Abstract
Aims:
While strong correlations exist between medication adherence and health economic outcomes in type 2 diabetes, current economic analyses do not adequately consider them. We propose a new approach to incorporate adherence in cost-effectiveness analysis.
Methods:
We describe a theoretical approach to incorporating the effect of adherence when estimating the long-term costs and effectiveness of an antidiabetic medication. This approach was applied in a Markov model which includes common diabetic health states. We compared two treatments using hypothetical patient cohorts: injectable insulin (IDM) and oral (OAD) medications. Two analyses were performed, one which ignored adherence (analysis 1) and one which incorporated it (analysis 2). Results from the two analyses were then compared to explore the extent to which adherence may impact incremental cost-effectiveness ratios.
Results:
In both analyses, IDM was more costly and more effective than OAD. When adherence was ignored, IDM generated an incremental cost-effectiveness of $12,097 per quality-adjusted life-year (QALY) gained versus OAD. Incorporation of adherence resulted in a slightly higher ratio ($16,241/QALY). This increase was primarily due to better adherence with OAD than with IDM, and the higher direct medical costs for IDM.
Conclusions:
Incorporating medication adherence into economic analyses can meaningfully influence the estimated cost-effectiveness of type 2 diabetes treatments, and should therefore be considered in health care decision-making. Future work on the impact of adherence on health economic outcomes, and validation of different approaches to modeling adherence, is warranted.
Introduction
Type 2 diabetes (T2D) presents a substantial health economic burden globally, and its prevalence and costs are only forecasted to increase in the coming years.Citation1–Citation3 Medication adherence, especially for patients with chronic diseases such as T2D, has long been viewed as a critical lever for improving outcomes and containing costs. Recent studies conclude that more than $100 billion is spent each year in the US on hospitalizations that could have been avoided with optimal medication adherence.Citation4 Indeed, growing evidence suggests that suboptimal T2D medication adherence eventually results in higher HbA1c levels, complication rates, and costs. Conceptual frameworks have been developed to describe these interrelations and their effect on reimbursement policies.Citation5–Citation8
In order to make appropriate judgments about resource allocation and cost-sharing (namely involving pharmaceuticals), decision-makers have progressively relied on health technology assessment and economic evaluation. The long-term impact of diabetes treatment on clinical outcomes, health events, quality-of-life (QoL), and costs is often mathematically modeled since notable complications may occur years after onset of diabetes.Citation9 However, current models used in T2D economic analysis do not adequately address or explain incorporation of real-world factors which influence medication adherence. These factors are wide-ranging and include patient preferences and behaviors, health care system factors, practice guidelines and patterns, and treatment characteristics.Citation8,Citation10 The exclusion of these factors can lead to inaccurate estimates on the cost-effectiveness of diabetes therapies, and thereby result in misinformed reimbursement decisions affecting patient access to treatment.
Several diabetes medications now in development have been criticized for being too similar to each other and not offering significant clinical benefit over existing therapies.Citation11 However, most of these new therapies and delivery systems differ greatly in other “nonclinical” ways, such as their route of administration, dosing schedule/convenience, and device ergonomics, all of which may influence patient preferences and behaviors (hence, medication adherence).Citation5,Citation6,Citation8 This highlights the increasingly important role that adherence could play in the health technology assessment of new anti-T2D therapies as they reach the market. In particular, this is noteworthy for injectable diabetes medications (IDM) (ie, insulin) versus oral antidiabetic (OAD) medications, since needle phobia, multiple daily injections, and varied dosing schedules (eg, meal timing) are inherently associated with IDM and may adversely affect adherence.Citation5
There is substantial opportunity to improve the methods used to estimate the cost-effectiveness of T2D medications by considering medication adherence.Citation8,Citation9 The objective of this study was to develop and apply an approach to integrate adherence in an economic model of diabetes.Citation8 In doing so, we compared the cost-effectiveness of IDM versus OAD before and after adjustment for adherence, hypothesizing that incorporation of medication adherence would reduce the cost-effectiveness of IDM versus OAD, since patients on IDM generally exhibit poorer adherence than OAD patients.
Research design and methods
We developed a novel approach to modeling medication adherence among T2D patients using conventional health economic techniques and software (Microsoft Excel and TreeAge Pro 2009). To test our approach, 2 separate economic analyses were conducted in a Markov/Monte Carlo format to provide a preliminary estimate of the impact of medication adherence on incremental cost-effectiveness ratios (ICERs):
Analysis 1: Base-case modeling of IDM vs OAD
Analysis 2: Adherence-adjusted modeling
Markov modeling in health care consists of developing a structure of a specified number of health states related to a given disease. Over time, patients will have a certain chance (or transition probability) to move from one health state to another.Citation12 This approach is particularly well suited for modeling chronic diseases, such as T2D, where numerous health states/complications exist and are repeatable, previous history of complications may impact future events, and where a long-term perspective for downstream outcomes is relevant. Monte Carlo simulations commonly serve as the analytic/mathematic engine to a Markov structure, as was done in our analyses. This allows for transition probabilities into and out of health states to be applied, including sensitivity analysis, whereby assumptions on reasonable variation for transition probabilities and the effects of random error may be accounted for.Citation13
Base case model
The model we developed consists of major health states that define the natural history of T2D and are consistent with those seen in previous models (see ).Citation10,Citation14 These states include the various diabetes complications, such as end-stage renal disease (ESRD), lower-extremity amputation, cardiovascular and coronary artery disease (CVD/CAD), nephropathy, stroke, neuropathy, retinopathy, and major hypoglycemia.
Figure 1 Base case model of fundamental T2D health states.
Abbreviations: IDM, injectable diabetes medication; OAD, oral antidiabetic drug; CVD, cardiovascular disease; CAD, coronary artery disease; ESRD, end-stage renal disease.
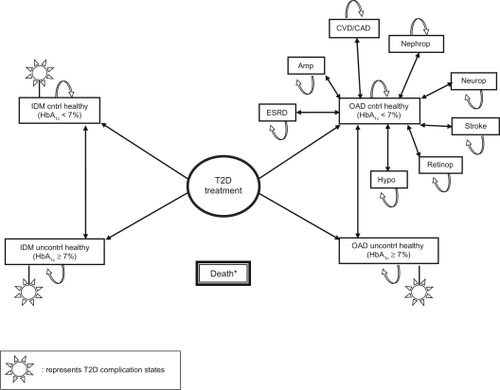
In this model, patients receive either IDM (insulin in our example) or OADs, chosen as mutually exclusive comparators because of the inherent differences between them which readily impact adherence. Patients on both therapies begin at a baseline HbA1c that is either <7% or ≥7%, and through subsequent annual iterations, may (1) remain in a ‘healthy’ state with controlled (<7%) or uncontrolled (≥7%) HbA1c, (2) progress between T2D complication states (with controlled or uncontrolled HbA1c), or (3) die. Probabilities for patients to move from one complication state to another are based on HbA1c over time, since patients with controlled HbA1c are much less likely to develop complications.Citation10,Citation14 Treatment effects (mean of −1.2% HbA1c and −0.8% HbA1c for IDM and OAD, respectively, to reflect greater potency of insulin use) were applied to assumed baseline HbA1c means of 8.1% and 6.8% for patients modeled to HbA1c ≥7% and <7%, respectively.Citation14,Citation15 An annual increase in HbA1c – or “creep up” effect – of + 0.14% per year was applied to initial levels of glycemic control to reflect the natural progression of underlying biological mechanisms affecting glycemic control over time, such as beta-cell loss and reduced insulin sensitivity.Citation10,Citation14 In the base case scenario, approximately 50% fewer IDM patients were, on average, likely to have baseline HbA1c <7% versus OAD patients, as those who are prescribed IDM frequently have further progressed, and/or more difficult to control, T2D. Citation14–Citation16 In both analysis 1 and analysis 2, the Death state may be transitioned to after any/all previous health state(s) and reflects all-cause mortality as well as adjustment for severity of T2D/CVD risk over time.
A lifetime (up to 35-year horizon), US third-party payer perspective was taken starting from the time of T2D treatment initiation.Citation10 Cohort characteristics and transition probabilities were derived from large epidemiologic studies, clinical trials, and recent national surveillance data.Citation10,Citation14,Citation15 Modeled output included life expectancy (LE), quality-adjusted life expectancy (QALE), cumulative incidence of T2D complications, direct medical and pharmacy costs, total lifetime costs, and ICERs.
Simulated cohorts and assumptions
The demographic and clinical characteristics used in the patient cohorts reflected those of typical US T2D populations, including the percentage of patients receiving specific OADs and IDM, the percentage of patients with baseline HbA1c <7% (for OADs 46.6%, for IDM 26.8%), age (mean of 51.1 years), gender (51.6% male), race/ethnicity (62.1% white, 17.4% black, 15.4% Hispanic, 5.1% other), presence of risk factors (eg, smokers 18.8%), and presence of preexisting complications.Citation10,Citation14–Citation16
The magnitude and duration of mean treatment effects remained consistent among IDM and OAD subclasses, and were extracted from large, long-term epidemiologic studies and literature reviews.Citation10,Citation14,Citation15 Applied risk factors included BMI, total cholesterol, systolic blood pressure, triglycerides, and smoking.Citation10
QoL utilities and disutilities for T2D health states were derived from previous studies.Citation10,Citation14 Mean pharmacy costs based on average 30-day wholesale price, and direct medical costs such as hospitalizations, were collected from published data and inflated to 2009 USD.Citation5,Citation6,Citation10,Citation15,Citation17 Of the patients who received IDM, it was assumed that 70% of them were given human insulin and 30% analog insulin. Within the OAD cohort, 70% of the patients were assumed to receive generic metformin ± sulfonylurea, and 30% nongeneric thiazolidinediones. This was done in analyses 1 and 2 to incorporate consistency for drug costs, and to approximate the use of generics in a real-world US scenario.Citation18,Citation19 A 3% discount rate for future costs and health was applied as a base case assumption; however, a range of 0%–6% was allowed during sensitivity analyses to address a range of variation in present value of benefits over time.Citation10
Adherence-adjusted model and theoretical approach
As depicted in , adjustments for adherence involved revisions to the degree of modeled glycemic control, risks of subsequent diabetes complications, and costs using current evidence on the impact of real-world medication adherence. Applied rates of adherence were primarily determined using medication possession ratios (MPR), and were derived from large observational studies.Citation5–Citation7,Citation18–Citation22 Just as the target of HbA1c <7% was applied to model the impact of glycemic control in analysis 1, MPR (≥80%) was added and applied to model the influence of optimal versus suboptimal (MPR < 80%) adherence in analysis 2. New health states were created, where categorical levels of MPR were combined with categorical HbA1c control, thereby enabling MPR to directly influence the likelihood of a patient moving to, and remaining in, either of the HbA1c categories (HbA1c < 7% or HbA1c ≥ 7%), and hence experience downstream T2D complications and costs. For example, recent data suggest that OAD and IDM patients with MPR ≥ 80% are more likely to achieve HbA1c < 7%, have reduced incidence of hypoglycemia and related resource use, and incur lower health care costs.Citation5,Citation7,Citation18–Citation22
Figure 2 Adherence-adjusted approach at modeling T2D.
Abbreviations: IDM, injectable diabetes medication; OAD, oral antidiabetic drug; CVD, cardiovascular disease; CAD, coronary artery disease; ESRD, end-stage renal disease; MPR, medication possession ratios.
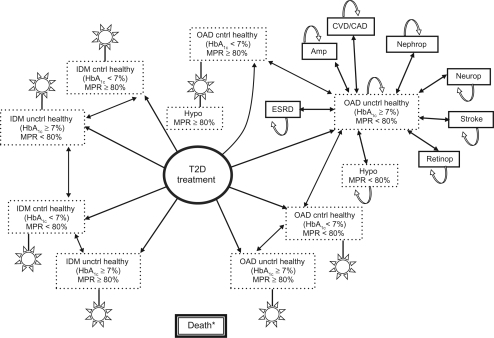
Specifically, revised transition probabilities in the adherence-adjusted model include means and distributions regarding adherence to anti-T2D treatment, likelihood of achieving HbA1c < 7%, and likelihood of major hypoglycemia. Up to 13% and 6% of patients on OADs and IDM, respectively, were allowed to exhibit MPR ≥ 80% (ranges: 36%–93% OADs, 54%–86% IDM). The chance of achieving target HbA1c was approximately 28% greater for patients with MPR ≥ 80%, and the chance of major hypoglycemia was 64% lower for patients with MPR ≥ 80% (74% chance overall, but only 47% chance for optimal adherence; OR: 0.36, P < 0.01).Citation5,Citation7,Citation18–Citation22 Medication adherence in both groups was modeled to worsen over time, at −0.3% per year.Citation6,Citation14
Statistical and sensitivity analyses
Probabilistic sensitivity analyses, wherein a range of distributions (uncertainty) for values of all input parameters are applied simultaneously, were conducted using cohort-level Monte Carlo simulation and bootstrapping. The progression of T2D was simulated in a 1000 × 1000 manner (each cohort of 1000 patients was simulated 1000 times), producing individual candidate bootstrap samples. The average costs and health for each cohort were first calculated, followed by an analysis of the 1000 average values (one per cohort). This resulted in means and standard deviations of the incremental costs, complication incidences, LE, and QALE for both IDM and OAD. Lastly, these means and standard deviations were calculated and compared for IDM versus OAD.
Results
Base case scenario: analysis 1
IDM use resulted in a longer LE (+0.56 years) and QALE (+0.92 years) than OAD use (). IDM also reduced the rates of major hypoglycemia, ESRD, CVD/CAD, nephropathy, and neuropathy.
Table 1 Summary of modeled results: IDM vs OADsTable Footnote*
Although IDM resulted in greater clinical effectiveness, IDM patients also incurred higher costs (by $11,166) than OAD patients, with pharmacy costs accounting for the majority ($8,768) of this difference. The ICER for IDM versus OAD was $12,097 per quality-adjusted life-year (QALY) gained, using total health care costs.
Adherence-adjusted model: analysis 2
After adjustment for adherence, IDM still led to a longer LE and QALE than OADs by +0.331 and +0.675 years, respectively, although the differences observed between IDM and OADs were smaller than without adjustment for adherence. While gaps between IDM and OADs in the incidence rates of some complications shrank, the rate of major hypoglycemia increased.
Total costs remained higher for IDM than for OAD (+$10,963) after incorporating adherence. The ICER of IDM versus OAD increased to $16,241 per QALY gained, again using total costs.
Impact of adherence on modeled output
Adjustment for adherence affected the differences between IDM and OAD treatment in a number of ways, including (1) generating smaller differences in LE and QALE; (2) smaller differences in all complication rates except for major hypoglycemia, which increased by 3.1%; and (3) a 34.3% higher ICER for IDM versus OADs ($16,241/QALY versus $12,097/QALY).
Comparing analysis 2 with analysis 1, LE and QALE for OADs improved 45% and 54% more than for IDM, and the relative difference in rates of ESRD, amputation, and neuropathy between IDM and OADs narrowed by 40%, 25%, and 53%, respectively, and CVD/CAD, nephropathy, and stroke by 2/3 each.
Sensitivity analyses
Probabilistic simulations did not meaningfully alter ICERs for IDM versus OADs in analysis 1 or 2, indicating a sufficient approach at limiting modeled uncertainty. Our base case model generated a range of ICERs from $7,155 to $18,300/QALY, and our revised model generated a range of $11,217 to $23,892/QALY.
Discussion
Utilizing a Markov model of fundamental T2D health states, we specifically aimed to (1) describe a new approach for modeling medication adherence, and (2) illustrate its impact on estimated cost-effectiveness. Our simulations demonstrated that adherence may be meaningfully incorporated into comparative economic analysis through its associations with level of glycemic control, which has an important impact on the risk of downstream diabetes complications (eg, major hypoglycemia) and costs.
In the adherence-adjusted model (analysis 2), we observed an increased ICER, resulting from several sources. Most notably, LE and QALE increased by up to 54% more for OADs than for IDM (although both cohorts saw slightly higher values than in analysis 1). The main reason for this effect was the increased number of patients modeled to HbA1c <7% after stratifying by MPR, and thus experiencing less T2D complications and associated QoL disutilities (ie, the range of applied MPR estimates caused up to 13% and 6% more OAD and IDM patients to have greater likelihood of HbA1c <7% in analysis 2). Secondly, although the differences for most complication rates between IDM and OADs decreased after adjustment for adherence, the difference in major hypoglycemia rates increased by 37%. Thirdly, a greater proportion of IDM lifetime costs were driven by direct medical costs, as opposed to pharmacy costs, after adjustment for adherence. These changes in modeled results likely occurred because more OAD patients were categorized into MPR ≥ 80% than IDM patients, and they therefore experienced less risk of major hypoglycemia (and related QoL disutility) as well as a greater chance of HbA1c <7%, resulting in fewer complications and increased QoL over time. Similarly, a higher frequency of MPR < 80% amongst IDM patients meant a higher risk of hypoglycemia, as well as a higher risk of other complications and higher costs. Furthermore, a balancing effect between greater treatment potency and overall poorer adherence likely occurred for IDM versus OADs. In this way, the greater HbA1c-lowering effect of insulin, which would reduce complication rates and thereby improve health outcomes, may have been partially offset by a higher frequency of MPR < 80% and HbA1c ≥ 7%, and thus a higher starting HbA1c level in the first year of treatment.
It is important to note several limitations to our modeling approach. Although the simulated diabetes population in our analyses was intended to represent a typical US T2D population, our assumptions regarding transition probabilities, actual pharmacy costs, and QoL values may not have fully achieved this. However, it is important to note that the primary goal of this study was to illustrate how adherence may be included in diabetes health economic models, and to then estimate the impact of adherence on ICERs. Our goal was not to evaluate the cost-effectiveness of a specific treatment, nor to focus on a specific population. In keeping the treatments and simulated populations constant between analyses 1 and 2, the isolated effect on cost-effectiveness of modeled adherence was revealed. Although outside the scope of our current analysis, it is important to recognize the increasing evidence that T2D disproportionally affects minority subpopulations in the US. It would therefore be interesting and worthwhile to model the impact of adherence in these subpopulations in a future study. The choice to compare IDM with OADs as hypothetical therapies occurred solely because they clearly differ in treatment modalities which may impact degree of adherence. Although our model could have allowed switches to occur between OADs and IDM throughout a patient’s life, we chose not to allow these switches, since it would have complicated the model and would not have contributed to illustrating the potential effect of incorporating adherence. That is, the incorporation of treatment changes would have generated a “mixed effect” of the impact of medication adherence and the relative effectiveness of different treatment strategies at various points in a patient’s disease progression. Thirdly, transition probabilities in the adherence-adjusted model are based largely on observational studies, which provide statistical associations that do not necessarily reflect cause–effect relationships. This underscores the importance of future work aimed at (1) developing more robust evidence on correlations between adherence and T2D outcomes, (2) identifying novel data sources and analytic techniques for observational, real-world research, and (3) further validation of different modeling approaches.
Modeling techniques worth exploring in future analyses include application of differential- and regression-based equations where adherence is a continuous parameter (variable) that influences glycemic control and complication rates over time, as opposed to a categorical approach, as was done in this study. Additionally, deterministic sensitivity analyses using point estimates could be performed to gain more direct insight into how much influence a specific input variable has, as opposed to allowing multiple parameters to vary simultaneously in a probabilistic sensitivity analysis. Lastly, it is important to note that although our adherence-adjusted approach produced an ICER that was approximately 34% higher than the ICER without adherence adjustment, IDM remained well within conventional thresholds of what is considered “good value for money”, especially when considering preferences and trade-offs in health care from a US societal perspective (up to $297,000/QALY).Citation23
Medication adherence is an important issue in chronic disease and, in particular, diabetes care. However, current economic models of diabetes pay too little attention to adherence, even though it may meaningfully influence the estimated cost-effectiveness of diabetes medicines. As new data and analytic techniques become available, and as new T2D compounds are commercialized, this will become increasingly important to inform health care decision-making for real-world settings.
Disclosure
The authors report no conflicts of interest in this work.
References
- RamachandranARamachandranSSnehalathaCIncreasing expenditure on health care incurred by diabetic subjects in a developing country: a study from IndiaDiabetes Care200730225225617259490
- NarayanKMBoyleJPGeissLSSaaddineJBThompsonTJImpact of recent increase in incidence on future diabetes burden: US, 2005–2050Diabetes Care20062992114211616936162
- BagustAHopkinsonPKMasloveLCurrieCJThe projected health care burden of type 2 diabetes in the UK from 2000 to 2060Diabet Med200219Suppl 41512121330
- CutlerDMEverettWThinking outside the pillbox – medication adherence as a priority for health care reformNew Engl J Med2010326171553155720375400
- CobdenDLeeWCBaluSJoshiAVPashosCLHealth outcomes and economic impact of therapy conversion to a biphasic insulin analog pen among privately insured patients with type 2 diabetes mellitusPharmacotherapy200727794896217594200
- LeeWCBaluSCobdenDJoshiAVPashosCPrevalence and economic consequences of medication adherence in diabetes: a systematic literature reviewManag Care Interface2006197314116898343
- RozenfeldYHuntJSPlauschinatCWongKSOral antidiabetic medication adherence and glycemic control in managed careAm J Manag Care2008142717518269302
- CobdenDNiessenLWRuttenFFHBarrCERedekopWKRelationships among self-management, patient perceptions of care, and health economic outcomes for decision-making and clinical practice in type 2 diabetesValue Health201013113814719695005
- WatkinsJBMinshallMESullivanSDApplication of economic analyses in US managed care formulary decisions: a private payer’s experienceJ Manag Care Pharm200612972673517249905
- ValentineWJPalmerAJNicklassonLCobdenDRozeSImproving life expectancy and decreasing the incidence of complications associated with type 2 diabetes: a modelling study of HbA1c targetsInt J Clin Pract20066091138114516939559
- NathanDMFinding new treatments for diabetes – how many, how fast … how good?New Engl J Med2007356543744017267901
- SonnenbergFABeckJRMarkov models in medical decision making: a practical guideMed Decis Making1993133223388246705
- FoneDHollinghurstSTempleMSystematic review of the use and value of computer simulation modelling in population health and health care deliveryJ Public Health Med200325432533514747592
- United Kingdom Prospective Diabetes Study GroupUnited Kingdom Prospective Diabetes Study 24: a 6-Year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapyAnn Intern Med19981281651759454524
- National Center for Health Statistics, Centers for Disease Control and PreventionNHANES 2007–2008 Dataset and Diabetes Fact Sheet Survey http://www.cdc.gov/nchs/fastats/diabetes.htm. Accessed Sep 22, 2009.
- DoddAHColbyMSBoyeKSFahlmanCKimSBriefelRRTreatment approach and HbA1c control among US adults with type 2 diabetes: NHANES 1999–2004Curr Med Res Opin20092571605161319469695
- 2009 Drug Red BookMontvale, NJMedical Economics Co.2009
- RodinHAHeatonAHWilsonARGarrettNAPlocherDWPlan designs that encourage the use of generic drugs over brand-name drugs: an analysis of a free generic benefitAm J Manag Care2009151288188820001169
- BriesacherBAAndradeSEFouayziHChanKAMedication adherence and use of generic drug therapiesAm J Manag Care200915745045619589012
- EvansJMDonnanPTMorrisADAdherence to oral hypoglycaemic agents prior to insulin therapy in type 2 diabetesDiabet Med200219868568812147151
- LawrenceDBRagucciKRLongLBParrisBSHelferLARelationship of oral antihyperglycemic (sulfonylurea or metformin) medication adherence and hemoglobin A1c goal attainment for HMO patients enrolled in a diabetes disease management programJ Manag Care Pharm200612646647116925454
- LunaBFeinglosMOral agents in the management of type 2 diabetes mellitusAm Fam Physician2001631747175611352285
- BraithwaiteRSMeltzerDOKingJTJrLeslieDRobertsMSWhat does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule?Med Care200846434935618362813