Abstract
Purpose
To compare the efficacy of brinzolamide in Japanese patients with primary open-angle glaucoma (POAG) or ocular hypertension (OH) after a change from timolol in combination therapy with latanoprost.
Methods
A 12-week, prospective, open-label, comparative study was performed in 20 patients [11 males and 9 females, mean age of 64.5 ± 11.0 (SD)y] with POAG or OH treated with both latanoprost once daily and timolol 0.5% twice daily. During the study brinzolamide was substituted for timolol. Intraocular pressure (IOP) was measured at baseline, 4, 8, and 12 weeks. Blood pressure (BP), pulse rate (PR), and adverse events were also recorded.
Results
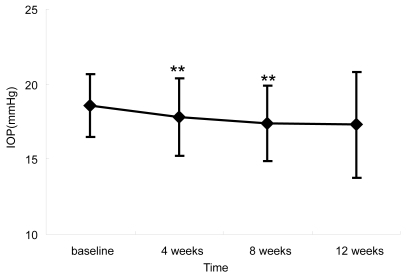
IOPs at baseline, 4, 8, and 12 weeks were 18.6 ± 2.1 mmHg, 17.8 ± 2.6 mmHg, 17.4 ± 2.5 mmHg, and 17.3 ± 3.5 mmHg, respectively. IOP reduction at 4 and 8 weeks was statistically significant (p < 0.05). The PR was significantly increased at 12 weeks (p < 0.01), but BP was not significantly affected. Four ocular adverse events were noted, but all were mild and transient.
Conclusions
Substituting brinzolamide 1% for timolol 0.5% in combination therapy with latanoprost 0.005% demonstrated significant IOP reduction with improvement in PR with POAG or OH. Combination therapy using latanoprost and brinzolamide may be recommended for better IOP control with fewer systemic adverse events.
Introduction
In recent years, significant advances have been achieved in the development of topical glaucoma medications (CitationCamras et al 1996; CitationSilver 1998; CitationBrubaker et al 2000), and options for combination therapy have increased. Because a significant proportion of glaucoma patients eventually need more than one agent for adequate control of intraocular pressure (IOP) (CitationKobelt-Nguyen et al 1998), it is important to reduce adverse ocular or systemic events in those treated with combination therapy. Using prostaglandin analogs as first-line treatment has given rise to numerous studies in search for the most effective and best-tolerated combination, from both a local and a systemic perspective. IOP reduction rates with various agents have been reported as follows: timolol 0.5% monotherapy, approximately 23.4% (CitationSchenker et al 1999); topical carbonic anhydrase inhibitors (CAIs) such as brinzolamide, 13.2%–16.7% (CitationSall 2000); and dorzolamide, 19.4% (CitationArici et al 1998). Meta-analysis of the IOP-lowering effects of several glaucoma medications demonstrated similar results (Citationvan der Valk 2005), with IOP reduction rates being highest with beta-blockers. The combination of a prostaglandin analog plus a beta-blocker has been extensively studied and is probably the most commonly used combination (CitationAlm et al 1995; CitationLarsson 2001; CitationHigginbotham et al 2002). It is noteworthy that the addition of a topical beta-blocker to latanoprost provides a slight additional IOP reduction (CitationBucci 1999; CitationO’Connor et al 2002). The efficacy of combination therapy using a topical CAI and latanoprost has not been studied to the same extent as that of latanoprost in combination with a beta-blocker; however, dorzolamide and brinzolamide have been reported to lower IOP at least as effectively as a beta-blocker when added to latanoprost (CitationO’Connor et al 2002; CitationShoji 2005). This option of latanoprost and topical CAI combination therapy may confer advantages for glaucoma patients as both drugs present an excellent systemic safety profile.
Brinzolamide (1% ophthalmic solution) is a topical CAI that, like dorzolamide, is known to lower IOP by inhibiting the production of aqueous humor (CitationSilver 1998; CitationSchenker et al 1999; CitationBrubaker et al 2000; CitationDeSantis 2000; CitationMarch and Ochsner 2000; CitationMichaud and Friren 2001). The IOP-lowering effect of brinzolamide 1.0% is reported to be equivalent to that of dorzolamide 2%, both as monotherapy (CitationBarnebey and Kwok 2000; CitationSall 2000) and in combination therapy with timolol (CitationMichaud and Friren 2001). Furthermore, when brinzolamide is substituted for dorzolamide, IOP is further decreased (CitationBarnebey and Kwok 2000) or is equal to IOP before substitution (CitationKobayashi et al 2004; CitationKubota et al 2004). In evaluations of ocular adverse events, dorzolamide has reportedly caused more intense ocular irritation than brinzolamide (CitationBarnebey and Kwok 2000; CitationSall 2000; CitationSilver 2000; CitationMichaud and Friren 2001; CitationSeong et al 2001; CitationKobayashi et al 2004; CitationStewart et al 2004; CitationTsukamoto et al 2005). The incidence of ocular discomfort or blurred vision for brinzolamide is significantly higher than that of dorzolamide in one study (CitationSilver 2000), but not statistically significant in other studies (CitationSall 2000; CitationMichaud and Friren 2001; CitationStewart et al 2004; CitationTsukamoto et al 2005). Thus brinzolamide 1% twice daily was selected as the topical CAI in this study.
The objective of this study was to evaluate the effects of a change in therapeutic regimen from combination therapy with timolol/latanoprost to combination therapy with brinzolamide/latanoprost in terms of: IOP, the cardiovascular functions of blood pressure (BP) and pulse rate (PR), and ocular adverse events.
Patients and methods
A 12-week, open-label, non-randomized, prospective, comparative study was conducted in Japanese patients with primary open-angle glaucoma (POAG) (17 patients) or ocular hypertension (OH) (3 patients) at Ryukyu University Hospital and Yamagata University Hospital. POAG was defined as glaucomatous optic neuropathy with repeatable visual field defects, and open angle. OH was defined as a normal visual field and normal optic disc appearance. Enrolled patients had been under treatment with both latanoprost (once daily, at 8 PM) and timolol 0.5% (twice a day, at 8 AM and 8 PM) for 3 months or longer and had not used any topical CAI prior to this study.
Inclusion criteria for the study were: (1) patients with primary open-angle glaucoma or ocular hypertension, (2) patients who had been treated with both latanoprost 0.005% once daily and timolol 0.5% twice daily for more than 3 months, (3) patients with IOP on latanoprost and timolol treatment ≥16 mmHg, (4) patients aged 20 years or over. The study included both males and females and inpatients and outpatients.
The following patients were excluded from the study: (1) patients with a history of chronic or recurrent inflammatory eye disease, ocular trauma, or ocular infection within the past 3 months, (2) those with corneal abnormalities preventing reliable applanation tonometry, (3) those who had had intraocular surgery, (4) those who had had laser surgery within the past 3 months, (5) those with severe, unstable, or uncontrolled cardiovascular or pulmonary disease that would preclude use of an ophthalmic beta-blocker, (6) those currently using any ophthalmic, dermatologic, or systemic corticosteroid or oral CAIs, (7) those with a history of severe hypersensitivity to oral CAIs, sulfonamide drugs, or any components of these medications, (8) those with dementia, and (9) those whom the investigator determined ineligible to participate in this study. Because enrolled patients were eligible for latanoprost and timolol combination therapy, those with hypersensitivity or allergy to either of these agents were excluded prior to the study.
The study protocol followed the guidelines of the Declaration of Helsinki. All patients were given a thorough explanation of the aims, protocol, and all procedures of the study before enrollment, and informed consent was then obtained.
Binzolamide 1% twice daily (at 8 AM and 8 PM) was given to each patient in place of timolol 0.5% twice daily to compare the efficacy and side effects from this change in therapeutic regimen. There was no wash-out period between the two treatment regimens. The prescription of brinzolamide 1% twice a day is common practice and is based on the label approved by the government in Japan.
IOP was analyzed on 1 eye per patient. If both eyes were eligible for inclusion, the eye with higher baseline IOP was selected as the study eye. When IOP was the same in both eyes, the right eye was selected. IOP was measured at the baseline visit and at 4, 8, and 12 weeks after enrollment using Goldmann applanation tonometry. The time of day at which IOP was measured during the study was the same as that at baseline. IOP measured immediately prior to substituting brinzolamide 1% was considered as the baseline. Resting BP (systolic and diastolic) and PR were also measured at the baseline and at 12 weeks. All patients underwent ophthalmic examinations at each visit during the follow-up period. Subjective ocular discomfort (mild irritation, blurred vision, and conjunctival hyperemia) and subjective ocular adverse events (stinging/intense irritation) after instillation of eye drops were investigated by patient interview. During clinic visits patients were asked whether they had a sense of ocular discomfort at the time of instillation and how long the ocular discomfort persisted after instillation. If a patient complained of any sense of irritation and/or blurred vision at least once during the follow-up period, the patient was regarded as having ocular discomfort.
IOP, BP, and PR values are shown as mean ± standard deviation. The Wilcoxon signed rank test was used for statistical analysis, and the difference was considered significant when the p value was less than 0.05.
Results
Of the 20 patients (11 males and 9 females) enrolled, no patients dropped out during the course of the study, and therefore 20 patients were accepted for analysis of IOP and local and systemic adverse events. shows the demograhpic characteristics of the patients. Briefly, the mean age of the patients was 64.5 ± 11.0 (mean ± standard deviation) years old. Seventeen patients (17 eyes) had POAG, and 3 patients (3 eyes) had OH. All had been treated with a combination of latanoprost 0.005% and timolol 0.5% for more than 3 months. shows the course of IOP at baseline and after the regimen change. Mean IOP at baseline, 4, 8, and 12 weeks after enrollment were 18.6 ± 2.1 mmHg, 17.8 ± 2.6 mmHg, 17.4 ± 2.5 mmHg, and 17.3 ± 3.5 mmHg, respectively. Significant reductions in mean IOP were observed at the 4-week and 8-week time points (p < 0.05) (). Mean IOP reduction from baseline was 0.8 mmHg (4.3% reduction) after 4 weeks, 1.2 mmHg (6.5% reduction) after 8 weeks, and 1.3 mmHg (7.0% reduction) after 12 weeks.
Figure 2 Change in IOP reduction rate after regimen change. Average IOP reduction rate 12 weeks after regimen change was 7.3%.
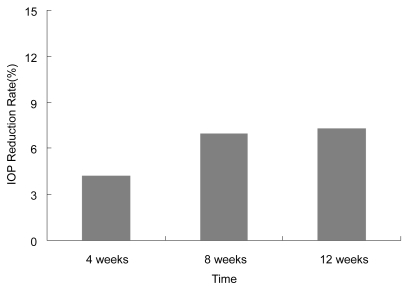
Table 1 Patients
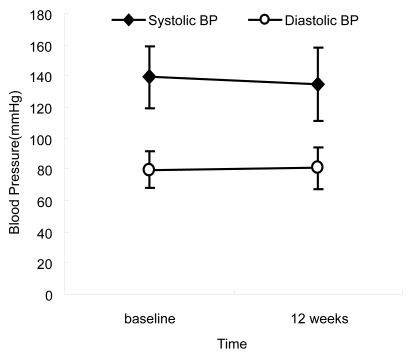
Mean systolic and diastolic BPs were 139 ± 20 mmHg and 80 ± 12 mmHg at baseline, and 135 ± 24 mmHg and 81 ± 13 mmHg at the 12-week point. Thus the substitution of brinzolamide had little effect on systolic and diastolic BP (p > 0.05) ().
Figure 3 Change in blood pressure after regimen change. No significant differences were seen in blood pressure (p > 0.05, Wilcoxon signed rank test).
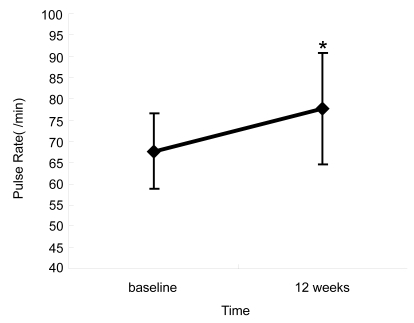
In contrast, mean PR was 68 ± 9 bpm at baseline, with a significant increase to 78 ± 13 bpm at the 12-week point (p < 0.01) (). Visual field defect and optic disc appearance did not change throughout the study period.
Figure 4 Change in pulse rate after regimen change. The pulse rate was significantly increased 12 weeks after substituting brinzolamide compared with baseline with timolol (*p < 0.01, Wilcoxon signed rank test).
No patient reported the adverse event of intense ocular irritation. After therapeutic substitution, ocular adverse events were observed in 4 cases (2 blurred vision, 1 mild irritation, and 1 mild hyperemia) (). All were mild and transient and disappeared without any treatment. Systemic adverse events were not observed.
Table 2 Ocular discomfort
Discussion
In this study, we compared the efficacy and safety of brinzolamide 1% twice daily as a second-line medication when substituted for timolol 0.5% in combination therapy with latanoprost in Japanese patients with POAG or OH. We concluded that substitution of brinzolamide for timolol significantly lowered IOP with significant improvement of PR without systemic or serious local adverse reactions.
Efficacy
Two recently developed topical CAIs, dorzolamide and brinzolamide, are now usually used as an adjunctive therapy to other anti-glaucoma eye drops. The IOP-lowering effect of brinzolamide 1% (twice daily) was equivalent to dorzolamide 2% (3 times daily) when added to timolol 0.5% (CitationMichaud and Friren 2001). The effect of topical CAIs as a second-line medication has been reported (CitationArici et al 1998; CitationShin 2000; CitationMichaud and Friren 2001; CitationO’Connor et al 2002; CitationMartinez-de-la-Casa et al 2004; CitationShoji et al 2005; CitationTsukamoto et al 2005). Brinzolamide was reported to show an additional IOP reduction of 5.3 mmHg (23.5%) when added to latanoprost (CitationShoji et al 2005). It has also been reported that the addition of dorzolamide to latanoprost decreased IOP by 3.9 mmHg (19.7%) compared with beta-blocker (2.5 mmHg; 12.3%) (CitationO’Conner et al 2002). Brinzolamide showed similar efficacy to timolol maleate when added to travoprost (CitationHollo et al 2006). In our study, IOP reductions from baseline at 4, 8, and 12 weeks after the substitution of brinzolamide 1% for timolol 0.5% were 0.8, 1.2, and 1.3 mmHg, respectively. The patients who enrolled in this study had already undergone treatment with latanoprost 0.005% and timolol 0.5%. Nevertheless, the substitution of brinzolamide for timolol 0.5% further significantly decreased IOP. This result was similar to the IOP reduction observed by CitationO’Conner et al (2002). The Early Manifest Glaucoma Trial Group reported that on follow-up, each mmHg reduction in IOP decreased the risk of disease progression by approximately 10% (CitationHeijl et al 2002). Therefore, even though the reduction in IOP after the substitution of brinzolamide for timolol was small (<2 mmHg), this IOP reduction could be considered clinically significant.
Ocular discomfort
The occurrence of ocular discomfort is related to the formula used for ophthalmic solutions. Brinzolamide is a white suspension. In our study, brinzolamide caused blurred vision (2 cases), and mild irritation and mild hyperemia (1 case) after switching from timolol. As with earlier studies, adverse events related to brinzolamide treatment generally occurred on instillation, were usually mild, resolved without treatment, and generally did not interrupt therapy continuation (CitationSilver 1998; CitationBarnebey and Kwok 2000; CitationMartinez-de-la-Casa et al 2004) (ie, no patient was discontinued from the current study due to adverse events). It has also been reported that in comparisons of brinzolamide and timolol monotherapy (CitationMarch and Ochsner 2000), brinzolamide produced fewer (3.3%) ocular adverse events (burning/stinging) than timolol (8.0%). The ocular discomfort of blurred vision was generally mild, and the medication was well tolerated. Therefore, evidence suggests that substituting brinzolamide for timolol may produce very few ocular adverse events in combination therapy with latanoprost.
Systemic effects
Non-selective and selective beta-blockers may have significant side effects on cardiovascular and respiratory organs, especially in elderly populations (CitationNelson et al 1986). When assessing a drug like timolol, which is used by a predominantly elderly population that is a priori at risk for cardiovascular and respiratory disease, it is often difficult to determine whether the suspect event is a consequence of the drug, the patients’ age, an underlying medical condition, or a combination of factors. In agreement with a previous study, no clinically significant adverse effect on PR and BP occurred with brinzolamide (CitationSilver 1998) and no systemic side effects were noted. Furthermore, increased PR is a potentially beneficial effect when substituting brinzomalmide for timolol. Topical beta-blockers can cause nocturnal hypotension in glaucoma patients (CitationHeyreh et al 1999). Glaucoma is a disease that generally requires lifelong treatment. The most effective medications for long-term therapy are those that are efficacious, well tolerated, and show few side effects. In those patients in whom beta-blockers are contraindicated, brinzolamide offers an effective alternative.
Conclusion
The latanoprost/brinzolamide combination can have a significant additive effect on IOP reduction compared with the latanoprost/timolol combination. IOP reduction has been established to be an effective means of treating glaucoma (CitationMao et al 1991; CitationCollaborative Normal-Tension Glaucoma Study Group 1998; CitationAGIS Investigations 2000; CitationHeijl et al 2002; CitationKass 2002; CitationTsukamoto et al 2005). To improve quality of life and vision, and to reduce the adverse events of glaucoma treatment, it is necessary to achieve a maximum IOP reduction with a minimum but adequate number of instillations. Substitution of brinzolamide 1% for timolol 0.5% in combination therapy with latanoprost 0.005% demonstrated superior and significant IOP reduction and improvement of PR. The ocular discomforts of blurred vision in 2 cases, mild irritation in 1 case, and mild hyperemia in 1 case were transient and mild after regimen change, and all resolved without treatment. The use of brinzolamide in place of timolol as a second-line medication in combination with latanoprost can be recommended for POAG or OH patients for better IOP control and fewer systemic side effects. However, this open non-randomized study evaluated a limited number of the patients treated in the short term, so that additional randomized studies on long-term administration and diurnal IOPs need to be considered.
Disclosures
None of the authors have any conflicts of interest to disclose.
References
- AGIS Investigations2000The Advanced Glaucoma Intervention Study (AGIS): The relationship between control of intraocular pressure and visual field deteriorationAm J Ophthalmol1304294011024415
- AlmAWidengardIKjellgrenD1995Latanoprost administrated once daily caused a maintained reduction of intraocular pressure in glaucoma patients treated concomitantly with timololBr J Ophthalmol791267880782
- AriciMKTopalkanaAGullerC1998Additive effect of latanoprost and dorzolamide in patients with elevated intraocular pressureInt Ophthalmol22374210090447
- BarnebeyHKwokSY2000Patients’ acceptance of switch from dorzolamide to brinzolamide for the treatment of glaucoma in a clinical practice settingClin Ther2212041211110231
- BrubakerRFIngnamCJSchoffEO2000Comparison of the efficacy of betaxolol -brinzolamide and timolol-dorzolamide as suppressors of aqueous humor flow in human subjectsOphthalmology107283710690826
- BucciMG1999Italian Latanoprost Study Group. Intraocular pressure-lowering effects of latanoprost monotherapy versus latanoprost or pilocarpine in combination with timolol: a randomized, observer-masked multi-center study in patients with open-angle glaucomaJ Glaucoma8243010084271
- CamrasCBAlmAWatsonP1996Latanoprost, a prostgalndin analog, for glaucoma therapy. Efficacy and safety after 1 year of treatment in 198 patients. Latanoprost Study GroupsOphthalmology1031916248942890
- Collaborative Normal-Tension Glaucoma Study Group1998The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucomaAm J Ophthalmol1264985059780094
- DeSantisL2000Preclinical overview of brinzolamideSurv Ophthalmol44s1192910665514
- HeijlALaskeMCBengtssonB2002Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma TrialArch Ophthalmol1202002
- HeyrehSSPodhajskyPZimmermanMB1999Beta-blocker eye drops and nocturnal arterial hypotensionAm J Ophthalmol128301910511024
- HigginbothamEJFeldmanRStilesM2002Fixed Combination Investigative Group. Latanoprost and timolol combination therapy vs monotherapy: one-year randomized trialArch Ophthalmol1209152212096962
- HollóGChiselitaDPetkovaN2006The efficacy and safety of timolol maleate versus brinzolamide each given twice daily added to travoprost in patients with ocular hypertension or primary open-angle glaucomaEur J Ophthalmol168162317191187
- KassMAHeuerDKHigginbothamEJ2002The Ocular Hypertension Treatment Study. A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucomaArch Ophthalmol1207011312049574
- KobayashiHKobayashiKOkinamiS2004Hypotensive effect and subjective symptoms after substitution of 1% dorzolamide by 1% brinzolamideJpn J Clin Ophthalmol582059
- Kobelt-NguyenGGerdthamUGAlmA1998Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective, observational chart review of newly diagnosed patients in Sweden and the United StatesJ Glaucoma7951049559495
- KubotaMHaraTKubotaS2004Ocular hypotensive effect of brinzolamide after switching from dorzolamideJpn J Clin Ophthalmol583013
- LarssonLI2001Effect on intraocular pressure during 24 hours after repeated administration of the fixed combination of latanoprost 0.005% and timolol 0.5% in patients with ocular hypertensionJ Glaucoma101091411316092
- MaoLKStewartWCShieldsMB1991Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucomaAm J Ophthalmol1115151985490
- MarchWFOchsnerLI2000The Brinzolamide Long-Term Therapy Study Group. The long-term safety and efficacy of brinzolamide 1.0% (Azopt) in patients with primary open-angle glaucoma or ocular hypertensionAm J Ophthalmol1291364310682964
- Martinez-de-la-CasaJMGarcia-FeijooCJMendez-HernandezC2004Concomitant administration of travoprost and brinzolamide versus fixed latanoprost/timolol combined therapy. Three-month comparison of efficacy and safetyCurr Med Res Opin201333915383180
- MichaudJEFrirenB2001International Brinzolamide Adjunctive Study Group. Comparison of topical brinzolamide 1% and dorzolamide 2% eye drops given twice daily in addition to timolol 0.5% in patients with primary open-angle glaucoma or ocular hypertensionAm J Ophthalmol1322354311476685
- NelsonWLFraunfelderFTSillsJM1986Adverse respiratory and cardiovascular events attributed to timolol ophthalmic solution, 1978–85Am J Ophthalmol102606113777080
- O’ConnorDJMartoneJFMeadA2002Additive intraocular pressure lowering effect of various medications with latanoprostAm J Ophthalmol133836712036683
- SallK2000The efficacy and safety of brinzolamide 1% ophthalmic suspension (Azopt) as a primary therapy in patients with open-angle glaucoma or ocular hypertension. The Brinzolamide Primary Therapy Study GroupSurv Ophthalmol44s1556210665518
- SchenkerHMaloneySLissC1999Patient preference, efficacy, and compliance with timolol maleate ophthalmic gel-forming solution versus timolol maleate ophthalmic solution in patients with ocular hypertension or open-angle glaucomaClin Therapeutics2113847
- SeongGJLeeSCLeeJH2001Comparisons of intraocular-pressure- lowering efficacy and side-effects of 2% dorzolamide and 1% brinzolamideOphthalmologica2151889111340389
- ShinD2000Adjunctive therapy with brinzolamide 1% ophthalmic suspension (Azopt) in patients with open-angle glaucoma or ocular hypertension maintained on timolol therapySurv Ophthalmol44s163810665519
- ShojiNOgataHSuyamaH2005Intraocular pressure lowering effect of brinzolamide 1.0% as adjunctive therapy to latanoprost 0.005% in patients with open angle glaucoma or ocular hypertension: an uncontrolled, open-label studyCurr Med Res Opin21503715899098
- SilverLH2000Ocular comfort of brinzolamide 1.0% ophthalmic suspension compared with dorzolamide 2.0% ophthalmic solution: Results from two multicenter comfort studies. The Brinzolamide Comfort Study GroupSurv Ophthalmol44s141510665516
- SilverLH1998The Brinzolamide Primary Therapy Study Group. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertensionAm J Ophthalmol12640089744373
- StewartWCDayDGStewartJA2004Short-term ocular tolerability of dorzolamide 2% and brinzolamide 1% versus placebo in primary open-angle glaucoma and ocular hypertension subjectsEye189051015002017
- TsukamotoHNomaHMatuyamaS2005The efficacy and safety of topical brinzolamide and dorzolamide when added to the combination therapy of latanoprost and a beta-blocker in patients with glaucomaJ Ocul Pharmacol Ther21170315857284
- van der ValkRWebersCABSchoutenJSAG2005Intraocular pressure-lowering effects of all commonly used glaucoma drugs. A meta-analysis of randomized clinical trialsOphthalmology11211778515921747