Abstract
The biology of both normal and tumor development clearly possesses overlapping and parallel features. Oncogenes and tumor suppressors are relevant not only in tumor biology, but also in physiological developmental regulators of growth and differentiation. Conversely, genes identified as regulators of developmental biology are relevant to tumor biology. This is particularly relevant in the context of brain tumors, where recent evidence is mounting that the origin of brain tumors, specifically gliomas, may represent dysfunctional developmental neurobiology. Neural stem cells are increasingly being investigated as the cell type that originally undergoes malignant transformation – the cell of origin – and the evidence for this is discussed.
Origin of brain tumors: historical insights
Over a century ago, the German pathologist Julius Cohnheim described the similarities between tumors and embryonic cells and suggested that “embryonic rests” were the source of tumors (CitationRather 1978). Later, the primitive cytoarchitecture and embryonic features of many malignant brain tumors was also described by CitationBailey and Cushing (1926). CitationSmyth and Stern’s (1938) observed that “subependymal glia may actually be the point of origin of tumors of the thalamus.”
These earlier descriptions were further advanced in 1944, Joseph Globus and Hartwig Kuhlenbeck called attention to the subependymal cell plate in the adult brain and described this structure as one with primitive cellular composition. Based on a study with human brain tumors, they stated that “one of the most important sources for such immature embryonal residue from which neuroectodermal tumors are likely to develop under certain still unknown conditions is the subependymal plate.” (CitationGlobus 1944).
The subependymal plate was better characterized as a “mitotically active and well defined subependymal layer is present in mammalian brains throughout life” and CitationLewis (1968) suggested that they could be a “possible source of different histological varieties of glioma, in particular those tumors in paraventricular situation and butterfly gliomas of the corpus callosum.”
In the late 1960s CitationHopewell and Wright (1969) demonstrated increased glial tumors with periventricular implantation of carcinogens in rats. In the 1970s, periventricular tumors were then demonstrated to occur in the subventricular region after intraventricular inoculation with avian sarcoma viruses, with a much higher rate of tumors occurring in neonatal rats versus adult rats (CitationCopeland et al 1975; CitationCopeland and Bigner 1977; CitationVick et al 1977). A single dose of ethylnitrosurea administration to pregnant rats also induced periventricular tumors in the offspring (CitationKoestner et al 1971).
Now clear evidence exists for the “subependymal plate, as described by Gobus and Kuhlenbeck is the subventricular zone (SVZ), know to be the largest cellular region of neural stem cells (NSCs) in the adult mammalian brain. The NSCs have been characterized by Buyalla and provide the migratory neuroblasts for neurogeneis in the olfactory bulb in rodents () (CitationSanai et al 2005). Recently, this migratory path for SVZ NSCs was also described in humans (CitationCurtis et al 2007).
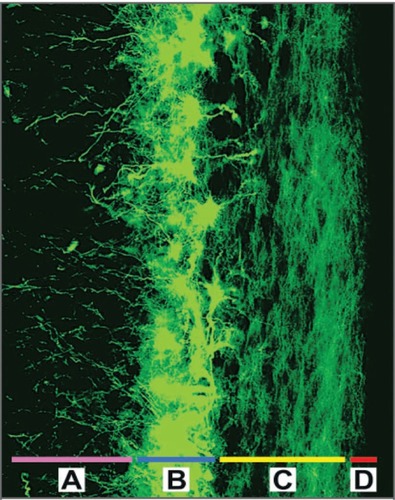
Figure 1 The cells of the subventricular zone, labeled with the astrocyte marker GFAP (shown in green), line the lateral walls of the lateral ventricles. This is the largest known region of adult neural stem cells in the human brain; it is composed of the deep subcortical white matter A), a periventricular ribbon of astrocytes that can function as neural stem cells B), a dense layer of astrocytic processes C), and the ependymal lining D). Throughout adult life, astrocytes from the subventricular zone exhibit a unique capacity for multipotency and self-renewal in vitro. Copyright © 2005. Reproduced with permission from CitationSanai N, Alvarez-Buylla A, Berger M. 2005. Neural stem cells and the origin of gliomas. N Engl J Med, 353:811–22.
Reappraising the prevailing theory of tumor genesis
It has been widely accepted that cancer occurs as a consequence of genetic and epigenetic alterations in a differentiated cell. These alterations could provide a proliferative advantage and ultimately lead to uncontrolled growth and spread of the malignant cells. This theory suggests that tumors, such as gliomas, result from mutations to terminally differentiated astrocytes and oligodendrocytes that “de-differentiate” into a less differentiated phenotype (CitationMabon et al 1950; CitationDoetsch et al 1999; CitationSakariassen et al 2007). Although the neoplastic transformation of fully differentiated glia is widely assumed to be the mechanism of gliomagenesis, this hypothesis has never been adequately tested.
Evidence for NSC as the cell of origin
The neurogenic zones within the human central nervous system (CNS) with their resident NSCs are considered the leading candidates for transformation leading to brain tumors. Specifically mitotically active cells (NSCs and their direct progeny, transit amplifying cells) are the cells that have the greatest probability of being the brain tumor cell of origin.
NSCs and gliomas share histological and biological similarities
Gliomas have long been described by pathologists for their remarkable cellular heterogeneity, and in fact the most aggressive and malignant glioma (glioblastoma multiforme) was termed based partly on the histological diversity comprising this tumor, hence “multiform.” A transformed NSC could provide this cellular landscape due to their mulitipotentiallity (ability to differentiate into the cell types that constitute their respective germ line). Mixed cell gliomas exist, such as oligoastrocytomas, and have both oligodendrocytes and astrocytes (CitationValtz et al 1991) and could be independent transformation of two differentiated cells, as suggested by the dedifferentiation theory of tumor genesis. More plausible would be the transformation of a single, bipotential progenitor cell such as a NSC or a transit amplifying cell (CitationChekenya and Pilkington 2002). These mixed cell gliomas also exhibit loss of heterozygosity on chromosomes 1p and 19q in both the astrocytic and oligodendrocytic components (CitationKraus et al 1995), suggesting that, in oligoastrocytomas, the astrocytes and oligodendrocytes comprising the tumor have a shared cell of origin.
Further, many glioma cells are undifferentiated, and lack expression of differentiated cell markers, as well as demonstrate staining with markers for nestin. Nestin expression is one hallmark feature of NSCs (CitationDahlstrand et al 1992; CitationTohyama et al 1992), and has become a reliable marker of NSCs (CitationLendahl et al 1990). Gliomas and NSCs also exhibit characteristic overlapping behavior () (CitationSanai et al 2005), such as high motility, association with vasculature and white matter tracts (CitationShoshan et al 1999; CitationDoetsch et al 2002; CitationPalmer et al 2000).
Table 1 Characteristics intrinsic to neural stem cells and gliomas. Copyright © 2005. Reproduced with permission from CitationSanai N, Alvarez-Buylla A, Berger M. 2005. Neural stem cells and the origin of gliomas. N Engl J Med, 353:811–22
NSCs more likely to accumulate oncogenic mutations
Accumulation of oncogenic genetic hits by cells is an infrequent stochastic event that most likely takes considerable time to result in transformation. NSCs, defined by their ability to self renew are both mitotically active and exist during the lifetime of the animal, allowing them to potentially accumulate the necessary multiple mutations for tumor formation. Accordingly, the cellular origin of gliomas would most likely occur from the proliferative zones in the mammalian CNS such as the SVZ contain at least two types of mitotically active cells: NSCs and transit amplifying cells (TACs) (CitationSeri et al 2004). Although TACs exist only briefly and then differentiate, their total cellular compartment is significantly larger than NSCs and total number of global number of divisions during their relatively short lifespan are comparable to NSCs that exist throughout life yet are less mitotically active () (CitationVescovi et al 2006).
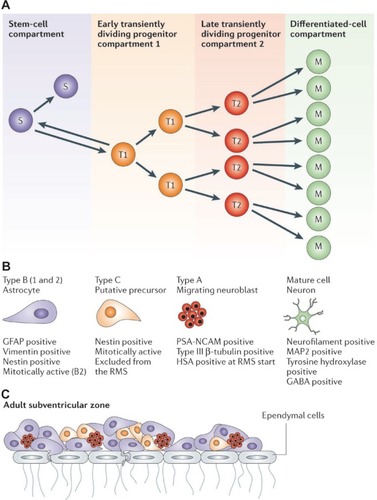
Figure 2 Hierarchical organization of the functional compartments in renewing tissues A) The stem-cell compartment (purple), early transiently dividing progenitor compartment (orange), late transiently dividing progenitor compartment (red) and differentiated-cell compartment (green) are schematically described. Cells in the stem-cell and transiently dividing progenitor compartments could be the target of the onco-transformation that leads to the formation of tumour stem cells B) The neural precursors that make up similar functional compartments in the neurogenetic regions of the adult brain and that might be the source of brain tumour stem cells C) The structure of the subventricular zone, showing how these precursors fit and are organized in the germinal neuroepithelium of the largest neurogenetic region of the adult brain.
Cancer stem cells
Approximately 150 years ago, pathologists Rudolph Virchow and Julius Cohnheim suggested there were histological similarities between the developing fetus and certain cancers (such as teratocarcinomas) and that both tissues have the capacity to differentiate and proliferate. This “embryonal-rest hypothesis” is the historical version of today’s cancer stem cell (CSC) hypothesis (CitationHuntly and Gilliland 2005). As defined at the American Association for Cancer Research workshop on cancer stem cells: cancer stem cells are cells that (1) self renew and (2) re-supply the tumor with the various lineages of cells of which it is comprised. Self renewal can only be defined experimentally by the ability to recapitulate the generation of a continuously growing tumor or tumor initiation cell () (CitationClarke et al 2006; CitationLee and Herlyn 2007).
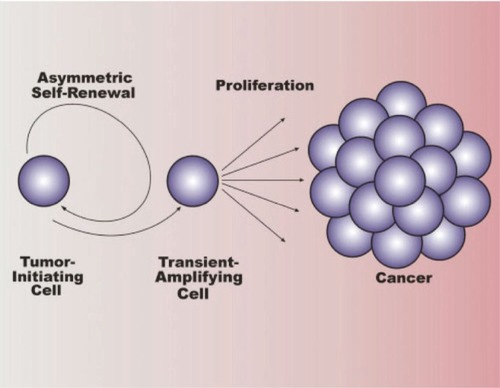
Figure 3 Overview of tumor stem cells in cancer. Cancer stem cells (tumor-initiating cells) divide asymmetrically, resulting in self-renewal of the tumor-initiating cell and production of a daughter cell known as a transient-amplifying cell (progenitor cell). The transient-amplifying cell is not thought to possess self-renewing capabilities, but instead divides indefinitely to contribute to cancer progression. Copyright © 2007. Reproduced with permission from CitationLee J, Herlyn M. 2007. Old disease, new culprit: tumor stem cells in cancer. J Cell Physiol, 213:603–9.
The original work establishing the CSC model was based on the hematopoietic system. Evidence for leukemia-CSCs was first reported in 1994 when Lapidot and colleagues isolated a rare population of CD34+CD38− cells from patients with acute myeloid leukemia. Infusion of these CD34+CD38− cells into severe combined immune-deficient mice resulted in leukemic blast generation; however, more differentiated cells (CD34+ CD38+) did not generate leukemia (CitationLapidot et al 1994; CitationAl-Hajj et al 2003; CitationBuzzeo et al 2007). On a molecular level, CSCs share properties with normal stem cells. They have similar markers and signaling pathways, respond to environmental cues, as well as telomerase activity, apoptosis clearance and increased membrane transporter activity (CitationSakariassen et al 2007).
The first report of cells with stem-like properties in brain tumors was by CitationIgnatova and colleagues (2002) where surgical specimens of glioblastoma multiforme were shown to have clonogenic neurosphere-forming cells that expressed both neuronal and glial markers upon differentiation () (CitationIgnatova et al 2002; CitationVescovi et al 2006). Subsequently, the Dirks group demonstrated CSC in brain tumors by 1 transplantation of CD133+ or CD133− populations into immunodeficient mice. With as few as 100 CD133+ cells from the primary tumor, a new phenocopy of the tumor could be created in the transplanted mice; and unsorted or CD133− primary tumor cells were unable to cause de novo tumor generation. As part of what has come to define CSCs-self-renewal capacity – was also shown by confirming the ability of serially transplanted CD133+ cells to recapitulate the original tumor (CitationSingh et al 2004; CitationBuzzeo et al 2007). These findings established the presence of brain tumor stem cells (BTSCs), cells which can differentiate into the neural lineages, and exhibit self renewal as demonstrated by recapitulation of primary tumors with serial transplantation. This has been shown for other types of brain tumors as well (CitationMerkle et al 2004; CitationTaylor et al 2005). The existence of these BTSCs adds further evidence toward the NSCs origin of gliomas by confirming that different brain tumors contain transformed, undifferentiated neural precursors that respond to the same mitogens that activate adult NSCs (CitationVescovi et al 2006). Second, they indicate that tumor stem-like cells possess some of the molecular features of NSCs. Third, BTSCs, through asymmetric division, could generate a BTSCs and a progenitor cell, the latter of which may migrate away to either form or contribute to the tumor mass (CitationVescovi et al 2006, CitationBerger et al 2004).
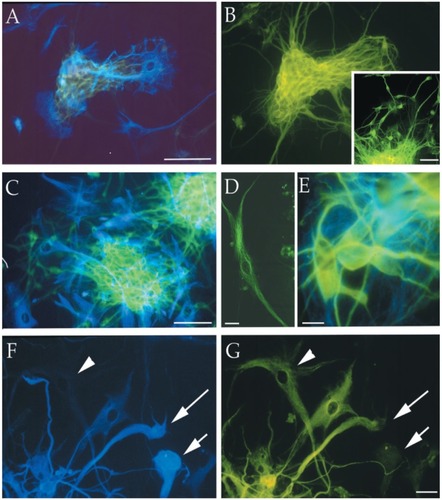
Figure 4 Immunofluorescence photomicrographs of representative MC-PL clones derived from different cortical glial tumor specimens (T1–T3, T6, and T7) processed for double (A, B, C, E, F) or single (B inset, D) labeling for different neural markers. The cells positive for the neuronal marker β-III tubulin are stained green (FITC), and the cells that expressed the astroglial marker GFAP are stained blue (AMCA). The arrowhead in F points out a cell that is not labeled for GFAP, but is immunopositive for β-III tubulin (arrowhead in G). The short arrow in F points out a cell that is immunolabeled for GFAP, but immunonegative for β-III tubulin (short arrow in G). The long arrow in F and G points out a cell that is labeled with both immunomarkers. Scale bars are 10 μm (F and G) and 100 μm (A–E). Copyright © 2002. CitationIgnatova T, Kukekov V, Laywell E, et al. 2002. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia, 39:193–206.
Gliomas and NSCs have common regulatory pathways
Neural stem cells and progenitor cells have activated cellular pathways, such as pro-mitotic genes, telomerase activity, and anti-apoptotic genes. This innate capacity overlaps with the mechanisms underlying tumor initiation, progression, or both. Thus, NSCs may require the least amount of mutations to become transformed.
EGFR expression is up-regulated in primary glioblastoma multiforme and transiently dividing progenitors (type C cells) (CitationMellinghoff et al 2005).
Fibroblast growth factors (FGFs) are involved in tumor proliferation and angiogenesis (CitationJoy et al 1997; CitationAuguste et al 2001) and also shown to regulate NSC proliferation and cell fate (CitationVescovi et al 1993; CitationGritti et al 1996; CitationPalmer et al 1999).
Notch receptors and signaling is involved in NSC renewal (CitationHitoshi et al 2002; CitationShen et al 2004) and related to proliferative capacity of gliomas (CitationPurow et al 2005).
PTEN is a tumor suppressor with an important function in the control of proliferation of neural stem cells and progenitor cells in vivo and in vitro (CitationBaker and McKinnon 2004; CitationGroszer et al 2001; CitationReya and Clevers 2005). PTEN is inactivated in glioblastomas (CitationRasheed et al 1999; CitationWechsler-Reya and Scott 2001) and its preservation in glioblastoma multiforme is clinically favorable (CitationMellinghoff et al 2005).
The Wnt-catenin pathway regulates adult neurogenesis (CitationChenn and Walsh 2003; CitationLie et al 2005) modulating its activity may increase glioma cell growth (CitationRoth et al 2000).
Recent experiments have also highlighted the increased ability of progenitor cells to be transformed versus differentiated cell types. If epidermal growth factor receptor (EGFR) is transfected into transgenic Ink4a-Arf-/- mouse (lacking genes for cell-cycle arrest) neural stem cells, the cells lead to glioma formation (CitationBachoo et al 2002). This contrasted with similar manipulation of differentiated mouse astrocytes. Further, if the undifferentiated mouse astrocytes were transfected with platelet-derived growth factor (pdgf) transgene and converted to a less differentiated state, they showed increased oncogenicity (CitationDai et al 2001; CitationBachoo et al 2002; CitationUhrbom et al 2002).
Mouse models of gliomas for investigation of glioma origin
Mouse models of gliomas are available and offer a unique opportunity to investigate tumor origin. These models, unlike glioma models in Drosophila and C. elegans, recapitulate the human pathology in terms of such characteristic structures as pleomorphic nuclei, diffusely infiltrative margins, secondary structures of Scherer, necrosis with pseudopalisading tumor cells, and microvascular proliferation. Xenograft models fail to phenocopy the classic histopathological features and are not an option for elucidating developmental mechanisms. Ultimately, these models of spontaneous tumor development and progression allows for the potential identification of novel mechanisms for tumorigenesis (CitationDing et al 2001).
Relevance of identifying the glioma cell of origin
Brain tumor classification with current histological criteria fails to accurately categorize patients as many patients with similar grade brain tumors have highly variable clinical outcomes. Clearly, this classification is one that at a minimum needs molecular modifiers. Defining the cell of origin, and confirming whether NSCs are indeed the cell of origin would improve not only glioma classifications, but also detection and treatment. The differential antigenic and molecular attributes of NSCs responsible for tumorigenesis could be exploited to target malignant cells prior tumor progression to clinical presentation. Indeed, defining cell of origin could help expand the concept of chemoprevention: targeting cells in the pre-morbid state.
Disclosure
The authors report no conflicts of interest in this work.
References
- Al-HajjMWichaMBenito-HernandezA2003Prospective identification of tumorigenic breast cancer cellsProc Natl Acad Sci U S A1003983812629218
- AugustePGürselDLemièreS2001Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and independent mechanismsCancer Res6117172611245488
- BachooRMaherELigonK2002Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axisCancer Cell12697712086863
- BaileyPCushingH1926A classification of the tumors of the glioma group on a histogenetic basis with a correlated study of prognosisPhiladelphia, PAJB Lippincott Company
- BakerSMcKinnonP2004Tumour-suppressor function in the nervous systemNat Rev Cancer41849614993900
- BergerFGayEPelletierL2004Development of gliomas: potential role of asymmetrical cell division of neural stem cellsLancet Oncol5511415288241
- BuzzeoMScottECogleC2007The hunt for cancer-initiating cells: a history stemming from leukemiaLeukemia2116192717541397
- ChekenyaMPilkingtonG2002NG2 precursor cells in neoplasia: functional, histogenesis and therapeutic implications for malignant brain tumoursJ Neurocytol315072114501220
- ChennAWalshC2003Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic miceCereb Cortex1359960612764034
- ClarkeMDickJDirksP2006Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cellsCancer Res6693394416990346
- CopelandDBignerD1977The role of the subependymal plate in avian sarcoma virus brain tumor induction: comparison of incipient tumors in neonatal and adult ratsActa Neuropathol (Berl)3816193346
- CopelandDVogelFBignerD1975The induction of intractranial neoplasms by the inoculation of avian sarcoma virus in perinatal and adult ratsJ Neuropathol Exp Neurol3434058166146
- CurtisMKamMNannmarkU2007Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extensionScience3151243917303719
- DahlstrandJCollinsVLendahlU1992Expression of the class VI intermediate filament nestin in human central nervous system tumorsCancer Res525334411382841
- DaiCCelestinoJOkadaY2001PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivoGenes Dev1519132511485986
- DingHRoncariLShannonP2001Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomasCancer Res6138263611325859
- DoetschFCailléILimD1999Subventricular zone astrocytes are neural stem cells in the adult mammalian brainCell977031610380923
- DoetschFPetreanuLCailleI2002EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cellsNeuron3610213412495619
- GlobusJHKuhlenbeckH1944The subependymal plate (matrix) and its relationship to brain tumors of the ependymal typeJ Neuropathol Exp Neurol3135
- GrittiAParatiECovaL1996Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factorJ Neurosci1610911008558238
- GroszerMEricksonRScripture-AdamsD2001Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivoScience2942186911691952
- HitoshiSAlexsonTTropepeV2002Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cellsGenes Dev168465811937492
- HopewellJWrightE1969The importance of implantation site in cerebral carcinogenesis in ratsCancer Res291927314311561
- HuntlyBGillilandD2005Leukaemia stem cells and the evolution of cancer-stem-cell researchNat Rev Cancer53112115803157
- IgnatovaTKukekovVLaywellE2002Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitroGlia3919320612203386
- JoyAMoffettJNearyK1997Nuclear accumulation of FGF-2 is associated with proliferation of human astrocytes and glioma cellsOncogene14171839010219
- KoestnerASwenbergJWechslerW1971Transplacental production with ethylnitrosourea of neoplasms of the nervous system in Sprague-Dawley ratsAm J Pathol6337564323476
- KrausJKoopmannJKaskelP1995Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligo-dendroglioma and oligoastrocytomaJ Neuropathol Exp Neurol549157815084
- LapidotTSirardCVormoorJ1994A cell initiating human acute myeloid leukaemia after transplantation into SCID miceNature36764587509044
- LeeJHerlynM2007Old disease, new culprit: tumor stem cells in cancerJ Cell Physiol213603917786956
- LendahlUZimmermanLMcKayR1990CNS stem cells express a new class of intermediate filament proteinCell60585951689217
- LewisP1968Mitotic activity in the primate subependymal layer and the genesis of gliomasNature21797454966809
- LieDColamarinoSSongH2005Wnt signalling regulates adult hippocampal neurogenesisNature4371370516251967
- MabonRSvienHAdsonA1950Astrocytomas of the cerebellumArch Neurol Psychiatry647488
- MellinghoffIWangMVivancoI2005Molecular determinants of the response of glioblastomas to EGFR kinase inhibitorsN Engl J Med35320122416282176
- MerkleFTramontinAGarcía-VerdugoJ2004Radial glia give rise to adult neural stem cells in the subventricular zoneProc Natl Acad Sci U S A101175283215574494
- PalmerTMarkakisEWillhoiteA1999Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNSJ Neurosci1984879710493749
- PalmerTWillhoiteAGageF2000Vascular niche for adult hippocampal neurogenesisJ Comp Neurol4254799410975875
- PurowBHaqueRNoelM2005Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferationCancer Res6523536315781650
- RasheedBWiltshireRBignerS1999Molecular pathogenesis of malignant gliomasCurr Opin Oncol11162710328589
- RatherL1978The Genesis of Cancer: A Study in the History of IdeasBaltimore MDJohns Hopkins University Press
- ReyaTCleversH2005Wnt signalling in stem cells and cancerNature4348435015829953
- RothWWild-BodeCPlattenM2000Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cellsOncogene1942102010980594
- SakariassenPImmervollHChekenyaM2007Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversiesNeoplasia98829218030356
- SanaiNAlvarez-BuyllaABergerM2005Neural stem cells and the origin of gliomasN Engl J Med3538112216120861
- SeriBGarcía-VerdugoJCollado-MorenteL2004Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrusJ Comp Neurol4783597815384070
- ShenQGoderieSJinL2004Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cellsScience30413384015060285
- ShoshanYNishiyamaAChangA1999Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumorsProc Natl Acad Sci U S A9610361610468613
- SinghSHawkinsCClarkeI2004Identification of human brain tumour initiating cellsNature43239640115549107
- SmythGSternK1938Tumors of the thalamus: a clinico-pathological studyBrain6133974
- TaylorMPoppletonHFullerC2005Radial glia cells are candidate stem cells of ependymomaCancer Cell83233516226707
- TohyamaTLeeVRorkeL1992Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cellsLab Invest66303131538585
- UhrbomLDaiCCelestinoJ2002Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated AktCancer Res625551812359767
- ValtzNHayesTNorregaardT1991An embryonic origin for medulloblastomaNew Biol3364712065021
- VescoviAGalliRReynoldsB2006Brain tumour stem cellsNat Rev Cancer64253616723989
- VescoviAReynoldsBFraserD1993bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cellsNeuron11951668240816
- VickNLinMBignerD1977The role of the subependymal plate in glial tumorigenesisActa Neuropathol (Berl)406371199034
- Wechsler-ReyaRScottM2001The developmental biology of brain tumorsAnnu Rev Neurosci2438542811283316