Abstract
Purpose:
To establish the efficacy and safety of intravitreal bevacizumab in reducing iris and anterior chamber angle neovascularization and managing neovascular glaucoma.
Design:
Prospective interventional case series.
Patient and methods:
Eleven eyes of 11 patients with iris and anterior chamber angle neovascularization with refractory intraocular pressure were treated with intravitreal injection of 1.25 mg bevacizumab (Avastin®). The study group included eight males and three females aged 23 to 77 years (average, 62 years). Out of the 11 cases, five had proliferative diabetic retinopathy, of whom two had undergone vitrectomy for tractional retinal detachment and vitreous hemorrhage, and six were secondary to ischemic central retinal vein occlusion (CRVO). All patients were followed for eight to 16 months (average, 10 months).
Results:
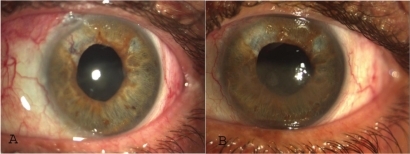
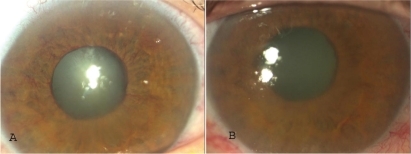
Iris and anterior chamber angle neovascularization receded in all eyes after one to three injections at monthly intervals. In five eyes, neovascularization recurred during the follow-up period. The intraocular pressure normalized in one eye. Four eyes were controlled with anti-glaucoma drops. A cyclodestructive procedure was required in two eyes. An Ahmet drainage valve was implanted in four eyes, including one controlled with additional antiglaucoma drops and one in which the intraocular pressure remained high while on maximum antiglaucoma medication and a cyclodestructive procedure was scheduled.
Conclusions:
Bevacizumab appears to be effective in reducing iris and anterior chamber angle neovascularization and is likely to extend our therapeutic options in the management of neovascular glaucoma.
Retinal ischemia and vascular endothelial growth factor (VEGF) have been implicated as major angiogenic stimuli responsible for retinal neovascularization. In addition, they are implicated in the growth of abnormal blood vessels on iris, anterior chamber angle, and in the development of neovascular glaucoma.
The standard treatment for iris and anterior angle neovascularization is panretinal photocoagulation (PRP), whereas in cases with intraocular pressure (IOP) rise, therapeutic options include PRP with topical antiglaucoma drops, per os carbonic anhydrase inhibitors, tube drainage procedures, photodynamic therapy,Citation1 or cyclodestructive procedures.
Bevacizumab (Avastin®; Genentech/Roche), a humanized antibody to VEGF, was recently reported to be of benefit in retinal and iris neovascularization due to diabetic retinopathy,Citation2–Citation5 in iris neovascularization due to central retinal vein occlusion (CRVO),Citation5,Citation6 central retinal artery occlusion,Citation6 and managing IOP in CRVO,Citation7 anterior ischemic syndrome,Citation8 and neovascular glaucoma of various etiologies.Citation9 In addition, intracameral bevacizumab followed by trabeculectomy reportedly results in good surgical outcomes.Citation10
This case series demonstrates the effectiveness and safety of intravitreal bevacizumab in the management of anterior chamber and iris neovascularization and as an adjunct treatment of neovascular glaucoma of various etiologies.
Patients and methods
Eleven eyes of 11 consecutive patients with iris and anterior chamber angle neovascularization and refractory IOP while on maximum antiglaucoma medication, were treated with intravitreal injections of 0.05 ml (1.25 mg) bevacizumab (Avastin®). Retreatment was based on biomicroscopic evidence of residual iris or anterior chamber neovascularization at monthly intervals until the disappearance of neovascularization.
The study group included eight male and three female patients, aged 23 to 77 years (average, 62 years). Out of the 11 cases, five had proliferative diabetic retinopathy, of whom two had undergone vitrectomy for tractional retinal detachment and vitreous hemorrhage, and six were secondary to ischemic CRVO.
The average IOP on presentation was 40.8 mmHg (standard deviation [SD] = 5.3) while on maximum anti-glaucoma medication. Seven patients had undergone prior panretinal laser photocoagulation (PRP), of whom three had additional PRP after bevacizumab injections. One additional patient commenced PRP after bevacizumab treatment, whereas the remaining three patients were not able to undergo any PRP because of dense vitreous hemorrhage ().
Table 1 Patient data on presentation and treatment
The patients were followed monthly for eight to 16 months (average, 10 months; SD = 2.6).
The study was approved by the University of Athens institutional ethical committee on the basis of existing literature. Each patient was informed of the study purposes, the alternative therapeutic options, the natural history of the disease, the off-label use of bevacizumab and signed the relative consent form. All the recommendations for intravitreal injections were followed.Citation11
Results
In seven eyes, iris and anterior chamber angle neovascularization biomicroscopically disappeared following the first injection, in one eye following the second one, whereas the other three eyes required a third injection.
The average IOP at the end of the follow-up period was 16.1 mmHg (range 10 to 32 mmHg). The patient who had had two injections, had his IOP controlled without further treatment. In four patients, the IOP was normalized with the use of antiglaucoma drops. In two patients, the IOP remained refractory and necessitated a cyclodestructive procedure. Implantation of an Ahmet drainage valve was completed in four patients, of whom two needed additional topical medication. In one of the latter two, the IOP remained high despite maximum antiglaucoma medication and a cyclodestructive procedure was scheduled.
Reappearance of iris and angle neovascularization during the follow-up period was noted in five eyes. Noteworthy, further laser treatment was not possible in any of these eyes ().
Table 2 Follow up and final results
No ophthalmic or systemic complications attributable to intravitreal injections were recorded, except insignificant conjunctival hematoma at injection site in some cases (, ).
Discussion
Intraocular bevacizumab has been tried as an adjunct treatment modality in anterior chamber neovascularization of various etiologiesCitation2–Citation6 and the short term efficacy of intraocular injection on iris neovascularization and IOP is well proven.Citation12–Citation14 In a retrospective case seriesCitation12 that included six eyes of three patients, all eyes manifested marked regression up to complete disappearance of iris and angle neovascularization after a single intravitreal injection. In three out of six eyes IOP decreased, whereas in the remaining patients IOP remained refractory and cyclophotocoagulation was decided. In another retrospective study including 41 eyes with significant follow-up (13.3 months), the authors concluded that intravitreal bevacizumab is well-tolerated, effectively stabilizing iris neovascularization and controlling IOP in patients with iris neovascularization alone or early-stage neovascular glaucoma without angle closure. In advanced neovascular glaucoma, intravitreal bevacizumab could not control IOP but could be used adjunctively to improve subsequent surgical results.Citation15
In our study, the intravitreal administration of bevacizumab in monthly intervals (one to three injections) resulted in considerable regression of iris and anterior chamber angle neovascularization. The results seem to be more favorable when “sufficient” elimination of retinal ischemia is attained, as was apparently the case in patient 1 following the reattachment of the retina. Iris neovascularization reappeared in all three eyes without laser treatment and in only two out of eight eyes that underwent PRP before or after intravitreal injection ().
Control of the IOP required additional medical treatment or surgical intervention in 10 out of 11 eyes. As a general trend, regression of iris neovascularization resulted in management of IOP without further treatment or with topical medical medication only (five of six cases), whereas recurrent neovascularization additionally required invasive treatments (valve implantation in two eyes and cyclodestruction in three eyes). The presence of extensive anterior synechiae predisposed to uncontrolled IOP that also demanded surgical intervention. Younger patients tended to respond better to treatment and develop less anterior synechiae ( and ).
The absence of ocular side effects or systemic complications is in agreement with the findings of a related metanalysis study comprising 127 eyes, which estimated that ophthalmic complications were under 0.78% without reported systemic complications.Citation14
In conclusion, intraocular bevacizumab targets the basic pathophysiological mechanism of retinal and anterior chamber neovascularization and may serve as an adjunct to PRP in cases of neovascular glaucoma. In our study, anti-VEGF treatment enabled continuation of PRP in one eye and initiation of PRP in three eyes (), reducing neovascularization until vitreous hemorrhage subsided and iris became more pliable to mydriasis. In this context, intraocular bevacizumab appears to be effective in reducing iris and anterior chamber angle neovascularization and has a role in the management of neovascular glaucoma.
Disclosures
The authors report no conflicts of interest in this work.
References
- ParodiMIaconoPPhotodynamic therapy for neovascular glaucomaOphthalmology200511218441845
- AveryRRegression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin®) treatmentRetina200626351354
- DavidorfFMouserGDerickRRapid improvement of rubeosis iridis from a single bevacizumab (Avastin®) injectionRetina20062635435616508439
- PaulaSJorgeRCostaARodrigues MdeLScottIUShort-term results of intravitreal bevacizumab (Avastin®) on anterior segment neovascularization in neovascular glaucomaActa Ophthalmol Scand20068455655716879583
- GrisantiSBiesterSPetersSTatarOZiemssenFIntracameral bevacizumab for iris rubeosisAm J Ophthalmol200614215816016815268
- MasonJAlbertMMaysAVailRRegression of neovascular iris vessels by intravitreal injection of bevacizumabRetina20062683984116963866
- BatioğluBAstamNÖzmertERapid improvement of retinal and iris neovascularization after a single intravitreal bevacizumab injection in a patient with central retinal vein occlusion and neovascular glaucomaInt Ophthalmol200828596117609852
- LeeSJLeeJJKinSYKimSDIntravitreal bevacizumab (Avastin®) treatment of neovascular glaucoma in ocular ischemic syndromeKorean J Ophthalmol20092313213419568367
- HasanreisogluMWeinbergerDMimouniKIntravitreal bevacizumab as an adjunct treatment for neovascular glaucomaEur J Ophthalmol20091960761219551676
- GuptaVJhaRRaoAKongGSihotaRThe effect of different doses of intracameral bevacizumab on surgical outcomes of trabeculectomy for neovascular glaucomaEur J Ophthalmol20091943544119396791
- JaissleGSzurmanPBartz-SchmitKRecommendation for the implementation of intravitreal injections – statement of the German Retina Society, the German Society of Ophthalmology (DOG) and the German Professional Association of Ophthalmologists (BVA)Klin Monatsbl Augenheilkd200522539039515912456
- IlievMDomigDWolf-SchnurrburschUWolfSSarraGIntravitreal bevacizumab (Avastin®) in the treatment of neovascular glaucomaAm J Ophthalmol20061421054105617157590
- Andrijević-DerkBVatavukZBencićGNovak-LausKMandićZIntravitreal bevacizumab for neovascular glaucomaActa Clin Croat20084717517919175068
- Martínez-CarpioPABonafonte-MárquezEHeredia-GarcíaCDBonafonte-RoyoSEfficacy and safety of intravitreal injection of bevacizumab in the treatment of neovascular glaucoma: systematic review]Arch Soc Esp Oftalmol20088357958818855277
- WakabayashiTOshimaYSakaguchiHIntravitreal bevacizumab to treat iris neovascularization and neovascular glaucoma secondary to ischemic retinal diseases in 41 consecutive casesOphthalmology20081151571158018440643