Abstract
Objective:
Inadequate asthma control may affect asthma resource use and treatment charges, consequently contributing to the growing economic burden of asthma. The study objective was to determine the impact of medication adherence and asthma control on resource use and charges in mild asthmatic patients treated with inhaled corticosteroids (ICSs).
Research design and methods:
A claims database was analyzed retrospectively from October 2001–December 2007 to identify mild asthmatic patients aged 12–65 years who began ICS treatment. Demographics, drug utilization, and resource use for each patient were identified for the 365-day period before and after the index date (pre-index and post-index periods, respectively). Patients were designated as having high control high adherence (HCHA) or low control low adherence (LCLA) based on post-index exacerbations and the percentage of days covered; not all patients who qualified for study inclusion met adherence designation requirements. Differences between the HCHA and LCLA cohorts in resource use (eg, asthma treatment days) and asthma-related treatment charges were assessed.
Results:
Compared with the HCHA cohort (n = 483), the LCLA cohort (n = 258) had more asthma treatment days (2.9 vs 3.9, respectively; P < 0.0001) and higher overall asthma treatment charges ($2655 vs $3345, respectively; P < 0.0001) in the post-index period. An adjusted odds ratio suggested that patients receiving mometasone furoate (MF) were approximately 5 times more likely to belong to the HCHA cohort than patients receiving any other ICS (P < 0.0001).
Conclusions:
Better asthma control and adherence to prescribed ICSs are associated with lower asthma-related resource use and charges. Mild asthmatic patients receiving MF were more likely to be in the HCHA cohort than patients receiving other ICSs, perhaps due to the once-daily dosing of MF. Current NAEPP guidelines recommend low-dose ICS monotherapy for mild persistent asthma; thus, it is critical to optimize mild persistent asthma control and limit unnecessary resource use and charges.
Introduction
The goal of asthma treatment is to achieve and maintain adequate asthma control, which can be measured by assessing the severity of asthma symptoms, the incidence of asthma-related exacerbations, and patient quality of life.Citation1 Current National Asthma Education and Prevention Program (NAEPP) guidelines define asthma control as reduced impairment (eg, prevention of chronic asthma symptoms and infrequent [≤2 days per week] use of short-acting β2-agonist [SABA] rescue medication) and reduced risk (eg, prevention of recurrent asthma exacerbations and minimization of the need for emergency department [ED] visits).Citation1 Inadequate asthma control may directly affect asthma resource use and treatment charges, which has the potential to significantly affect the growing global economic burden of asthma.Citation2 For example, uncontrolled asthma leads to increased use of SABAs and oral corticosteroids (OCSs) as well as increased ED, hospital, and outpatient visits,Citation3,Citation4 and has been shown to correlate with higher asthma-related direct medical expenditures and indirect charges.Citation3 In particular, the cost of rescue SABA therapy has recently increased because of new US Food and Drug Administration regulations that eliminate the use of ozone-depleting chlorofluorocarbon (CFC) propellants in all metered-dose inhalers (MDIs).Citation5 Although this new regulation is environmentally important, it has resulted in the replacement of generic CFC SABA inhalers with more costly non-generic hydrofluoroalkane (HFA) inhalers and has increased the economic burden associated with overreliance on SABAs. In 2004, the average cost of a HFA albuterol MDI across all payer types was 2.9 times greater than a generic CFC albuterol MDI ($39.50 vs $13.50, respectively).Citation5
One key element in achieving asthma control and thereby reducing healthcare utilization and costs, is proper adherence to prescribed asthma treatment.Citation1 Nonadherence to medical treatment regimens is estimated to cost the US healthcare system $100 billion annually.Citation6 Among patients with asthma, reduced adherence to prescribed medication has been associated with the need for increased medical care and reduced asthma control, including increased ED visits, increased OCS use, increased exacerbations, and/or worse asthma symptom scores.Citation7–Citation10 Because current NAEPP guidelines indicate that low-dose inhaled corticosteroids (ICSs) are the recommended treatment for mild persistent asthma,Citation1 ICS monotherapy is the most important type of treatment to consider for assessing the association between adherence and mild persistent asthma control. Factors that may affect adherence for patients with mild persistent asthma include ease and frequency of ICS administration and patients’ perceptions of treatment effectiveness. Previous data indicate that patients with asthma are more likely to be highly adherent to once-daily ICS therapy compared with twice-daily ICS therapy.Citation11 However, adequate formal analyses of the difference in charges and resource utilization of ICS treatment between highly adherent and minimally adherent patients with mild asthma are lacking from the current biomedical literature.
The primary objective of the current study was to determine the impact of adherence and asthma control on resource use and asthma treatment charges in patients with mild asthma having ICS claims. A secondary objective was to determine which ICS treatment was associated with the greatest likelihood of achieving high control and high adherence, which may indicate which ICS therapies are best for optimizing asthma control.
Patients and methods
Study design
A claims database (Ingenix LabRx, Eden Prairie, MN, USA) was analyzed retrospectively from October 2001 through December 2007 to identify patients with mild asthma who began treatment with an ICS, including but not limited to mometasone furoate (MF; Asmanex®, Merck & Co., Inc., Whitehouse Station, NJ, USA), fluticasone propionate (FP; Flovent®, GlaxoSmithKline, Research Triangle Park, NC, USA), FP HFA (Flovent® HFA, GlaxoSmithKline, Research Triangle Park, NC, USA), budesonide (Pulmicort®, Astra-Zeneca LP, Wilmington, DE, USA), or beclomethasone dipropionate (Qvar®, 3M Drug Delivery Systems, Northridge, CA, USA). During the timeframe used for this analysis, the database included approximately 37 million patients who primarily resided in the Southern or Midwestern United States (South, 43%; Midwest, 33%; West, 13%; Northeast, 11%). For each patient, the date of the first ICS prescription fill was considered the index date. Demographic information, drug utilization, and resource use for each patient were identified for the 365-day period before the index date (pre-index period) and the 365-day period following the index date (post-index period). Information collected during the pre-index period included total number of SABA canister prescriptions filled; incidence of comorbidities, including pneumonia, sinusitis, acute bronchitis, acute laryngitis, upper respiratory infection, acute nasopharyngitis, gastroesophageal reflux disease, and rhinitis; total asthma-related charges; pre-index asthma days; and pre-index asthma records. Asthma days was defined as the number of distinct days in which the patient had a medical record with any diagnosis of asthma in the pre-index period. Pre-index asthma records was defined as the number of distinct asthma-related records, identified by the presence of an asthma-related International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) code in which the patient had a medical record with a diagnosis of asthma in the pre-index period; note that a single office visit may have resulted in >1 asthma record.
Patients
All patients met the following inclusion/exclusion criteria: received an ICS prescription between October 2001 and December 2007; enrolled in health plans at least 1 year before and 1 year after index date; were 12 to 65 years of age; had no other chronic pulmonary condition (eg, identified by ICD-9-CM codes 415X, 416X, 417X, 491X, 491.2X, 492X, 493.2X, 494X, or 770.2X); received no combination therapy within 7 days of the index date; did not switch asthma therapy during post-index period; were designated as having mild asthma using an algorithm developed previously;Citation12 and were designated as having high control, high adherence (HCHA) or low control, low adherence (LCLA). Patients with mild asthma were defined as those who had been diagnosed with an ICD-9-CM code of 493.0X, 493.1X, or 493.9X and did not experience an asthma exacerbation or use >2 SABA canisters during the 365-day pre-index period. Although these criteria for mild asthma are not clinically based, similar methods have been used in other analyses.Citation12–Citation14 An asthma exacerbation was defined as an asthma episode that required hospitalization, treatment in an emergency room, or an outpatient visit during which nebulization or a prescription for OCSs was given, as previously described.Citation12 For adherence, the percent days covered (PDC) was defined as the percentage of days that patients had access to medication assuming daily drug use. The HCHA cohort included patients with no exacerbation events (high control) and ≥60% PDC (high adherence) during the post-index period. The LCLA cohort included patients with ≥2 exacerbation events (low control) and <10% PDC (low adherence) during the post-index period. Low adherence was selected as <10% PDC because this corresponds with patients who generally had 1 and only 1 prescription (eg, a median 30-day supply) during the 1-year post-index period. These cutoffs were based on distributions of exacerbations and PDC using upper and lower quartiles (eg, no priori determinations were made regarding patient cutoff points for designation as HCHA or LCLA).
Assessments
The primary outcomes of this analysis were post-index comparisons between the HCHA and LCLA cohorts in asthma-related resource use (eg, asthma-related medical records, asthma treatment days, and OCS claims) and charges (eg, outpatient charges, inpatient charges, pharmaceutical charges, and total asthma charges). All asthma charges were adjusted to 2008 US dollars.
Secondary outcomes of this analysis included the percentage of patients in each ICS treatment group who belonged in the HCHA cohort and odds ratios for the likelihood of belonging to the HCHA cohort based on ICS treatment initiated on the index date. This analysis was limited to ICSs for which patient sample sizes in the database were ≥500.
Statistical analyses
Bivariate analyses of the HCHA and LCLA cohorts were performed to determine differences in asthma resource use and overall asthma treatment charges. A 2-by-2 contingency table chi-square analysis was preformed to compare the unadjusted odds of patients belonging to the HCHA cohort based on ICS treatment. A forward stepwise logistic regression model was built to evaluate the odds ratio of a patient on a specific ICS therapy belonging to the HCHA cohort, adjusting for other covariates in the model.
Results
A total of 741 patients were included in the current analysis (). Because this analysis was designed to compare patients at the extreme ends of the adherence/control spectrum, only 4% of patients who met all other inclusion/exclusion criteria qualified for either the HCHA or LCLA cohort (). The majority of patients were female; the mean age of patients was 44.2 and 33.4 years in the HCHA and LCLA cohorts, respectively (). The HCHA cohort had a higher age, a lower proportion of female patients, a larger number of asthma days and asthma-related records, and higher asthma-related charges during the pre-index period (P < 0.0001; ). Both cohorts were similar in the proportions of patients with measured comorbidities, with the exception of rhinitis (higher in the HCHA cohort [P < 0.0001; ]) and upper respiratory tract infection (higher in the LCLA cohort [P < 0.0001; ]).
Figure 1 Patient selection. Patients were identified from a commercial insurance database and analyzed retrospectively to identify those with mild asthma who initiated treatment with an ICS. Patients who met all inclusion and exclusion criteria were assigned to 1 of 2 control/adherence cohorts based on the number of exacerbation events and percent days covered in the pre-index period: HCHA, 0 exacerbation events and ≥60% PDC; LCLA, ≥2 exacerbation events and <10% PDC.
*Criterion included owing to the initial existence of a fluticasone propionate with salmeterol cohort, which is not presented in the current analysis.
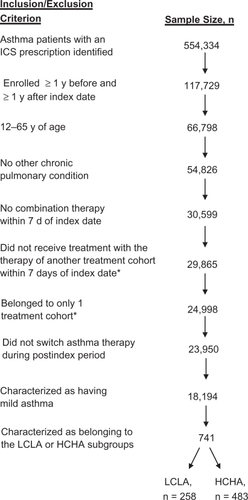
Table 1 Demographics and characteristics at index date
Resource use and treatment charges: bivariate analyses
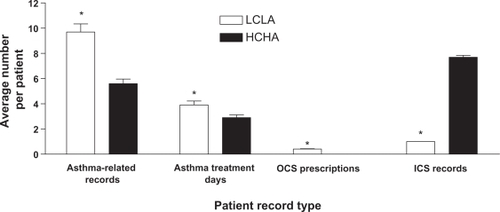
Compared with the HCHA cohort, the LCLA cohort was associated with more mean asthma-related medical records (5.6 vs 9.7, respectively; P < 0.0001), more mean asthma treatment days (2.9 vs 3.9, respectively P < 0.0001), more mean OCS prescriptions (0.0 vs 0.4, respectively; P < 0.0001), and fewer mean ICS records (7.7 vs 1.0, respectively; P <0.0001) in the post-index period ().
Figure 2 Resource utilization. The mean number of post-index asthma-related medical records, asthma days, OCS prescriptions, and ICS records among patients assigned to the HCHA or LCLA cohort are depicted. Error bars represent standard error of the mean.
*P < 0.0001.
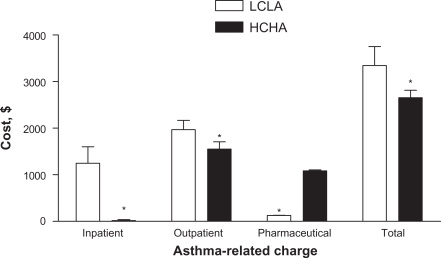
The HCHA cohort was associated with lower total mean asthma charges in the post-index period compared with the LCLA cohort ($2655 vs $3345, respectively, P < 0.0001; ). Mean pharmaceutical asthma charges were significantly higher for the HCHA cohort compared with the LCLA cohort ($1085 vs $129, respectively, P < 0.0001; ), as one would expect based on the cohort definitions. The lower overall mean asthma treatment charges in the HCHA cohort were primarily driven by lower resource use (ie, inpatient/outpatient charges) compared with the LCLA cohort (inpatient charge, $19 vs $1248, respectively, P < 0.0001; outpatient charge, $1551 vs $1968, P =0.0001; ).
Figure 3 Asthma-related charges. The mean post-index asthma-related inpatient, outpatient, pharmaceutical, and total charges among patients assigned to the HCHA or LCLA cohort are depicted. Error bars represent standard error of the mean.
*P ≤ 0.0001.
ICS use associated with HCHA: multivariate analyses
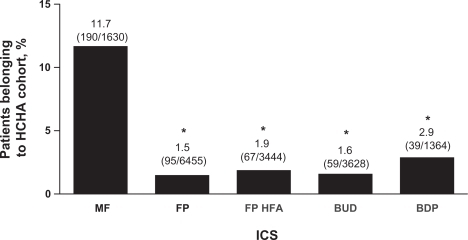
Among patients who met all inclusion criteria and received an ICS that was prescribed to ≥500 patients in the database (n = 16,521), a total of 450 patients belonged to the HCHA cohort. A significantly higher percentage of patients in this subanalysis who received MF belonged in the HCHA cohort than any other ICS (P < 0.0001; ). Using an unadjusted chi-square analysis, MF patients were 7.425 times more likely than patients receiving other ICS agents to be in the HCHA cohort (P < 0.0001). Calculation of odds ratios from the logistic regression model revealed that patients receiving MF were 5.081 (95% CI, 4.144–6.230) times more likely than patients receiving other ICS agents to be in the HCHA cohort (P < 0.0001) after adjusting for all other pre-index variables.
Figure 4 ICS use associated with HCHA. The percentages of patients included in the current analysis that were treated with MF, FP, FP HFA, BUD, or BDP and assigned to the HCHA cohort are depicted. Percentages for each ICS group represent the number of patients who were treated with each respective ICS and qualified for the HCHA cohort divided by the total number of patients who were treated with each respective ICS. Error bars represent standard error of the mean.
*P < 0.0001 vs MF.
Discussion
The results of the current analysis suggest that increases in asthma control and adherence to prescribed asthma medication correlate with decreases in total asthma-related charges. Among patients with a low level of asthma control, who were minimally adherent to their prescribed ICS medication, the incidence of asthma-related records, asthma treatment days, and the number of OCS prescriptions were higher compared with patients who had a superior level of asthma control and were more adherent to their prescribed ICS medication. This finding is not surprising given the strong correlation between asthma patient adherence to prescribed medication and asthma control,Citation7–Citation10 and the increased costs associated with asthma exacerbationsCitation15 and/or extra doctor visitsCitation16 that often accompany poorly controlled asthma. In a large retrospective analysis of a managed care database by Stern and colleagues (N = 97,743), patients who were more highly compliant to their prescribed controller medication (including ICSs) were significantly less likely to experience an asthma-related exacerbation (P < 0.001).Citation9 Other studies that specifically assessed adherence to ICS treatment have found that enhanced patient adherence is associated with increased asthma control as measured by overall asthma control scores,Citation7,Citation8 emergency department visits,Citation10 and OCS prescriptions.Citation10 In a prospective cohort study by Krishnan and colleagues (N = 60), asthma patients with poor adherence had significantly worse asthma symptom scores (P = 0.04) 2 weeks after hospital discharge following an asthma exacerbation.Citation7 In a large, descriptive, observational study by Molimard and Le Gros (N = 4362), asthma control was deemed inadequate among 62.7% of persistent asthma patients who missed ≥4 ICS doses per week, but only 37.5% of patients who missed <4 ICS doses per week.Citation8 In a retrospective claims database analysis of asthma patients by Williams and colleagues (N = 405), ICS adherence correlated negatively and significantly to emergency department visits and OCS prescriptions; furthermore, each 25% increase in the percentage of time that patients were without ICS therapy doubled the asthma-related hospitalization rate.Citation10
The lower asthma-related days and records observed in the HCHA cohort compared with the LCLA cohort is in contrast to the index date data, when these parameters were higher in the HCHA cohort. This may suggest subjects in the HCHA cohort had slightly more severe (although still mild) asthma resulting in more asthma-related visits. The resulting decrease in asthma-records and days during the post-index period may be due to implementation of ICS treatment resulting in control (prior ICS use was not part of the inclusion criteria), a switch to more effective therapy, or an emphasis on drug adherence by the prescribing physician.
Collectively, these data demonstrate that enhanced adherence to prescribed ICS therapy correlates with improved asthma control. As such, it is critical to optimize patient adherence to not only improve patient outcomes, but also to lower the global economic burden of asthma care. The increased asthma-related exacerbations, emergency room visits, and inpatient and outpatient doctor visits associated with poorly controlled asthma result in significant pharmacoeconomic burden.Citation15,Citation16 A recent retrospective analysis of the 2004 Medical Expenditure Panel Survey data by Kamble and Bharmal estimated that asthma afflicts 6.4 million children and 14.8 million adults in the United States alone and costs approximately $1000 and $2000, respectively, per person annually in 2007 US dollars.Citation16 All medical visits combined (including inpatient, outpatient, emergency room, and office-based medical visits) accounted for approximately 60% of all asthma-related expenditures in children and approximately 40% of all asthma-related expenditures in adults. Given the significant overall economic burden of asthma and the association between asthma control and patient adherence to prescribed medication, improvements in patient adherence and asthma control have the potential to significantly reduce asthma-related healthcare expenditures.
As expected, data from the current study suggested that reduced asthma control and adherence to prescribed asthma medication correlated with increased asthma-related total charges driven by increased inpatient and outpatient charges. Previous studies have demonstrated similar correlations between asthma control, adherence, and resource use and charges. Among asthma patients (N = 527) stratified to 1 of 4 quartiles of asthma control that ranged from “good control” to “poor control,” Accordini and colleagues found that hospitalizations and doctor visits increased significantly with decreasing levels of controller medication use and poor asthma control, and generally correlated with higher asthma-related direct medical expenditures and indirect costs.Citation3 For all 5 mean annual cost parameters assessed (ie, doctor visits and lab visits, pharmacological treatment, hospitalization, indirect costs, and total), the estimated mean annual cost per patient increased as the level of disease control decreased (P < 0.001).Citation3 In parallel with results from the current study, patients with poor asthma control experienced the lowest percentage of direct charges due to pharmacological treatment and the highest percentage of direct charges due to hospitalization, whereas patients with good asthma control experienced notably higher pharmacological treatment charges and lower direct charges.Citation3 Collectively, these data suggest that the total expenses associated with asthma-related charges are lower for patients with superior asthma control, driven by reductions in hospital and physician-related services (ie, inpatient and outpatient visits, hospitalizations, and ED visits) charges, but not reductions in prescription medication charges. This finding is somewhat surprising because the greatest contributor to asthma care charges in the United States is prescription drugs, which are estimated to account for 43% of the $16.1 billion spent on asthma care annually.Citation17 However, it is possible that minimally adherent patients with mild persistent asthma who have poorly controlled asthma have lower pharmaceutical asthma-related charges because they do not adhere to their prescribed dosing regimens of controller medications, such as ICSs. As such, these patients may use less of their prescribed medication, which may lead to complications that result in asthma exacerbations, hospitalizations, ER visits, and/or more frequent trips to see physicians. The current data suggest that highly adherent asthma patients who achieve a high level of asthma control may have more charges for prescription medication than patients with a lower level of adherence and asthma control, but the tradeoff for this higher expense is fewer inpatient and outpatient physician visits, which corresponds with lower total medical care charges.
There are several reasons why patients who received MF were approximately 5 times more likely to be in the HCHA cohort than patients who received the other ICSs. In general, there are limited data that suggest clinically relevant differences between ICSs in clinical efficacy.Citation18 However, study outcomes that may be related to patient adherence (ie, asthma control) will tend to be minimal in well organized clinical trials because such trials are often designed to limit differences between treatment groups in adherence. The once-daily dosing regimen of MF may be the most likely reason why a higher percentage of patients receiving MF were in the HCHA cohort compared with patients who received one of the other ICSs. Previous data suggest that adherence to prescribed medication, regardless of disease state, increases as daily doses decrease.Citation19 Guest et al specifically investigated the change in adherence when patients with asthma were switched from a twice-daily ICS to once-daily ICS treatment.Citation11 As might be expected, patients who switched to a once-daily ICS were more likely to be highly adherent and had lower asthma-related charges compared with patients who switched to another twice-daily ICS dosing regimen.Citation11 In addition, Price et al found that for MF specifically, adherence was significantly higher for once-daily dosing than twice-daily dosing.Citation20 NAEPP guidelines note the importance of assessing and encouraging asthma patient adherence to prescribed therapy,Citation1 and point out that adherence to a therapeutic plan is enhanced when daily doses are minimized.Citation21–Citation23 Further analyses in larger sample sizes of patients are necessary to verify if MF treatment is associated with lower asthma-related charges among highly adherent, high asthma control patients compared with other ICSs because of a superior dosing regimen. Furthermore, it is important to note that although most patients with asthma treated with MF are prescribed a once-daily dosing regimen, some patients (ie, those ≥12 years of age receiving oral OCSs) may receive a twice-daily regimen,Citation24 and it is impossible to determine how many patients were prescribed once-daily MF versus twice-daily MF in the current claims database analysis. However, because of their designation as mild asthma patients, it is likely that the majority of patients in the current study who received MF were prescribed a once-daily regimen.
An alternative or additional explanation for the increased adherence with MF patients is that the MF device (Twisthaler®, Merck & Co., Inc., Whitehouse Station, NJ, USA) is easy to use. There are only 3 steps in the dosing process and the device has an integrated dose counter.Citation25 In a head-to-head comparison of the MF Twisthaler and a fluticasone propionate metered-dose inhaler, there was no significant difference in the number of subjects who rated the inhalers “easy to use,” but significantly more subjects using Twisthaler “liked it a lot” (47% vs 22%, P = 0.01).Citation26 Ease of use and patient satisfaction with an inhaler may contribute to adherence, but this area of research has not been adequately explored.
One limitation of the overall design of the current study was that it only included patients with a low level of asthma control and adherence to their prescribed ICS medication, or a high level of asthma control and adherence to their prescribed ICS medication. In real-world clinical settings, many asthma patients fall somewhere in between this continuum, with “medium” levels of both asthma control and adherence to therapy. Previous data suggest that, regardless of asthma severity, improved asthma control is associated with lower asthma-related healthcare costsCitation3 and higher patient adherence to prescribed therapy.Citation7–Citation10 However, specific analyses of patients with a low level of asthma control and good adherence to prescribed therapy, or a low level of asthma control and poor adherence to prescribed therapy are lacking from the biomedical literature. One reason for this gap in the literature may be because it is rare to find patients who are not misdiagnosed who have unparalleled levels of asthma control and adherence to therapy. For all patients, physicians should promote adherence to prescribed treatment in accordance with current NAEPP guidelinesCitation1 to optimize each individual patient’s respective asthma control potential and minimize healthcare costs. For patients with a low level of asthma control who are highly adherent to their prescribed therapy, it may be necessary for physicians to re-evaluate the treatment plan being employed to determine if alternate therapeutic options should be pursued.
There are several limitations to this type of observational claims database analysis. The retrospective nature of the study limited the amount and type of information that could be collected, such as information about why a specific ICS was chosen or how adherence could be measured. Also, it is difficult to accurately categorize asthma severity without using predefined clinical outcome measures;Citation13 one previous claims-based analysis categorized patients as having mild asthma less frequently than did pulmonary function testing in the same population.Citation14 In addition, the definitions of HCHA and LCLA used in this analysis were not standardized and it is difficult to verify the accuracy of data found in a claims database. However, these limitations are inherent to any claims database analysis and do not preclude the development of important and clinically relevant conclusions about the effect of asthma control and adherence on asthma-related charges and resource use.
Conclusions
Collectively, these data suggest that better asthma control and adherence to prescribed ICSs are associated with lower overall asthma-related resource use and charges. Furthermore, patients with mild asthma receiving MF were more likely to be in the HCHA patient cohort than patients receiving a different ICS, which may have been due to the once-daily dosing regimen of MF. Of critical importance to optimizing mild persistent asthma control and limiting asthma-related resource use and charges is following current NAEPP guidelines, which indicate low-dose ICS monotherapy as the preferred treatment for mild persistent asthma.Citation1
Transparency
Declaration of funding
This work was supported by Schering-Plough Corporation, now Merck & Co., Inc.
Declaration of financial/other relationships
Prakash Navaratnam is a paid consultant for Merck & Co., Inc.
Howard S Friedman is the owner of Analytic Consulting, LLC which performs consulting work in the pharmaceutical industry, including Merck & Co., Inc.
Eduardo Urdaneta was an employee of Schering-Plough Corporation when the study was conducted.
Acknowledgements
Medical writing assistance was provided by Brett Mahon, PhD and was funded by Schering-Plough Corporation, now Merck & Co., Inc. Additional assistance was provided by John McLaughlin, MSPH.
References
- National Asthma Education and Prevention ProgramExpert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma 2007 Available at: http://www.nhlbi.nih.gov/guidelines/asthma/index.htm [Last accessed April 2008].
- MasoliMFabianDHoltSBeasleyRThe global burden of asthma: executive summary of the GINA Dissemination Committee reportAllergy20045946947815080825
- AccordiniSBugianiMArossaWPoor control increases the economic cost of asthma. A multicentre population-based studyInt Arch Allergy Immunol200614118919816899987
- BriggsAHBousquetJWallaceMVCost-effectiveness of asthma control: an economic appraisal of the GOAL studyAllergy20066153153616629780
- Food and Drug AdministrationUse of ozone-depleting substances; removal of essential-use designations; final ruleFed Regist2005701716817192
- HorneRCompliance, adherence, and concordance: implications for asthma treatmentChest200613065S72S16840369
- KrishnanJARiekertKAMcCoyJVCorticosteroid use after hospital discharge among high-risk adults with asthmaAm J Respir Crit Care Med20041701281128515374842
- MolimardMLe GrosVImpact of patient-related factors on asthma controlJ Asthma20084510911318350401
- SternLBermanJLumryWMedication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care dataAnn Allergy Asthma Immunol20069740240817042149
- WilliamsLKPladevallMXiHRelationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthmaJ Allergy Clin Immunol20041141288129315577825
- GuestJFDavieAMRuizFJGreenerMJSwitching asthma patients to a once-daily inhaled steroid improves compliance and reduces healthcare costsPrim Care Respir J200514889816701704
- FriedmanHSYawnBPResource utilization in asthma: combined fluticasone propionate/salmeterol compared with inhaled corticosteroidsCurr Med Res Opin20072342743417288696
- ColiceGWuEQBirnbaumHHealthcare and workloss costs associated with patients with persistent asthma in a privately insured populationJ Occup Environ Med20064879480216902372
- BirnbaumHGIvanovaJIYuAPAsthma severity categorization using a claims-based algorithm or pulmonary function testingJ Asthma200946677219191141
- LaneSMolinaJPlusaTAn international observational prospective study to determine the cost of asthma exacerbations (COAX)Respir Med200610043445016099149
- KambleSBharmalMIncremental direct expenditure of treating asthma in the United StatesJ Asthma200946738019191142
- American Lung AssociationTrends in Asthma Morbidity and MortalityNew York, NYEpidemiology and Statistics Unit New York, NY
- BarnesNCThe properties of inhaled corticosteroids: similarities and differencesPrim Care Respir J20071614915417530144
- ClaxtonAJCramerJPierceCA systematic review of the associations between dose regimens and medication complianceClin Ther2001231296131011558866
- PriceDRobertsonABullenKImproved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label studyBMC Pulm Med10120051135
- BenderBGBenderSEPatient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnairesImmunol Allergy Clin North Am20052510713015579367
- EisenSAMillerDKWoodwardRSThe effect of prescribed daily dose frequency on patient medication complianceArch Intern Med1990150188118842102668
- HaynesRBYaoXDeganiAInterventions to enhance medication adherenceCochrane Database Syst Rev2005CD00001116235271
- Asmanex® Twisthaler® (mometasone furoate)Full Prescribing InformationMerck & Co Inc.Whitehouse Station, NJ, USA2008
- KarpelJPAn easy-to-use dry-powder inhalerAdv Ther20001728228611317831
- WardlawALariveePEllerJEfficacy and safety of mometasone furoate dry powder inhaler vs fluticasone propionate metered-dose inhaler in asthma subjects previously using fluticasone propionateAnn Allergy Asthma Immunol200493495515281471