US$352.65 per DFE gained.
Probabilistic sensitivity analysis
Holding the salaries, fringe levels, and telephony charges constant at their base case levels, we repeated our analysis reflecting the uncertainty in the intervention effectiveness and the number and duration of calls required, using 1,000 bootstrap samples from the original clinical trial results.
presents statistics describing the various types of costs, total costs, and cost-effectiveness ratio in this sensitivity analysis.
Table 1 Bootstrap statistics for cost components, total costs, and cost-effectiveness ratio
Discussion
Our base case estimate of approximately US$427 per DFE gained is the product of a limited analysis. A full cost-utility analysis would require estimating the number of years of improved vision that result from these DFEs. To truly estimate this would entail long-term follow-up of the study participants, which was outside the scope of this study. It is possible, indeed likely, that sustained DFE utilization would require periodic “boosters” of the intervention as well. For these reasons, we have limited our analysis to estimating the cost per additional DFE obtained. The fact that the additional DFEs yielded an additional 16.4 new diagnoses of diabetic retinopathy suggests that, at least initially, this screening process is efficient.
The intervention we have studied goes far beyond a mere “reminder” call. The health educators spent time with participants promoting both self-motivation and problem-solving assistance. Because such an intervention is labor intensive and cannot be carried out by untrained staff, not surprisingly, health educator labor accounts for the vast majority of total costs of this intervention.
Our intervention is considerably more effective than mailed reminders. Within our own study, the telephone group’s participation in DFE screening exceeded that of the print group by 74%. Similar contrasts have been found by others. For example, Halbert et al increased DFE uptake by only 1.6 DFEs per 100 persons using multiple mailed reminders (CitationHalbert et al 1999). They did not report the costs of their approach.
Relatively few cost-effectiveness analyses of interventions aimed at increasing utilization of established preventive measures have been published. CitationFishman et al (2000) compared 3 approaches to enhance mammography utilization in a managed care population of women who did not respond to a letter advising them to schedule a screening mammogram: a post-card reminder, a simple reminder call, and a motivational call. In their study, a simple reminder call cost US$92 for each mammogram generated. They found that motivational calling was more expensive but no more effective than simple reminder calling, so they did not calculate a cost-effectiveness ratio for that strategy. Their estimate of mammograms generated is questionable, however, because lacking a control group, they simply assumed that half of the observed mammograms would have occurred in the absence of intervention. Our intervention is more complex than theirs in that we made multiple calls (3.1 per participant on average). Fishman’s population, women served by Group Health Cooperative of Puget Sound in western Washington state, probably enjoys many socioeconomic advantages compared with our study population. And the barriers to arranging a DFE for our participants were also more formidable than the barriers to scheduling a mammogram for Fishman’s managed care members, especially considering that Fishman’s callers, unlike ours, had access to the appointment scheduling and could make the appointment for the patient during the call.
CitationMcDowell et al (1986) compared 3 methods of recalling patients for influenza vaccination in four Canadian primary care practices: personal reminder by physician, a letter, or a telephone call from a nurse. They found the telephone intervention to be the most effective, increasing vaccination probability from 9.8% among controls to 37.0%. Their single telephone call lasted, on average, 2 min 43 sec. They did not count time spent attempting incomplete calls, nor do they include any costs for training or supervision. Their cost-effectiveness ratio, which assumed nurses were paid US$40,000 per year, was approximately US$4 per vaccination gained. Obtaining a vaccination can be very quick and simple, particularly if it has been pre-ordered by the physician: a short visit to the nurse is all that is required. By contrast, to have a DFE one must have an appointment with an ophthalmologist or optometrist, which typically entails a wait, and which may be subject to last-minute rescheduling. Mydriatic drops are administered, and one waits while they take effect. While the examination itself may last only a few moments, the patient may not be able to resume normal activities until the effect of the dilating drops wears off some time later. Thus the barriers to obtaining a flu shot are substantially less than those for a DFE
We found no publications examining the cost-effectiveness of interventions that were fully comparable to ours in duration or intensity, in accounting methods, or in the difficulties to be overcome by the intervention, especially in our low-income urban minority population.
We see that our intervention is more expensive per unit outcome than other published approaches to increasing the use of preventive services, because our intervention is far more labor intensive than the others. We could have saved slightly more than 17% of the cost of our intervention had we terminated it after 5 telephone calls, and not a single DFE would have been lost through this modification. Fewer calls might have been needed, and the intervention might have been more effective, in a setting where interventionists had the ability to schedule appointments for the patients. Other approaches to reducing the cost of this intervention are not apparent. Some health care providers can obtain telephone service for lower charges than we have assumed, but telephony charges are only a small fraction of our total costs.
Labor costs dominate our expenses. The intervention may not be effectively delivered by personnel less skilled in health education and counseling than we budgeted in our analysis, and the amount of training and supervision provided was modest by any standard. Conceivably, outsourcing the telephone calls to health educators in a lower-wage country might result in savings, but callers’ foreign accents or other aspects of language or cultural differences, and unfamiliarity with our health care system, might well reduce the effectiveness of the intervention. The cost-effectiveness ratio may also be improved if a health system with an efficient appointment system adopted our intervention, so that the two activities were linked.
Diabetic retinopathy strikes approximately 50% of people with diabetes during their lifetimes, and despite the availability of effective treatment for early disease, it remains the leading cause of blindness among adults. It accounts for 10% of the total cost of diabetic complications (CitationCaro et al 2002), amounting to just over US$4,700 over 30 years for each person with type 2 diabetes. Although the technical aspects of diagnosis and treatment are well refined, the logistics of identifying early, treatable diabetic retinopathy are daunting. Complementary to efforts like ours that enhance participation in DFE screening, others have sought alternatives to DFE screening, such as retinal photography in the primary care setting, which is not as demanding of patient time and effort.
Clearly the challenge of diabetic retinopathy is no longer solely a biomedical one: it must also be solved through effective delivery of health services, proactive providers, and more motivated and empowered consumers. The barriers to care faced by poor, urban minority populations are difficult to surmount, and their vulnerability to diabetic complications is great. Yet we have shown that much can be achieved if we are willing to pay a moderate cost. If not, society will pay the medical, social, and personal costs for preventable cases of vision loss and blindness. A just society would bear this cost to give its poorest people their best chance at preserving eyesight.
Acknowledgments
This research was supported by National Institutes of Health grant EY13497 and partially by DK 20541. The authors express their appreciation to William H. Herman, MD MPH for his advice about this analysis.
Disclosures
None of the authors has any conflicts of interest to disclose.
References
- AielloLPGardnerTWKingGL1998Diabetic retinopathy (technical review)Diabetes Care21143569538986
- American Diabetes Association2004Position statement: Retinopathy in diabetesDiabetes Care27S84714693935
- BecklesGLWilliamsonDFBrownAF2007Agreement between self-reports and medical records was only fair in a cross-sectional study of performance of annual eye examinations among adults with diabetes in managed careMed Care458768317712258
- BrechnerRJCowieCCHowieLJ1993Ophthalmic examination among adults with diagnosed diabetes mellitusJAMA270171488411502
- CaroJJWardAJO’BrienJA2002Lifetime costs of complications resulting from type 2 diabetes in the U.SDiabetes Care254768111874933
- FishmanPTaplinSMeyerD2000Cost-Effectiveness of strategies to enhance mammography useEff Clin Pract32132011185326
- HalbertRJNicholJMLeungK-M1999Effect of multiple patient reminders in improving diabetic retinopathy screening: a randomized trialDiabetes Care22752510332676
- HuninkMGBultJRde VriesJ1998Uncertainty in decision models analyzing cost-effectiveness: the joint distribution of incremental costs and effectiveness evaluated with a nonparametric bootstrap methodMed Decis Making18337469679999
- McDowelINewellCRosserW1986Comparison of three methods of recalling patients for influenza vaccinationCMAJ1359917
- PayScale, Inc.2008Accessed Jan 3, 2008 URL: www.payscale.com
- TaylorCRMerinLMSalungaAM2007Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill studyDiabetes Care30574817327323
- WalkerESchechterCBCabanA2008Telephone intervention to promote diabetic retinopathy screening among the urban poorAm J Prev Med341859118312805
- ZhangXNorrisSSaadineJ2007Effectiveness of interventions to promote screening for diabetic retinopathyAm J Prev Med333183517888859