Abstract
Chronic infection with hepatitis C virus (HCV) is a major public health problem, with perhaps 180 million people infected worldwide. A significant proportion of these will eventually develop clinical complications, such as cirrhosis, liver decompensation and hepatocellular carcinoma. Sustained virological response (SVR) to antiviral therapy is associated with improvement in liver histology and survival free of liver-related complications. Great effort has been made to improve SVR rate by adapting the duration of therapy according to HCV genotype and to on-treatment response. Rapid virological response (RVR, undetectable HCV RNA at week 4) usually has a high positive predictive value for achieving SVR and early virological response (EVR, ≥ 2 log reduction or undetectable HCV RNA at week 12) exhibits a high negative predictive value for non-response. Individualized approach can improve cost-effectiveness of HCV antiviral therapy by reducing side effects and the costs of therapy associated with unnecessary exposure to treatment and through extending therapy for those with unfavorable features. This article summarizes recent data on strategies of individualized treatment in naïve patients with mono-infection by the different HCV genotypes. The management of common side effects, the impact of HCV infection on health-related quality of life and the potential applications of host genomics in HCV therapy are also briefly discussed.
Introduction
Chronic infection with hepatitis C virus (HCV) is a major public health problem, with perhaps 180 million people infected worldwide.Citation1 Natural history studies indicate that around 20% to 30% of patients with chronic hepatitis C will eventually develop clinical complications, such as cirrhosis, liver decompensation and hepatocellular carcinoma.Citation2,Citation3 The estimated number of deaths associated with HCV-related complications is 8000 to 10,000 in the United States and more than 86,000 in Europe, annually.Citation4,Citation5 In addition, HCV infection is the leading cause of liver transplantation both in the United States and Europe.Citation6,Citation7 A population-based study identified that HCV-related mortality has increased significantly between 1995 and 2004, particularly among persons aged 55 to 64 years, males, African-Americans, and Native Americans.Citation8 It has also been estimated that HCV-related deaths will likely continue to increase over the next two decades.Citation9–Citation12
The ultimate goal of HCV therapy is to prevent liver-related complications and death. Since chronic hepatitis C usually exhibits a very slow progression, these events occur several decades after infection. For that reason, a sustained virological response (SVR), defined as the absence of HCV RNA from serum by a sensitive PCR assay 24 weeks following discontinuation of therapy has been considered a “virological cure” and a surrogate marker of benign long-term clinical outcome.Citation13 In fact, eradication of HCV is associated with improvement in liver histology and survival free of liver-related complications.Citation14–Citation16
This article summarizes recent data on strategies of individualized treatment in naïve mono-infected patients infected by different HCV genotypes. The management of common side effects, the impact of HCV infection on health-related quality of life and the potential applications of genomics in HCV therapy are also briefly discussed.
Individualized therapy – historical perspective
The effectiveness of HCV treatment has steadily increased since the first trials of monotherapy with standard interferon alpha (IFNα) in 1986 to the large registration trials of pegylated IFNα (PEG-IFNα).Citation17–Citation23 In the first period, 10% to 20% of patients would achieve a sustained virological response (SVR) to monotherapy with IFNα.Citation17,Citation18 In a second period, the addition of ribavirin to IFNα increased the SVR rate to 40%.Citation20,Citation21 Finally, SVR was obtained in 55% of cases after administration of PEG-IFNα in combination with ribavirin.Citation22,Citation23
Comparison of nucleotide sequences of HCV variants in patients of different risk groups and geographic areas has revealed at least 6 distinct genotypes (genotypes 1, 2, 3, 4, 5, 6). These variants/genotypes differ in 30% to 35% of nucleotide sites.Citation24,Citation25 The greatest variability is seen within genes encoding for envelope proteins E1 and E2; core genes and genes encoding for nonstructural proteins are preserved. Within each genotype, subtypes have been identified that differ in nucleotide sites by 15% to 25%.Citation26
The major features of HCV infection are shared by all genotypes, though steatosis develops more frequently in patients with genotype 3 than in patients with other genotypes.Citation27 The main biologic difference among HCV genotypes is the striking variation in response to IFNα. Only 10% to 20% of patients with genotype 1 achieve SVR with IFNα monotherapy, whereas the rate is 50% in patients with genotype 2 or 3. With the pegylation of IFNα and the addition of ribavirin, 40% to 50% patients with genotype 1 achieve SVR compared with 80% of those with genotypes 2 or 3.Citation22,Citation23,Citation28 A basic understanding of the mechanisms behind these differences remains unknown, although a study by Neumann and colleagues suggests that IFNα more effectively blocks viral replication, and increase free virion clearance and death rate of cells infected with HCV genotype 2 compared to genotype 1.Citation29
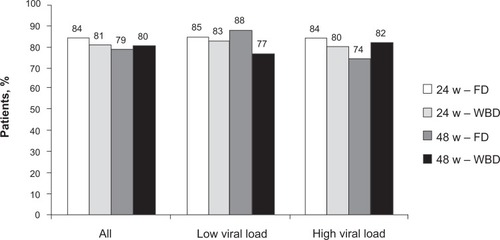
The high response rate observed in genotype 2 and 3 patients suggested that these would need shorter and less intense therapy than patients with other genotypesCitation30,Citation31 and this was well demonstrated in a registration trial authored by Hadziyannis et al.Citation28 In this study, patients were treated with PEG-IFNα2a and ribavirin and randomized to 4 treatment arms: A, 24 weeks of treatment with fixed dose of ribavirin (800 mg/day); B, 24 weeks of treatment with weight-based dose of ribavirin (1000 to 1200 mg/day); C, 48 weeks of treatment with fixed dose of ribavirin; and D, 48 weeks of treatment with weight-based dose of ribavirin. Patients with genotype 1 had the highest SVR (52%) if treated with high weight-based ribavirin dose for 48 weeks and the lowest (29%) if treated with low fixed dose of ribavirin for 24 weeks. Stratifying patients according to baseline viral load confirmed a higher SVR rate in those with low viral load than high (). However, a benefit of prolonged therapy with high dose of ribavirin was observed in both groups. In this study, patients with genotype 2 and 3 were grouped together. In these patients, it was found that there was no benefit from either prolonging therapy beyond 24 weeks or by increasing the dose of ribavirin to more than 800 mg ().
Figure 1 SVR in patients with genotype 1 and high (>800,000 IU/mL) and low (≤800,000 IU/mL) viral load. Patients were randomly treated for 24 and 48 weeks with pegylated interferon α2a (180 μg/week) and a low fixed dose (FD, 800 mg/day) or a higher weight-based dose (WBD, 1000 to 1200 mg/day) of ribavirin.Citation28
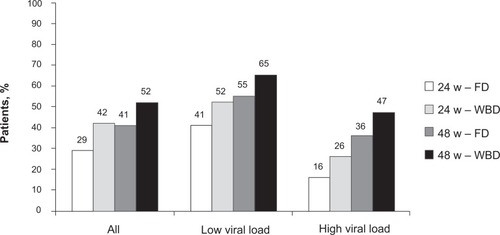
Figure 2 SVR in patients with genotype 2/3 and high (>800,000 IU/mL) and low (≤800,000 IU/mL) viral load. Patients were randomly treated for 24 and 48 weeks with pegylated interferon α2a (180 μg/week) and a low fixed dose (FD, 800 mg/day) or a higher weight-based dose (WBD, 1000 to 1200 mg/day) of ribavirin.Citation28
Mainly based on the Hadziyannis trial,Citation28 most experts have recommended standard HCV treatment for genotype 1 infection as a 48-week therapy with PEG-IFNα (2a or 2b) associated with weight-based dose of ribavirin. For genotype 2/3 patients, it is proposed a 24-week treatment with PEG-IFNα (2a or 2b) combined with a fixed dose of 800 mg of ribavirin.Citation32 Later research has attempted to further tailor therapy on the presumption that maybe some patients could achieve non-inferior SVR rates with therapy shorter than 24 weeks and some may need treatment longer than 48 weeks. This individualized approach can improve cost-effectiveness of HCV antiviral therapy by reducing side effects and costs of therapy associated with unnecessary exposure to treatment and through extending therapy for those with slow responses.Citation33
Virological responses
The probability of achieving SVR can be accurately estimated by assessing on-therapy response. In fact, the evaluation of viral load decay during therapy has been considered the main parameter to determine optimal duration of HCV treatment, a strategy known as “response-guided therapy”. Hence, several types of virological responses have been defined based on the kinetics of serum HCV RNA decline:
Rapid virological response (RVR): undetectable HCV RNA at week 4 of therapy, using a test with a lower limit of detection of 50 IU/mL.
Early virological response (EVR): defined as a ≥2 log reduction (compared to the baseline level) or undetectable HCV RNA in serum at week 12 of treatment. EVR can be further divided into partial EVR and complete EVR, according to HCV RNA status (still detectable in partial EVR and undetectable in patients with complete EVR).
RVR is observed in approximately 12% to 47% of Western genotype 1 patients,Citation34–Citation41 43% to 68% in Asian genotype 1 subjects,Citation42,Citation43 and in 61% to 78% of those with genotype 2 or 3, irrespective of origin.Citation44–Citation50 Patients with RVR have a high probability of achieving SVR, with positive predictive values ranging from 71% to 96% in different studies.Citation34–Citation52 Likewise, EVR is more frequently seen in genotype 2 or 3 patients than in those infected with genotype 1 (94%–97% vs 73%–81%, respectively).Citation38,Citation51,Citation53 The absence of EVR is the most accurate predictor of failure to achieve SVR, with negative predictive value of 93% to 100%.Citation23,Citation38,Citation51–Citation54
Treatment of HCV infection with genotype 1
Standard therapy
The combination of PEG-IFNα and ribavirin is the current standard regimen for the treatment of chronic hepatitis C.Citation32 There are two licensed pegylated interferons, PEG-IFNα2b (Peg-Intron®; Schering Plough Corp) with a 12-kDa linear polyethylene glycol (PEG) molecule, and PEG-IFNα2a (Pegasys®; Hoffmann-La Roche) with a 40-kDa branched PEG, both given subcutaneously. The newly published IDEAL study suggested that there is no significant difference in SVR rates between the two PEG-IFNs.Citation59 In addition, this study showed that the standard dose of PEG-IFNα2b in the treatment of genotype 1 infection should be 1.0 μg/kg/week and not 1.5 μg/kg/week. Daily dose of oral ribavirin should be weight-based (800 mg if weight ≤65 kg; 1000 mg if weight is between 65 and 85 kg; 1200 mg for patients with 85 to 105 kg; and 1400 mg for those with >105 kg).Citation22,Citation56 PEG-IFNα2a is administered at a fixed dose of 180 μg/week. With this type of PEG-IFNα, patients with weight ≤75 kg and those with >75 kg should receive 1000 mg/day and 1200 mg/day of ribavirin, respectively.Citation23 Standard duration of therapy for genotype 1 patients is 48 weeks, irrespective of the PEG-IFNα molecule.
Stopping rule
Retrospective analysis of the two pivotal studies with pegylated interferon suggested that patients unable to achieve a decrease of serum HCV RNA ≥ 2 log at week (EVR) were very unlikely to benefit from prolonged treatment.Citation23,Citation54 Various studies have further confirmed that only ∼3% of subjects without EVR eventually achieve SVR.Citation38,Citation51–Citation53 Hence, it is recommended that antiviral therapy should be stopped in these patients.Citation32,Citation57–Citation60
Short treatment to rapid responders ()
Several studies have investigated the response to 24 weeks of treatment in selected genotype 1 patients (). A post hoc analysis of the study by Hadziyannis et alCitation28 sought to identify genotype 1 patients that did not profit from increasing PEG-IFNα2a treatment from 24 to 48 weeks.Citation35 It was observed that 20% (146/729) had a RVR and that these patients had a SVR rate of 88% after only 24 weeks of treatment compared to 83% after 48 weeks. The limited number of patients did not permit an adequate analysis of the effect of ribavirin dose. Even though these results are encouraging, definite conclusions cannot be drawn from this study alone.
Table 1 Summary of the main studies evaluating individualized therapy in patients with genotype 1
Ferenci et al found RVR in 150/516 (29%) in a prospective study including predominantly genotype 1 (87%) treated with PEG-IFNα2a and ribavirin.Citation39 In patients with RVR, treatment was stopped after 24 weeks. The SVR rate was 80% and the virological relapse rate was 7%. No control group was included in this study.
Mangia et al randomized genotype 1 patients into 2 main groups: a control group treated with a 48-week standard therapy with PEG-IFNα2a or PEG-IFNα2b (n = 237) and an individualized duration group (n = 459), in which different durations were used according to the time when HCV RNA first became undetectable.Citation40 In patients with RVR (185/696, 27%), SVR was observed in 77% and 87% of subjects (P = 0.12) and the relapse rates were 19% and 10% for 24 and 48 weeks, respectively (P = 0.13). In the subgroup of RVR patients with baseline viremia <400.000 IU/mL, SVR rate was 83% in the 48-week group and 84% in those treated by 24 weeks (P = 0.83).
Two studies from Taiwan with genotype 1 subjects confirmed that a 24-week therapy is associated with higher relapse and lower SVR rates.Citation42,Citation43 Both studies also suggested that RVR patients with low baseline viremia present comparable SVR rates when treated for 24 or 48 weeks.
Altogether, based on the available data, one cannot as yet recommend 24 weeks therapy to all genotype 1 patients with RVR. However, based on case-by-case analysis, a 24-week treatment could be considered in genotype 1 subjects with RVR that experience significant side effects, particularly in those with low viremia at baseline. It remains to be defined which cutoff is the most accurate value to define low viremia, 400,000, 600,000 or 800,000 IU/mL.
Long treatment to slow responders ()
Only 50% of genotype 1 patients achieve SVR. A subgroup of patients seems to respond slowly to treatment. The question has been raised whether these slow responders would have a better chance of achieving SVR if treatment was prolonged beyond 48 weeks to 72 weeks (). Pearlman et al defined slow response as having a viral load drop of 2 log or more within 12 weeks, being HCV RNA positive at week 12, and being HCV RNA negative at week 24.Citation61 In this single center US trial with PEG-IFNα2b and weight-based ribavirin, a slow response was observed in 112/361 patients (32%) and 101 subjects were randomized to 48 or 72 weeks of therapy. SVR rates were 18% and 38% after 48 and 72 weeks of treatment, respectively (P = 0.03) and the relapse rates were 59% and 20%, respectively (P = 0.004). Although these results favored extended treatment, it should be noted that this trial included a high number of African Americans (48%), with a well described poor response to interferon. Thus, these results should be interpreted with caution.
In the study by Mangia et al, both PEG-IFNα2a and 2b were used together with weight-based ribavirin. Slow response was defined as being HCV RNA positive at week 8 but negative week 12.Citation40 Using this definition, slow response was observed in 73/696 patients (10%). Patients with slow response were treated for 48 (n = 21) or 72 (n = 52) weeks. A SVR was achieved in 38% after 48 weeks and 63% after 72 weeks (P = 0.068). A virological relapse was seen in 43% and 15% after 48 and 72 weeks treatment, respectively (P = 0.057).
Recently, data from the SUCCESS trial, a large, multicenter, European study with genotype 1 slow responders treated with PEG-IFNα2b and weight-based ribavirin has been published in abstract form.Citation62 In this randomized controlled trial, like in Pearlman’s study,Citation61 patients with detectable HCV RNA and a viral load decline ≥2 log at week 12 but with undetectable HCV RNA at week 24 were considered as slow responders. Undetectable HCV RNA at week 12 was observed in 816 subjects (57%), who were treated with a standard duration therapy of 48 weeks. At week 24, 159/1419 (11%) slow responders were randomized to 48- or 72-week therapy. No difference was seen in SVR rates between groups (43% vs 48%, after 48 and 72 weeks, respectively; P = NS).
Two other trials have assessed the impact of prolonged treatment on SVR in slow responders.Citation34,Citation36 Both trials used PEG-IFNα2a and 800 mg/day of ribavirin as a fixed dose and found a benefit with a 72-week treatment vs 48 weeks. However, the use of a non-standard dose of ribavirin makes the interpretation of these studies difficult.
In the study by Berg et al,Citation34 dropout rates were significantly higher in the extended treatment arm, as compared to the standard duration one (41% vs 24%, respectively). Likewise, in the SUCCESS trial,Citation62 dropouts were also more frequent in the 72-week arm, but in this trial even among those treated per protocol, no significant gain was achieved by prolonging treatment beyond 48 weeks.
The discrepant results between the above-mentioned trials have at least two possible explanations: the use of fixed vs weight-based ribavirin and the use of different PEG-IFNs. As the IDEAL trial failed to show significant difference between PEG-IFNs regarding SVR rates,Citation55 we believe that the dosing of ribavirin has had the strongest impact on treatment outcome in these trials. Hence, optimizing ribavirin dose may be more important than prolonging treatment. When optimal dosing proves impossible to maintain, prolonged therapy may be considered.
In summary, although a 72-week prolonged treatment may result in improved SVR rates in a subset of slow responders, available data are insufficient to clearly identify which patients would benefit from extended antiviral therapy. Hence, a recommendation to slow responders regarding prolonged treatment beyond 48 weeks cannot be made at present.
Treatment of HCV infection with genotypes 2 and 3
Standard therapy
Combination therapy with PEG-IFNα 2a or 2b plus ribavirin represents also the standard of care for patients infected with genotypes 2 or 3.Citation28,Citation63 Recommended doses for PEG-IFNα 2b is 1.5 mμg/kg/week and 180 mμg/week for PEG-IFNα2a. Patients with genotypes 2 and 3 can be treated with low dose ribavirin (800 mg) and for only 24 weeks, without a negative impact on SVR rates.Citation28
Stopping rule
The 12-week stopping rule is not clinically useful in genotype 2/3 subjects, since more than 95% of these patients clear HCV by the week 12. A modified stopping rule based on failure to achieve RVR does not seem to be helpful either, given its low negative predictive value for SVR in those infected by genotype 2 and 3, when treated for 24 weeks (∼40%).Citation38,Citation49
Short treatment to rapid responders ()
The study by Hadziyannis et al showed a high and equal response to 24 and 48 weeks combination treatment with PEG-IFNα2a in patients with genotype 2 or 3, regardless of ribavirin dosage (SVR rates ranging from 79% to 84%).Citation28 The next question is whether these individuals (or a subgroup of them) would do as well with even shorter treatment. This was assessed in an uncontrolled pilot trial by Dalgard et al.Citation44 In this study, RVR was obtained by 95/122 (78%) subjects with genotype 2 or 3. Patients with RVR received only 14 weeks of treatment while the remaining received the standard 24-week treatment. PEG-IFNα2b was used and ribavirin was dosed high and according to weight (800 to 1400 mg/day). In the group who received 14-week treatment, 90% achieved SVR and among those without RVR the SVR rate was 56%. These good results were confirmed by Mangia et al, who randomized 283 patients 3:1 to either standard 24-week therapy (1.0 μg/kg/week of PEG-IFNα2b plus ribavirin) or to a variable schedule with 12 weeks to those with RVR and 24 weeks to the remaining.Citation45 Among subjects with RVR, SVR was achieved in 85% and 91% after 12 and 24 weeks of therapy, respectively (difference of −6%; 95% CI, −16 to +4).
Table 2 Summary of the main studies evaluating individualized therapy in patients with genotype 2 and 3
In a third trial, Dalgard et al randomized patients with RVR to 14 and 24 weeks of combination treatment with 1.5 μg/kg/week of PEG-IFNα2b plus ribavirin.Citation50 In this study, RVR was achieved in 299/432 (71%). In a modified intention to treat analysis, the SVR rates were 86% and 93% after 14 and 24 weeks, respectively (difference of −6.9%; 95% CI, −0.1 to +13.9). Considering only those patients who were treated per protocol, SVR rates were 90% in the 14-week group and 94% in the 24-week group. Overall, it was found that patients with genotype 2 responded better than those with genotype 3 and that those with low viral load responded better than those with high viremia (>400.000 IU/mL). However, prolongation of treatment was not shown to be more beneficial to “difficult-to-treat” patients (genotype 3 with high viremia), as compared to those “easy-to-treat” subjects (genotype 2 with low viremia) ().
Table 3 Impact of HCV baseline viral load and genotype on sustained virological response therapy in patients with genotype 2 and 3
A post hoc analysis of a large genotype 2/3 registration trial largely confirmed the above findings.Citation47 In this trial, called ACCELERATE, 1465 patients were randomized to 16- or 24-week treatment with PEG-IFNα2a and fixed dose of 800 mg of ribavirin, among whom 65% had RVR. In patients with RVR treated for 16 weeks, the SVR rate was 79% and in those treated for 24 weeks it was 85% (P = 0.02).
In view of these trials, it seems that the antiviral response to short treatment is very good, but not as good as after a 24-week course. The difference in response seems to be around 5% in favor of longer treatment. Therefore, the number needed to treat for 24 weeks, instead of 12 to 14 weeks, to achieve one SVR would be of approximately 20. What the most cost-effective approach would be remains to be determined.
Approximately 10% to 15% relapse after short treatment. Mangia et al studied the effect of retreating these patients for 24 weeks and found that 30/43 (70%) of such patients achieved SVR after a second therapy.Citation40 Dalgard et al found that treating all RVR patients with short therapy and retreating the relapsers would be more cost-effective than treating all for 24 weeks.Citation50
Thus, European and Asian studies suggest that patients with genotype 2 or 3 infection and RVR may be treated for 12 weeks and retreated in case of relapse. However, it should be emphasized to the patient that this approach carries a 10% to 15% risk of a second therapy of 24 weeks being necessary. Patients without RVR should be treated for 24 weeks. If short therapy is chosen, ribavirin should be dosed according to weight. There are several subpopulations in which short therapy approach has not yet been well evaluated, like HIV-HCV co-infected patients, cirrhotic individuals, liver transplanted subjects and those of African origin. Until new data become available, these patients should receive standard therapy.
Long treatment to slow responders
Up to now, there are no published trials addressing the efficacy of longer treatment in genotype 2 or 3 patients exhibiting slow virological response. However, a retrospective pooled analysis of Fried’s and Hadziyannis’ trials suggested that a combination of higher doses of ribavirin (1000 to 1200 mg/day) and a longer duration of therapy (48 weeks) improved SVR in genotype 2 and 3 subjects without RVR.Citation64 This population will be included in the ongoing NCORE 2/3 trial, a randomized, open-label study comparing 24 vs 48 weeks of combination therapy with PEG-IFNα 2a plus weight-based ribavirin.Citation65
Treatment of HCV infection with other genotypes
Genotype 4
The efficacy of combination therapy with PEG-IFNα plus ribavirin in genotype 4 subjects was confirmed in four prospective studies, three of them with a randomized controlled design.Citation39,Citation66–Citation68 Originally, based on preliminary studies with SVR rates similar to those achieved in genotype 1 infection, the recommended duration of therapy for these patients was 48 weeks. In fact, Kamal et al observed a lower SVR rate after 24-week combination treatment with PEG-IFNα2b plus ribavirin as compared to 36- and 48-week regimens (29% vs 66% and 69%, respectively; P = 0.001).Citation66 Although global SVR rates were not different between 36- and 48-week groups (66% vs 69%, P = 0.3), 65% of subjects with high viral load at baseline (>2 million copies/mL) achieved higher SVR rate when treated for 48 weeks, as compared to 35% in the 24-week group (P = 0.04). In this study, irrespective of treatment duration, no patient without EVR achieved SVR, suggesting that genotype 4 patients without EVR would not benefit from continuing therapy. In a second trial with 378 patients, the feasibility of response-guided treatment in this population has been confirmed.Citation68 In this trial, 60/378 (16%) of subjects were randomized to a control group treated with standard dose of PEG-IFNα2b plus 10.6 mg/kg/day of ribavirin for 48 weeks. The remaining patients (n = 318, 84%) were treated according to on-therapy response: those with RVR were treated for 24 weeks (n = 69, 22%), subjects with complete EVR (undetectable HCV RNA at week 12) were treated for 36 weeks (n = 79, 25%), and those with partial EVR (≥2 log drop and detectable HCV RNA after 12 weeks) received therapy for 48 weeks (n = 160, 50%). The overall SVR rate of the variable-duration groups was comparable to 48-week fixed duration group (68% vs 58%), with better compliance and fewer side effects as compared to controls. Within each variable-duration group, SVR rates were 86%, 76% and 56% in patients with RVR, complete EVR and partial EVR, respectively. Nevertheless, as opposed to the previous trial, EVR showed a low negative predictive value to predict failure to achieve SVR, in both variable-duration and control groups (40% and 54%, respectively). Finally, Ferenci et al observed SVR in 26/30 genotype 4 patients with RVR (87%) after 24 weeks of treatment with PEG-IFNα 2a and ribavirin.Citation39
To sum up, genotype 4 patients should receive combination therapy of PEG-IFNα (standard dosage) and high, weight-based dose of ribavirin (1000 to 1200 mg/day) for 48 weeks. We still lack sufficient data to recommend individualized therapy to this group. In the future we hope to see further investigations elucidating this promising approach.
Genotype 5
Most cases of HCV genotype 5 infections occur in South Africa, where it has been reported as the most prevalent genotype.Citation69,Citation70 Since there are insufficient data on optimal schedule, these subjects should receive the same standard regimen as proposed for those with genotype 1.
Genotype 6
In some countries of Southeast Asia (Vietnam, Thailand), the reported prevalence of HCV infection is as high as 6%, and genotype 6 accounts for approximately 30% of the cases.Citation71 Two studies have assessed virological response to PEG-IFNα combined with ribavirin in HCV genotype 6 infection.Citation72,Citation73 These studies have shown SVR rates of 75% and 86% but they included only 56 patients in total. Compared to a standard 48-week schedule, therapy for 24 weeks was associated with lower SVR rate (39% vs 75%, P = 0.044).Citation72 Hence, it is currently recommended that subjects with HCV genotype 6 infection should be treated like genotype 1 and 5 patients.
Side effects of antiviral therapy
Treatment with PEG-IFNα and ribavirin is hampered by frequent and sometimes serious side effects. Among the latter are autoimmune diseases, significant hemolytic anemia and severe depression. Except from hemolytic anemia, side effects are believed to be mostly IFN-related. It should however be mentioned that ribavirin can carry an increased risk of birth defects. Therefore, proper contraception during and within 6 months after therapy must be used. Other minor side effects like cough and skin rash also seem to be mainly associated with ribavirin.
A few hours after the first injection with PEG-IFNα, the majority of patients will experience a flu-like syndrome with myalgia, headache, fatigue and fever. Generally, these symptoms are managed by common analgesics and by improving oral hydration. After 4 to 6 weeks, the flu-like syndrome gradually resolves.
Depression may be induced by IFNα, with an incidence of 20% to 30%.Citation74 Most commonly, it develops after three months of treatment. Mild and moderate depression can be handled with conservative measures by nonpsychiatrist professionals. However, the development of moderate depression may require reduction of PEG-IFNα dosage with or without prescription of an antidepressant (usually, a selective inhibitor of serotonin uptake). If severe depression is diagnosed, HCV treatment should be stopped and the patients should be immediately referred to a psychiatrist.
Several autoimmune diseases have been reported to occur during HCV treatment, including systemic lupus erythematosus, psoriasis, type I diabetes mellitus, Graves’ disease and hypothyroidism.Citation75–Citation77
IFNα exerts a myelosuppressive effect, sometimes leading to significant thrombocytopenia and leucopenia.Citation22,Citation23 This toxic effect of IFNα increases the problem of ribavirin-induced hemolytic anemia, which develops to some degree in most patients. Thrombocytopenia during IFNα treatment is rarely associated with clinical significant complications and it seems safe to continue with full dose as long as platelet count is superior to 30 × 109/mL and no bleeding episodes has been recorded. In selected cirrhotic subjects with low platelets at baseline, eltrombopag therapy may help to raise counts in order to allow the initiation of HCV therapy.Citation78 However, this is an expensive therapy and it its effect on the SVR rate is unknown.
As with thrombocytopenia, IFNα-related leucopenia is usually benign and it is safe to maintain the dose of PEG-IFNα if neutrophils count is above 0.5 × 109/mL without signs of infections.
If hemoglobin falls to less than 10 g/dL or a very rapid decline in hemoglobin level is seen, it is advisable to reduce the dose of ribavirin. Nonetheless, in selected subpopulations (HIV co-infection, liver transplanted patients and end-stage renal disease subjects), the use of erythropoietin may be justified. However, the positive impact of this approach on SVR rate remains to be documented. Interestingly, a post-hoc analysis of the IDEAL study confirmed previous findings suggesting that those who experience anemia have a greater chance of achieving SVR than those who do not.Citation55 This suggests that anemia may be a biological marker of adequate ribavirin exposure.
Impact of HCV infection on quality of life
Health-related quality of life (HRQOL) is reduced among HCV patients.Citation79 Whether this is due to the chronic viral infection, due to the psychological effect of carrying a potentially deadly virus or it is a reflection of the special population (ie, drug users) that most commonly carries the virus is debatable.Citation80 Successful virus eradication improves HRQOL either through a beneficial psychological or a biological effect.Citation79
Genomics in chronic hepatitis C
A poorer response to HCV therapy has been noted in those of African and Latino origin as compared to Caucasians.Citation55,Citation81,Citation82 It has also been suggested that East Asians have higher SVR rates than patients of European ancestry.Citation43,Citation83 Recent data derived from the IDEAL trial found a strong association between a polymorphism near the IL28B gene (encoding interferon-λ-3) and SVR, both among patients of European ancestry and African-Americans.Citation84 This finding clearly suggests that host genomics plays a major role in antiviral response and provides a new approach for individualization of therapy in HCV chronic infection.
There are more than 32,000 protein-encoding genes, but the exact number of functional transcripts that are expressed in the liver is unknown.Citation85,Citation86 High-throughput genomics methods like microarray (MA) technology and large-scale real-time reverse transcription polymerase chain reaction (RT-PCR) has been used to identify genes associated with liver fibrogenesis and host immune response, shedding light into the intrinsic mechanisms involved in major issues of HCV chronic infection, like viral persistence after acute infection,Citation87,Citation88 liver fibrosis progression,Citation89–Citation92 and response to antiviral therapy.Citation93–Citation103 While MA analysis allows a broader search of gene expression signatures among several hundreds to thousands of mRNA transcripts in a single experiment, RT-PCR represents a targeted approach, in which it is possible to quantify the expression of relevant genes, previously selected through a hypothesis-driven research.Citation104,Citation105 Several groups have reported both gene expression profilesCitation93–Citation97 as well as single nucleotide polymorphisms (SNPs)Citation98–Citation103 that may affect response to antiviral therapy in chronic hepatitis C. Although there is some variation among the implicated genes, the vast majority of these are IFN-stimulated genes (ISGs), involved in regulatory IFN pathways. A common finding of these gene expression studies has been a higher baseline expression of ISGs in liver tissue of nonresponders, compared to patients with SVR, suggesting that ISGs are already maximally induced in non-responders before IFNα therapy. Whether (and how) this pretreatment higher expression of ISGs actually prevents an adequate response to exogenous PEG-IFNα remains to be defined. Based on the exciting findings of the above-mentioned studies, it is conceivable that, within the next few years, genomic tools will become available in clinical practice, allowing the individualization of treatment by identifying before treatment those patients with higher probability to respond to antiviral therapy.
Future therapies
New therapies like HCV NS3/4A protease inhibitors and HCV NS5B polymerase inhibitors are being developed and are eagerly anticipated. Initial results have been particularly promising with the protease inhibitors telaprevir and boceprevir, with SVR rates ranging from 61% to 75% in naïve genotype 1 patients.Citation106–Citation108 It is out of the scope of this review to deal with these drugs in depth, but it should be mentioned that it is likely that their use in clinical practice will be individualized as well. For instance, host genomics and/or on treatment viral response may be used to identify those who will only need double therapy with PEG-IFNα and ribavirin in contrast to those who will require triple therapy with PEG-IFNα, ribavirin and a new antiviral drug.
Future research agenda
Several issues on the use of viral kinetics for the optimization of HCV therapy need further clarification, including:
Health-economics of individualized therapy;
Individualized treatment in genotype 4, 5 and 6 patients;
Differences between genotype 2 and 3 patients;
Influence of key baseline factors, like initial HCV viral load, advanced fibrosis, insulin resistance;
Optimal dosing of ribavirin;
HCV kinetics in special populations, including HIV and HBV co-infected subjects, end-stage renal disease patients, and ethnic minorities;
Incorporation of innovative molecular biology tools, such as transcription mediated amplification test (TMA), high-throughput genomic methods, and characterization of the liver and serum proteomes; and
Individualized treatment in triple therapy with new drugs like protease and polymerase inhibitors.
Conclusion
Despite major advances in therapy, only about 50% of patients clear HCV infection with the current available treatment. Hence, the development of new drugs and novel treatment strategies are deemed necessary to improve outcome in chronic hepatitis C. A growing body of evidence suggests that baseline host and viral characteristics, as well as viral kinetics during treatment should be used to individualize HCV therapy. This adapted approach can improve treatment cost-effectiveness by avoiding unnecessary exposure to drugs and by identifying those requiring extended therapy. The use of viral genomics in the management of HCV infection has since long been well established. The last five years have witnessed a significant development in the field of viral kinetics as well and it is expected that the growing knowledge in host genomics will further enhance the efficacy of HCV antiviral therapy.
Disclosures
The authors declare no conflicts of interest.
References
- Hepatitis C fact sheetGenevaWorld Health Organization URL: http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed July 13, 2009.
- BenvegnùLGiosMBoccatoSAlbertiANatural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complicationsGut200453574474915082595
- NiederauCLangeSHeintgesTPrognosis of chronic hepatitis C: results of a large, prospective cohort studyHepatology1998286168716959828236
- Cdc.gov [homepage on the Internet]Centers for Disease Control and Prevention (US). Division of Viral Hepatitis. US disease burden data: 1980–2003 URL: http://www.cdc.gov.myaccess.library.utoronto.ca/ncidod/diseases/hepatitis/resource/PDFs/disease_burden2004.pdf. Accessed July 18, 2008.
- MühlbergerNSchwarzerRLettmeierBSroczynskiGZeuzemSSiebertUHCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortalityBMC Public Health200922934
- MukherjeeSSorrellMFControversies in Liver Transplantation for Hepatitis CGastroenterology200813461777178818471554
- PrietoMBerenguerMRimolaALiver transplantation in hepatitis C. A Spanish multicentre experienceEur J Gastroenterol Hepatol19981097717769831272
- WiseMBialekSFinelliLBellBPSorvilloFChanging trends in hepatitis C-related mortality in the United States, 1995–2004Hepatology20084741128113518318441
- DavisGLAlbrightJECookSFRosenbergDMProjecting future complications of chronic hepatitis C in the United StatesLiver Transpl20039433133812682882
- Deuffic-BurbanSWongJBValleronAJCostagliolaDDelfraissyJFPoynardTComparing the public health burden of chronic hepatitis C and HIV infection in FranceJ Hepatol200440231932614739105
- SypsaVTouloumiGPapatheodoridisGVFuture trends of HCV-related cirrhosis and hepatocellular carcinoma under the currently available treatmentsJ Viral Hepat200512554355016108772
- Deuffic-BurbanSPoynardTSulkowskiMSWongJBEstimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United StatesJ Viral Hepat200714210711517244250
- MaylinSMartinot-PeignouxMMoucariREradication of hepatitis C virus in patients successfully treated for chronic hepatitis CGastroenterology2008135382182918593587
- BrunoSStroffoliniTColomboMfor Italian Association of the Study of the Liver Disease (AISF)Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective studyHepatology200745357958717326216
- VeldtBJHeathcoteEJWedemeyerHSustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosisAnn Intern Med20071471067768418025443
- MalletVGilgenkrantzHSerpaggiJBrief communication: the relationship of regression of cirrhosis to outcome in chronic hepatitis CAnn Intern Med2008149639940318794559
- HoofnagleJHMullenKDJonesDBTreatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary reportN Engl J Med1986315157515783097544
- LinRRoachEZimmermanMStrasserSFarrellGCInterferon alfa-2b for chronic hepatitis C: effects of dose increment and duration of treatment on response rates: results of the first multicentre Australian trialJ Hepatol1995234874968583134
- PoynardTBedossaPChevallierMA comparison of three interferon alfa-2b regimens for the long-term treatment of chronic non-A, non-B hepatitisN Engl J Med1995332145714627739681
- McHutchisonJGGordonSCSchiffERInterferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy GroupN Engl J Med199833921148514929819446
- PoynardTMarcellinPLeeSSRandomized trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT)Lancet19983529138142614329807989
- MannsMPMcHutchisonJGGordonSCPeginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trialLancet2001358928695896511583749
- FriedMWShiffmanMLReddyKRPeginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infectionN Engl J Med20023471397598212324553
- SimmondsPHolmesECChaTAClassification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 regionJ Gen Virol199374Pt 11239123998245854
- SimmondsPGenetic diversity and evolution of hepatitis C virus – 15 years onJ Gen Virol200485Pt 113173318815483230
- ZeinNNClinical significance of hepatitis C virus genotypesClin Microbiol Rev200013222323510755999
- Rubbia-BrandtLQuadriRAbidKHepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3J Hepatol200033110611510905593
- HadziyannisSJSetteHJrMorganTRfor PEGASYS International Study GroupPeginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin doseAnn Intern Med2004140534635514996676
- NeumannAULamNPDahariHDifferences in viral dynamics between genotypes 1 and 2 of hepatitis C virusJ Infect Dis20001821283510882578
- TsubotaAChayamaKIkedaKFactors predictive of response to interferon-alpha therapy in hepatitis C virus infectionHepatology1994195108810948175130
- DiodatiGBonettiPTaggerARelationship between serum HCV markers and response to interferon therapy in chronic hepatitis C. Evaluation of HCV genotypes during and after long-term follow-upDig Dis Sci19943911249725027525169
- GhanyMGStraderDBThomasDLSeeffLBDiagnosis, management, and treatment of hepatitis C: An updateHepatology20094941335137419330875
- SiebertUSroczynskiGAidelsburgerPClinical effectiveness and cost effectiveness of tailoring chronic hepatitis C treatment with peginterferon alpha-2b plus ribavirin to HCV genotype and early viral response: a decision analysis based on German guidelinesPharmacoeconomics200927434135419485429
- BergTvon WagnerMNasserSExtended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirinGastroenterology200613041086109716618403
- JensenDMMorganTRMarcellinPEarly identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapyHepatology200643595496016628671
- Sánchez-TapiasJMDiagoMEscartínPfor TeraViC-4 Study GroupPeginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatmentGastroenterology2006131245146016890599
- ZeuzemSButiMFerenciPEfficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremiaJ Hepatol20064419710316290907
- YuJWWangGQSunLJLiXGLiSCPredictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirinJ Gastroenterol Hepatol200722683283617565637
- FerenciPLaferlHScherzerTMfor Austrian Hepatitis Study GroupPeginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological responseGastroenterology2008135245145818503773
- MangiaAMinervaNBaccaDIndividualized treatment duration for hepatitis C genotype 1 patients: A randomized controlled trialHepatology2008471435018069698
- de Segadas-SoaresJAVillela-NogueiraCAPerezRMNabucoLCBrandão-MelloCECoelhoHSIs the rapid virologic response a positive predictive factor of sustained virologic response in all pretreatment status genotype 1 hepatitis c patients treated with peginterferon-alpha2b and ribavirin?J Clin Gastroenterol200943436236619077732
- YuMLDaiCYHuangJFRapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trialHepatology20084761884189318508296
- LiuCHLiuCJLinCLPegylated interferon-a-2a plus ribavirin for treatment-naive asian patients with hepatitis C virus genotype 1 infection: a multicenter, randomized controlled trialClin Infect Dis200847101260126918834319
- DalgardOBjøroKHellumKBTreatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot studyHepatology20044061260126515558712
- MangiaASantoroRMinervaNPeginterferon alfa-2b and ribavirin for 12 vs 24 weeks in HCV genotype 2 or 3N Engl J Med2005352252609261715972867
- von WagnerMHuberMBergTPeginterferon-alpha-2a (40 KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis CGastroenterology2005129252252716083709
- ShiffmanMLSuterFBaconBRfor ACCELERATE InvestigatorsPeginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3N Engl J Med2007357212413417625124
- YuMLDaiCYHuangJFA randomized study of peginterferon and ribavirin for 16 versus 24 weeks in patients with genotype 2 chronic hepatitis CGut200756455355916956917
- LaggingMLangelandNPedersenCfor NORDynamIC Study GroupRandomized comparison of 12 or 24 weeks of peginterferon alpha-2a and ribavirin in chronic hepatitis C virus genotype 2/3 infectionHepatology20084761837184518454508
- DalgardOBjøroKRing-LarsenHfor North-C GroupPegylated interferon alfa and ribavirin for 14 versus 24 weeks in patients with hepatitis C virus genotype 2 or 3 and rapid virological responseHepatology2008471354317975791
- FerenciPFriedMWShiffmanMLPredicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40 KD)/ribavirinJ Hepatol200543342543315990196
- Martinot-PeignouxMMaylinSMoucariRVirological response at 4 weeks to predict outcome of hepatitis C treatment with pegylated interferon and ribavirinAntivir Ther200914450151119578235
- DiagoMOlveiraASoláRTreatment of chronic hepatitis C genotype 1 with peginterferon-alpha2a (40 kDa) plus ribavirin under routine clinical practice in Spain: early prediction of sustained virological response rateAliment Pharmacol Ther200725889990617402993
- DavisGLWongJBMcHutchisonJGMannsMPHarveyJAlbrechtJEarly virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis CHepatology200338364565212939591
- McHutchisonJGLawitzEJShiffmanMLIDEAL Study TeamPeginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infectionN Engl J Med2009361658069319625712
- JacobsonIMBrownRSJrFreilichBPeginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trialHepatology200746497198117894303
- DhumeauxDMarcellinPLereboursETreatment of hepatitis C. The 2002 French consensusGut200352121784178714633963
- McCaughanGWOmataMAmarapurkarDfor Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working PartyAsian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infectionJ Gastroenterol Hepatol200722561563317444847
- OrlentHVrolijkJMVeldtBJSchalmSWHepatitis C 2002 guidelines: summary and annotationsScand J Gastroenterol2003Suppl 239105110
- ShermanMBainVVilleneuveJPThe management of chronic viral hepatitis: A Canadian consensus conference 2004Can J Infect Dis Med Microbiol200415631332618159509
- PearlmanBLEhlebenCSaifeeSTreatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow respondersHepatology20074661688169418046717
- ButiMLurieYZakharovaNGExtended treatment duration in chronic hepatitis C genotype 1-infected slow responders: final results of the SUCCESS study [abstract]J Hepatol2009501 SupplS58
- ZeuzemSHultcrantzRBourliereMPeginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3J Hepatol200440699399915158341
- WillemsBHadzyiannisSJMorganTRShould treatment with peginterferon plus ribavirin be intensified in patients with HCV genotype 2/3 without a rapid virological response? [abstract]J Hepatol20072 SupplS6
- Hoffmann-La Roche; Hoffmann-La Roche (Clinical Trials, Study Director)A study of combination therapy With PEGASYS (peginterferon alfa-2a (40 kD) and Copegus (ribavirin) in patients with chronic hepatitis C genotype 2 or 3 who do not achieve a rapid viral response In: ClinicalTrialsgov [Internet]Bethesda (MD)National Library of Medicine (US)2000 - [cited 2009 Jul 28]. URL: http://clinicaltrials.gov/show/NCT00623428 NLM Identifier: NCT00623428
- KamalSMEl TawilAANakanoTPeginterferon alpha-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological responseGut200554685886615888797
- ZayadiARAttiaMBarakatEMFResponse of hepatitis C genotype-4 naive patients to 24 weeks of peginterferon-a2b/ribavirin or induction-dose interferon-a2b/ribavirin/amantadine: a non-randomized controlled studyAm J Gastroenterol2005100112447245216279899
- KamalSMEl KamarySSShardellMDPegylated interferon alpha-2b plus ribavirin in patients with genotype 4 chronic hepatitis C: The role of rapid and early virologic responseHepatology20074661732174017943989
- SmutsHEKannemeyerJGenotyping of hepatitis C virus in South AfricaJ Clin Microbiol199533167916817650216
- Prabdial-SingNPurenAJMahlanguJBarrowPBowyerSMHepatitis C virus genotypes in two different patient cohorts in Johannesburg, South AfricaArch Virol2008153112049205818946631
- NguyenMHKeeffeEBChronic hepatitis C: Genotypes 4 to 9Clin Liver Dis200593411426vi16023974
- NguyenMHTrinhHNGarciaRNguyenGLamKDKeeffeEBHigher rate of sustained virologic response in chronic hepatitis C genotype 6 treated with 48 weeks versus 24 weeks of peginterferon plus ribavirinAm J Gastroenterol200810351131113518477343
- FungJLaiCLHungIChronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirinJ Infect Dis2008198680881218657036
- DieperinkEWillenbringMHoSBNeuropsychiatric symptoms associated with hepatitis C and interferon alpha: A reviewAm J Psychiatry2000157686787610831463
- SchreuderTCGelderblomHCWeeginkCJHigh incidence of type 1 diabetes mellitus during or shortly after treatment with pegylated interferon alpha for chronic hepatitis C virus infectionLiver Int2008281394618031478
- DalgardOBjøroKHellumKThyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapyJ Intern Med2002251540040611982739
- SelmiCLleoAZuinMPoddaMRossaroLGershwinMEInterferon alpha and its contribution to autoimmunityCurr Opin Investig Drugs200675451456
- McHutchisonJGDusheikoGShiffmanMLTPL102357 Study GroupEltrombopag for thrombocytopenia in patients with cir rhosis associated with hepatitis CN Engl J Med2007357222227223618046027
- McHutchisonJGWareJEJrBaylissMSfor Hepatitis Interventional Therapy GroupThe effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivityJ Hepatol200134114014711211891
- DalgardOEgelandASkaugKVilimasKSteenTHealth-related quality of life in active injecting drug users with and without chronic hepatitis C virus infectionHepatology2004391748014752825
- MuirAJBornsteinJDKillenbergPGAtlantic Coast Hepatitis Treatment GroupPeginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whitesN Engl J Med2004350222265227115163776
- Rodriguez-TorresMJeffersLJSheikhMYLatino Study GroupPeginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis CN Engl J Med2009360325726719144941
- YanKKGuirgisMDinhTTreatment responses in Asians and Caucasians with chronic hepatitis C infectionWorld J Gastroenterol200814213416342018528940
- GeDFellayJThompsonAJGenetic variation in IL28B predicts hepatitis C treatment-induced viral clearanceNature2009816 [Epub ahead of print]10.1038/nature08309
- YamashitaTHashimotoSKanekoSComprehensive gene expression profile of a normal human liverBiochem Biophys Res Commun2000269111011610694486
- YamashitaTHondaMTakatoriHNishinoRHoshinoNKanekoSGenome-wide transcriptome mapping analysis identifies organ-specific gene expression patterns along human chromosomesGenomics200484586787515475266
- BiggerCBBraskyKMLanfordREDNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infectionJ Virol200175157059706611435586
- SuAIPezackiJPWodickaLGenomic analysis of the host response to hepatitis C virus infectionProc Natl Acad Sci U S A20029924156691567412441396
- AsselahTBiècheILaurendeauILiver gene expression signature of mild fibrosis in patients with chronic hepatitis CGastroenterology200512962064207516344072
- BiècheIAsselahTLaurendeauIMolecular profiling of early stage liver fibrosis in patients with chronic hepatitis C virus infectionVirology2005332113014415661146
- LauDTLuxonBAXiaoSYBeardMRLemonSMIntrahepatic gene expression profiles and alpha-smooth muscle actin patterns in hepatitis C virus induced fibrosisHepatology200542227328115986378
- SmithMWWaltersKAKorthMJGene expression patterns that correlate with hepatitis C and early progression to fibrosis in liver transplant recipientsGastroenterology2006130117918716401481
- JiXCheungRCooperSLiQGreenbergHBHeXSInterferon alfa regulated gene expression in patients initiating interferon treatment for chronic hepatitis CHepatology200337361062112601359
- ChenLBorozanIFeldJHepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infectionGastroenterology200512851437144415887125
- HayashidaKDaibaASakaiAPretreatment prediction of interferon-alfa efficacy in chronic hepatitis C patientsClin Gastroenterol Hepatol20053121253125916361052
- FeldJJNandaSHuangYHepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment responseHepatology20074651548156317929300
- AsselahTBiecheINarguetSLiver gene expression signature to predict response to pegylated interferon plus ribavirin combination therapy in patients with chronic hepatitis CGut200857451652417895355
- HwangYChenEYGuZJGenetic predisposition of responsiveness to therapy for chronic hepatitis CPharmacogenomics20067569770916886895
- LinEHwangYWangSCGuZJChenEYAn artificial neural network approach to the drug efficacy of interferon treatmentsPharmacogenomics2006771017102417054412
- HuangYYangHBorgBBA functional SNP of interferon-gamma gene is important for interferon-alpha-induced and spontaneous recovery from hepatitis C virus infectionProc Natl Acad Sci U S A2007104398599017215375
- MorganTRLambrechtRWBonkovskyHLfor HALT-C Trial GroupDNA polymorphisms and response to treatment in patients with chronic hepatitis C: results from the HALT-C trialJ Hepatol200849454855618619701
- PersicoMCapassoMRussoRElevated expression and polymorphisms of SOCS3 influence patient response to antiviral therapy in chronic hepatitis CGut200857450751517881539
- WelzelTMMorganTRBonkovskyHLfor HALT-C Trial GroupVariants in interferon-alpha pathway genes and response to pegylated interferon-Alpha2a plus ribavirin for treatment of chronic hepatitis C virus infection in the hepatitis C antiviral long-term treatment against cirrhosis trialHepatology20094961847185819434718
- AsselahTBiècheISabbaghAGene expression and hepatitis C virus infectionGut200958684685819074178
- WaltersKAKatzeMGUsing high-throughput genomics to study hepatitis C: what determines the outcome of infection?Antiviral Res200981319820819135090
- McHutchisonJGEversonGTGordonSCPROVE1 Study TeamTelaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infectionN Engl J Med2009360181827183819403902
- HézodeCForestierNDusheikoGPROVE2 Study TeamTelaprevir and peginterferon with or without ribavirin for chronic HCV infectionN Engl J Med2009360181839185019403903
- KwoPLawitzEMcConeJHCV SPRINT-1 final results: SVR 24 from a phase 2 study of boceprevir plus PegIntron™ (Peginterferon alfa-2b)/ribavirin in treatment-naive subjects with genotype-1 chronic hepatitis C [abstract]J Hepatol2009501 SupplS4