Abstract
Alefacept is the first biological agent approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe chronic plaque psoriasis. It is a full human fusion protein binding to CD2 on T cells. With its dual mechanism of action, alefacept blocks the interaction between the leukocyte-function-associated antigen (LFA)-3 and CD2 and thereby impedes the activation and proliferation of T cells. In addition, alefacept induces apoptosis of activated memory T cells. This paper presents an overview about the clinical studies on alefacept, its mechanism of action, and the results of the clinical trials focused on efficacy and safety of alefacept in different populations. Further on, data available on the use of alefacept in combination with other therapeutic agents are discussed.
Introduction
Psoriasis is a chronic, inflammatory skin disorder, which affects up to 2% of the population worldwide (CitationChristophers 2001). It has been shown that genetic and environmental factors influence the severity of the disease (CitationElder et al 2001; CitationLebwohl 2003). The most common type, chronic plaque psoriasis, shows well demarked plaques covered by silver scales which are often located symmetrically and bilaterally. The extensor surfaces (elbows and knees), the lower back, scalp, and the nails are involved most often. In severe cases the plaques can affect the whole body. Approximately 30% of the patients suffer from psoriatic arthritis, which is an inflammatory, sero-negative arthritis with variable course (CitationKorman and Moul 2005. Mild forms of psoriasis can be controled by topical treatment (such as topical steroids, vitamin D derivates, selective retinoids, anthralins), whereas the therapy of moderate to severe forms consists of phototherapy (ultraviolet B light or psoralen plus ultraviolet A light) combined with a variety of systemic treatment forms, such as methotrexate, oral retinoids, cyclosporine, 6-azathioprine, and fumaric acid. However, all of these therapeutic options are limited: topical treatment is unsuitable to treat larger areas; chronic steroid use has common side-effects, such as skin atrophy, striae, and teleangiectasiae. Phototherapy can lead to photo-aging of the skin and to an increased risk of skin cancer (CitationKorman and Moul 2005. Besides that, long-term use of the systemic agents is limited by serious side-effects including myelosuppression, hepatotoxicity, impairment of the renal function and teratogenicity (CitationLuba and Stulberg 2006).
Psoriasis is regarded as a Th1 pre-dominated inflammatory autoimmune disease. It is supposed that after contact with an unknown antigen, a subset of T cells develops into memory CD4+ and CD8+ T cells. These cells proliferate and migrate from the lymph nodes to the skin where they initiate a cutaneous inflammatory reaction and the production of pro-inflammatory mediators (CitationKrueger 2002).
Alefacept: mechanisms of action
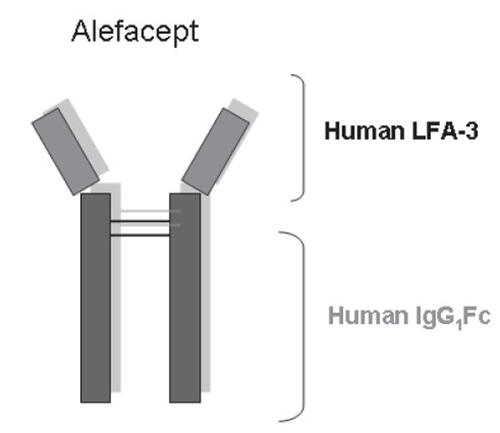
Alefacept (Amevive®, Astellas Pharma US, Inc.) is a full dimeric human fusion protein, which consists of an extracellular portion of the human leukocyte function antigen-3 (LFA-3) fused to the Fc portion of immunoglobulin G1 ().
Figure 1 Overview of the structure of the human fusion protein alefacept.
Abbreviations: IgG, immunoglobulin G; LFA, leukocyte function antigen.
Two main signals are required to initiate the activation, proliferation, and cytokine secretion of T cells (CitationRobert and Kupper 1999: (1) The presentation of an antigen in association with the major histocompatibility class complex (MHC) of antigen presenting cells (APC) to the T cell receptor (TCR) combined with (2) the activation of T cells via costimulatory signals by APC which can be mediated by bridging of the lymphocyte function antigen (LFA)-3 on APC and CD2 on T cells.
Alefacept binds competitively to the CD2 on the surface of T cells with the LFA-3 portion of the drug and efficiently interferes with LFA-3/CD2 binding and thereby T cell activation (CitationMiller et al 1993), whereas the Fc portion of alefacept engages the immunoglobulin receptor FcγRIII on the surface of natural killer cells resulting in apoptosis of specific memory T cell subsets (CitationMajeau et al 1994). Since the CD2 expression is higher on memory than on naïve T cells (CitationSanders et al 1988, alefacept binds mainly to memory T cells and induces a selective reduction of specific T cell subtypes by apoptosis. This means that alefacept can inhibit T cell activation on the one hand and selectively deplete memory T cells on the other hand (CitationLangley et al 2005).
Pharmacokinetics and pharmacodynamics of alefacept
Alefacept is detectable 6 hours after intramuscular injection in the sera of the patients and the concentration peaks after a time period of 24–192 hours. Once absorption is complete, the elimination has a half-life of approximately 12 days (CitationVaishnaw and TenHoor 2002) and the excretion follows a first order elimination (CitationLiu et al 2004). The effect of renal or hepatic impairment on the pharmacokinetic of alefacept has not been evaluated in detail yet.
Following its mechanism of action alefacept reduces circulating memory T cells (CD4+CD45RO+ and CD8+CD45RO+) thereby decreasing the count of total lymphocytes, whereas the number of naïve T cells (CD4+CD45RA+ and CD8+CD45RA+) remains approximately normal. Additionally, alefacept does not have any functional effect on CD19+ B cells or CD16+/CD56+ natural killer (NK) cells (CitationGordon et al 2003 CitationOrtonne et al 2003). The selective effect of alefacept on memory T cells is of particular immunologic importance, since it has been shown previously that the reduction of pathogenic memory T cells goes along with the improvement of psoriasis (CitationEllis Krueger 2001; CitationOrtonne et al 2003). Most importantly, in patients receiving multiple doses of alefacept no cumulative effect mirrored by further reduction of the total number of lymphocytes or the number of T cell subsets has been observed (CitationGordon et al 2003 CitationOrtonne et al 2003 CitationLowe et al 2003).
Dosage and administration of alefacept
Alefacept administration was studied as a weekly intravenous (i.v.) 7.5 mg administration as well as intramuscular (i.m.) 15 mg injection. In different studies both formulations showed similar safety and efficacy profiles (CitationVaishnaw and TenHoor 2002).
The currently recommended dose is 7.5 mg given once a week as an i.v. bolus or 15 mg administered i.m. once a week for 12 weeks and an interval of 12 weeks without treatment between courses. Although an i.m. application is more convenient for drug administration, the drug injection side reactions occur more often after i.m. than after i.v. application. Since both ways of administration require application by a health care professional, patients have to visit the doctor weekly. In particular for patients with low compliance these weekly visits ensure continuous drug applications and the control of the respective clinical response.
Recently an open-labeled study, which evaluated the bioequivalence of subcutaneous (s.c.) application compared with i.m. administration of alefacept (CitationSweetser et al 2006), showed very similar pharmacodynamic effects for both ways of application. Even the total number of lymphocytes and lymphocyte subsets was similar for both routes of administration, so that s.c. application of alefacept might offer a convenient alternative way of administration especially for compliant patients who are unable to organize weekly office visits and who want to self-administer the drug at home.
Efficacy of alefacept
In most of the studies the clinical efficacy of alefacept was evaluated with the help of the Psoriasis Area Severity Index (PASI), a score ranging from 0 (no psoriasis) to 72 (the most severe form of the disease possible) combining scores for the degree of erythema, induration, desquamation, and the body surface area affected (CitationLiu et al 2004).
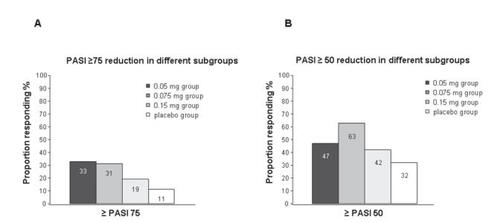
Different phase II and III clinical trials evaluated the efficacy of a single as well as of multiple courses of alefacept. In an early placebo-controlled, double-blind multi-center phase II study completed in 2001, 229 patients receiving a 30-second i.v. bolus alefacept at varying weight-adjusted doses (0.025, 0.075, and 0.150 mg/kg body weight) or placebo weekly for 12 weeks with a 12-week follow-up period have been examined (CitationEllis and Krueger 2001). According to the results of this study, a dosage of 0.075 mg alefacept was most effective. In 63% of the patients treated with 0.075 mg alefacept a >50% reduction of the PASI (PASI 50) was achieved and in about 31% of patients a 75% reduction of the PASI (PASI 75) was reached 12 weeks after treatment () (CitationEllis and Krueger 2001). In comparison with other studies, it has to be remembered that in this study the PASI achievements were based on cumulative success rates. Using the evaluation criterion of other studies, which means PASI 75 achieved at week 14, about 21% of patients achieved PASI 75 at week 14.
Figure 2 Schematic summary of studies on the reduction of the psoriasis area severity index (PASI) induced by alefacept treatment (from data of CitationEllis CN, Krueger GG. 2001) (A) PASI ≥75 and (B) PASI ≥50.
The first phase III study completed in 2002 evaluated the efficacy of 2 courses of alefacept (CitationKrueger et al 2002). 553 patients with chronic plaque psoriasis received two courses with i.v. application of a non-weight-adjusted dose of 7.5 mg alefacept or placebo once a week for 12 weeks each, followed by a 12-week phase without treatment. In this study a PASI 75 was achieved in 28% of the patients and a PASI 50 in 56% of the alefacept-treated patients during treatment and follow-up control after the first course. In patients receiving a second course, the efficacy increased significantly: in 40% of those patients a 75% reduction was achieved and in 71% of the patients a 50% or greater reduction of the PASI was reached (). In both courses the decrease of the PASI peaked 8 weeks after the treatment course.
Figure 3 Schematic summary of the study data on different courses of Alefacept treatment (from data of CitationKrueger et al 2002) (A), and results achieved with intramuscular application of different doses of alefacept versus placebo (from data of CitationLebwohl et al 2003) (B).
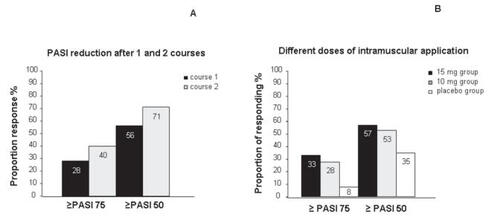
Concerning the efficacy of i.m. application of alefacept, an international, randomized, double-blind, placebo-controlled phase III study was completed in 2003 (CitationLebwohl et al 2003). In this study 507 patients were randomized to receive 2 different doses of alefacept (10 mg or 15 mg) or placebo administered i.m. once a week for a total of 12 weeks with a 12 week follow-up period. An overall PASI 50 response was achieved in 57% patients treated with alefacept 15 mg i.m., and a >50% reduction in PASI was achieved in 53% of patients treated with 10 mg i.m. in comparison with a 35% reduction of PASI in the placebo group. At least a 75% reduction in PASI throughout the study period has been achieved in 33% of patients treated with 15 mg i.m., in 28% of patients treated with 10 mg alefacept i.m., and in 8% of the placebo group (). Data are consistent with the results from earlier studies mentioned above, in which alefacept was administered by an i.v. bolus (CitationEllis and Krueger 2001; CitationKrueger et al 2002); therefore, the i.m. application of alefacept represents a convenient alternative route of administration. An overview of the results of studies on alefacept is provided in .
Table 1 Summary of studies on alefacept
Safety and tolerability of alefacept
During all studies the incidence of (opportunistic) infections and malignancies was monitored. The patients were also tested for development of specific antibodies against alefacept. In different phase II and III clinical trials the safety and tolerability of alefacept has been reported in detail (CitationKrueger et al 2002 CitationLebwohl et al 2003 CitationLowe et al 2003). The incidence of serious adverse events, discontinuations, infections, malignancies, and the development of anti-alefacept antibodies was low. No correlation was noted between the overall incidence of infections and decreased CD4+ T cell counts and no cases of significant sustained T cell suppression have been observed. Further, no opportunistic infections such as reactivation of herpes simplex virus have been observed (CitationLebwohl et al 2003). Most malignancies observed were cutaneous carcinomas such as basal cell and squamous cell carcinomas. The rate of malignancy was not greater than in the average population (CitationWong and Lebwohl 2003). The only side-effects observed in greater frequency in the group treated with alefacept were common colds occurring soon after treatment but limited to the early treatment phase (CitationKrueger et al 2002). In spite of the fact that alefacept leads to a reduction in T cell subsets, the comparable T cell responses of patients treated with alefacept and healthy individuals could be explained mainly by the fact that alefacept targets only pathogenic T cells (CitationGottlieb et al 2003).
In 2002 a safety report based on data of 1359 patients who have received i.v. or i.m. alefacept in placebo – controlled trials was published. The most common adverse events reported were headache, nasopharyngitis, upper respiratory tract infections, pruritus, flu-like symptoms, dizziness, and nausea, whereas the rate of adverse events was similar in alefacept treated patients and controls. In five patients, malignancies occurred concomitantly to treatment but turned out to be independent from the study drug. Additionally, no correlation has been observed between the overall incidence of infections and decreased CD4+ T cell counts.
Concerning the injection site reactions, only one case led to a discontinuation of the alefacept therapy. In a clinical trial in which alefacept was administered i.m., 16% of the patients treated with alefacept compared with 8% of the patients who received a placebo developed injection site reactions such as pain, inflammation, bleeding, oedema, unspecific reactions, or very rarely hypersensitivity (CitationScheinfeld 2005a).
To evaluate the effect of alefacept treatment on T cell mediated immune responses, primary and secondary immune responses to a harmless T cell-dependent neo-antigen (øX174, a bacteriophage, ie, a virus that infects bacteria) and acquired immune response to a recall antigen (tetanus toxoid) under alefacept-treatment have been evaluated in a prospective, multicenter, randomized parallel-group phase II clinical study published in 2003 (CitationGottlieb et al 2003). In this study it has been shown that a 12-week course of alefacept treatment had no effect on the primary or secondary antibody response in vitro. The in vitro response to both the neoantigen and the known antigen of T cells of patients treated with alefacept was comparable to the response of T cells of the control group.
Long-term efficacy and safety of alefacept
Concerning the remission times, a study accomplished in 2003 showed that a PASI 50 has been maintained for a median period of 7 months in a subgroup of 54 patients treated with alefacept (CitationLebwohl et al 2003; CitationPapp 2006). In another group of patients, a reduction of the PASI greater or equal to 50% was reached and maintained for a period of over 4 months.
Repeated treatment courses are often necessary in order to control the chronic relapsing course of psoriasis. Patients treated with more than one 12-week course showed a longer duration of response compared with those treated with a single course only. In different phase III studies, the safety and efficacy of up to two courses of alefacept has been evaluated. Data presented in 2005 at the 63rd Annual Meeting of the American Academy of Dermatology showed an increase of the PASI 75 response rates for alefacept given i.v. from 29% during the first course of therapy (n = 521) to 54% during the fifth course (n = 39). PASI 50 was achieved in 56% of patients during the first course and was enhanced to a maximum of 74% of patients during the fifth course (CitationMenter et al 2006). However, because of the fact that the number of patients involved decreased from 521 to 39 it cannot be excluded that only responders have been positively selected in this study and non-responders dropped out, which would profoundly influence the efficacy of the treatment. This should be kept in mind when evaluating the overall efficacy of such a therapy for the average cohort of psoriasis patients.
The result of long-term use of the i.m. formulation was evaluated using the physician global assessment scale (PGA), a seven point scale, which the physician uses to assess the severity of the psoriasis plaques. 21% of the patients (n = 457) have been classified using PGA as cleared or almost cleared after one course of alefacept given i.m. The PGA response increased to 41% during course 4 (n = 100). However, during course 5 the response rate decreased to less than 30% (n = 50). Therefore, further studies need to evaluate if there is a possible limitation to the improvement of the disease after several courses of alefacept treatment and if it would be helpful to select only responders for the treatment, and, if so, which read-out parameters for the selection of responders and differentiation from non-responders would be the best in order to save time and costs by not treating non-responders for a long time.
In 2005, an integrated analysis of data from 13 clinical trials with 1869 patients affected by chronic plaque psoriasis who had received up to 9 courses of alefacept over a 5-year period was published. In this study the safety and tolerability of alefacept for long-term treatment was assessed (CitationGoffe et al 2005). Consistent with early reports, the most common infectious side-effects, which remained similar between courses, were headache (14%) nasopharyngitis (10%), influenza (8%), upper respiratory tract infections (8%), and pruritus (8%). The infections were mild to moderate and resolved with conventional treatment.
Consistent with its composition as a full human fusion protein, alefacept has a low immunogenicity although the development of antibodies against alefacept is possible. Less than 1% of the patients developed a low antibody titer (<1:40), but consistent with previous studies (CitationLebwohl et al 2003) anti-alefacept antibodies were not associated with hypersensitivity, did not amplify the treatment course, and most probably did not neutralize the efficacy of the drug. Additionally, no increase in the number of patients developing antibodies against alefacept occurred during the treatment with alefacept over multiple courses. The data show that the incidence of adverse effects and discontinuations because of adverse effects, infections, and malignancies does not increase in patients treated with multiple courses in comparison with patients with one course. Additionally, no evidence for a rebound phenomenon or worsening of psoriasis with discontinuation of alefacept has been reported. Despite these positive results there are no sufficient data to exclude a potential negative influence of a long-term use of alefacept. Therefore, further studies and observations on the long-term safety and efficacy of alefacept therapy are indispensable (CitationPapp 2006).
Safety and efficacy of alefacept in specific risk-groups
Recently the safety and efficacy of alefacept in patient groups at higher risk for adverse events (old, obese, and diabetic patients) was evaluated (CitationGottlieb et al 2005). Based on the data of these studies, the safety profile of alefacept administered to specific risk-groups did not differ from the safety profile of the general population. Even multiple courses of alefacept did not lead to increased adverse effects in high-risk patients. However, due to the limited sample sizes additional data are required in order to confirm these results. With regard to the increasing incidence of infections and malignancies with increasing age, careful evaluation of the patients’ history and associated diseases is recommended, particularly in older patients.
In 2005 a report of two patients who received alefacept while testing positive for hepatitis C virus (HCV) was published (CitationThaci et al 2005). Because of its mechanism of action, alefacept led to transient decreases in CD4+ and CD8+ T cell counts in these patients. However, these reductions did not increase the HCV viral load or exacerbate the HCV infection. In both cases liver enzymes remained stable throughout the treatment and follow-up periods. The use of alefacept in patients suffering from an active or reactive chronic viral infection has to be evaluated carefully in future, considering the risk of reactivation or aggravation of the existing infection by an immunosuppressive agent like alefacept.
A small number of women included in clinical trials became pregnant while being treated with alefacept. Although there have been no reports of any birth defects caused by alefacept, the effects on the fetal immune system and the post-natal immune functions in humans are still unknown.
Safety and efficacy of alefacept combination therapy
The safety and efficacy of alefacept in combination with various therapies including topical steroids, methotrexate, cyclosporine, narrowband and broadband ultraviolet B light (UVB), systemic retinoids, vitamin D derivatives, and prednisone is currently being evaluated in an ongoing international study, in which approximately 400 patients with chronic plaque psoriasis receive multiple courses of alefacept administered i.m. in combination with different other therapies CitationGordon et al 2005. In general, patients who are candidates for alefacept combination therapy should have required systemic therapy before because of the severity of their disease which is not responsive to topical therapy and should have normal circulating CD4+ counts. Preliminary data available on effectiveness of alefacept in combination with topical therapies, UVB, oral retinoids, methotrexate, or cyclosporin did not show any increase of the adverse events compared with alefacept monotherapy (CitationGordon et al 2005). The incidence of serious infections remained low (0.7%), and no opportunistic infections and no increased liver toxicity from methotrexate or renal toxicity from cyclosporine has been observed (CitationGordon et al 2005).
A combination of etanercept, a recombinant tumor-necrosis factor (TNF)-α fusion protein, and alefacept was evaluated in three patients who did not show sufficient clinical improvement under etanercept monotherapy. After the addition of alefacept the clinical response increased (CitationKrell 2006), indicating that even the combination of different biologicals with synergistic modes of action might be successful in single cases.
Side-effects and contraindications of alefacept
Because of the important role T cells play as defender cells of the immune system, a selective reduction of specific T cell subsets by alefacept might predispose patients receiving alefacept to various infections and to the development of malignancies. Although no evidence for an increased risk of malignancies has been reported, it will take long-term follow-up studies to finally determine whether or not the risk of malignancies increases after long-term treatment with alefacept. Alefacept is contraindicated in all patients with a history of systemic malignancies such as lymphoma or leukemia. If a patient develops a malignancy under alefacept treatment the administration of the drug should be discontinued. Due to the fact that treatment with alefacept results in a selective reduction of memory T cells, monitoring of T cell counts is recommended. The US product labeling recommends monitoring of CD4+ T cell counts every 2 weeks during the 12-week treatment course. At the onset of the alefacept treatment the CD4+ lymphocyte counts should be at least ≥400 cells/μL. If these counts decrease below 250 cell/μL the dose should be reduced and monitoring every week instituted. If the counts remain below this level for a month, alefacept should be discontinued (CitationBiogen Idec Inc. 2005).
Alefacept should not be administered to patients infected with human immunodeficiency virus (HIV) because of a possible disease acceleration and complication after a significant reduction of CD4+ T cells. Further, the use of alefacept is contraindicated if there is a known hypersensitivity to alefacept. In breast-feeding mothers application is not recommended because of the potential excretion of the drug into human milk (CitationBiogen Idec Inc. 2005).
Alefacept in psoriatic arthritis
In 2002 a prospective, open-labeled pilot study was performed to evaluate the clinical effect and changes in the synovium of patients suffering from active psoriatic arthritis (PsA) receiving treatment with alefacept (CitationKraan et al 2002). A dosage of 7.5 mg alefacept was administered i.v. in weekly intervals during a total time period of 12 weeks. At baseline as well as after 4 and 12 weeks clinical evaluations were conducted, which included an examination of the joints for swelling and tenderness, morning stiffness, and pain which was classified by a visual analogue scale. At the same time arthroscopic synovial biopsies were taken. They showed an improvement in the clinical picture as well as changes of the inflammation in synovial tissue after treatment with alefacept.
Alefacept in palmoplantar pustular psoriasis (PPP)
Palmoplantar pustular psoriasis (PPP) is a form of psoriasis characterized by recurrent crops of sterile pustules on the palms and soles, which erupt repeatedly over months or years. Many different treatments have been used for PPP but none has been generally accepted as being reliably effective. Recently a case of successful treatment of a patient suffering from a severe form of PPP with alefacept has been reported (CitationYeung-Yue et al 2005). After treatment with a total of 17 doses of alefacept the lesions on his palms and soles improved: erythema as well as the number of pustules was clearly regressive. Twelve weeks after the last alefacept treatment nearly all lesions were cleared. The clinical improvement remained for a minimum of 19 weeks after the last application of alefacept. An additional report described the successful use of alefacept in 2 patients with extensive and recalcitrant palmoplantar psoriasis (CitationMyers et al 2005). In one of the two patients, clinical improvement could be observed after just 5 doses of alefacept. After the completion of the first course (12 doses of 15 mg alefacept weekly) this patient showed further improvement. The second patient improved after the first course of therapy, too. However, 5 weeks after the last application, diffuse erythema of the pales and soles with moderate scaling occurred and substantial improvement could be achieved only after a second course of alefacept treatment. In order to confirm these data of alefacept as a useful treatment alternative for patients suffering from palmoplantar psoriasis, controlled clinical trials are necessary.
Alefacept in nail psoriasis
Involvement of the nails is common in patients with psoriasis. Psoriatic nail disease can be refractory to treatment because therapeutic options are limited. Recently an open-labeled study aimed to determine the efficacy and safety of alefacept in patients suffering from nail psoriasis (CitationParrish et al 2006). Fifteen patients with moderate to severe nail psoriasis measured according to the Nail Psoriasis Severity Index (NPSI) were treated with alefacept administered i.m. 33% of the treated patients reached a reduction of NPSI of at least 50% whereas the median decrease was 38%. These data show that alefacept might represent an additional therapeutic option for nail psoriasis but further studies are necessary in order to confirm the positive results of this study.
Effect of alefacept on the quality of life (QOL) of psoriasis patient’;s
Psoriasis can seriously affect patient’s QOL. The impact of the chronic disease on social relations, psychological status, and daily activities was evaluated recently by the National Psoriasis Foundation. In a large inquiry (more than 17,000 respondents) a profound influence of psoriasis on patients’ QOL could be shown, including adverse psychosocial effects, impaired daily activities, anxiety, and depression (CitationKrueger et al 2001. Many patients reported feeling restrained in their lifestyle. Approximately 50% of the patients were not satisfied with their therapies and 75% of the patients with severe disease were frustrated with the lack of efficacy on their current treatment.
In phase II and III studies with alefacept, QOL was measured with the dermatology-specific QOL index, a practical questionnaire technique (DLQI) (CitationFinlay and Khan 1994). Significant improvements of QOL in patients treated with alefacept compared with placebo treatment could be observed. In a phase II study accomplished in 2003 an association between the clinical effect of alefacept on psoriasis and improvement in patients’ QOL could be shown. Patients treated with alefacept had significantly greater improvement of QOL compared with patients receiving placebo (CitationEllis et al 2003 Citationvan de Kerkhof et al 2005). In a phase III study alefacept also improved QOL in patients with chronic plaque psoriasis. This benefit was maintained for at least 12 weeks after treatment. A second course of alefacept was demonstrated to lead to a further increase of the QOL in these patients (CitationFinlay et al 2003 CitationFeldman et al 2004 Citationvan de Kerkhof et al 2005).
Conclusion
Clinical data from different studies confirm the efficacy of alefacept, which improves psoriasis with durable remission without any significant impact on the immune function of T cells. The data currently available show a favorable safety profile over short-term treatment (CitationGoffe et al 2005) with few apparent side-effects and no clear link to serious infections, malignancies, or other serious adverse events (CitationScheinfeld 2005a). Until now no correlation between the reduced CD4+ and CD8+ T cell counts under alefacept treatment and an increased risk for the development of serious infections or malignancies has been observed. Further, alefacept has been shown to be well-tolerated in a broad spectrum of patients’ including old, diabetic, and obese patients (CitationGottlieb et al 2005). Unfortunately the number of non-responders is relatively high and the long-term remission has been shown only in patients achieving PASI 75, which means only one third of all treated patients. Clinical studies should further evaluate the possibility of coupling alefacept with topical or ultraviolet light therapy in order to enhance the patient’s clinical results.
Even if most of the studies published on the treatment of chronic plaque psoriasis seem to provide promising data, it has to be remembered that the significance of these studies might be limited by high drop-out rates which may bias analyses because patients not responding to treatment may selectively drop out of studies, skewing the results to good responses. Further, it is always possible that studies with lower response rates and less success might remain unpublished.
In summary alefacept represents a safe alternative therapeutic option for the treatment of adult people suffering from moderate to severe chronic plaque psoriasis with contraindications or resistance to traditional systemic therapies. Potential limitations for the use of alefacept include high costs of treatment, insufficient data available for long-term follow-up, a high number of non-responders, and the fact that it often takes several months of treatment to discover that the patient does not respond to alefacept because of missing valuable and early read-out parameters for responders and non-responders. Additionally it should be noted that the transient decrease of CD4+ T cells under therapy requires monitoring of lymphocytes counts as well as regular re-evaluation of potential signs of infections or malignancies during treatment with alefacept.
Ongoing research should focus on the use of alefacept in combination with other treatment forms for specific recalcitrant cases, the expansion of safety assessment studies in patients of different risk groups, and the efficacy of alefacept treatment in patients with other psoriasis-related disorders such as psoriasis arthritis or nail psoriasis (CitationKorver et al 2006). Much effort should be put into identifying parameters to define subgroups of patients with a high probability to respond or not respond to the treatment at the earliest possible time point during treatment or, if applicable, before the start of treatment.
Abbreviations
| APC | = | antigen presenting cell |
| DLQI | = | dermatology-specific quality of life index |
| FcγR | = | Fc gamma receptor |
| i.m. | = | intramuscular |
| i.v. | = | intravenous |
| LFA | = | lymphocyte function antigen |
| MHC | = | major histocompatibility complex |
| NPSI | = | Nail Psoriasis Severity Index |
| PASI | = | Psoriasis Area Severity Index |
| LTP | = | long-term potentiation |
| PGA | = | physician global assessment scale |
| PPP | = | palmoplantar pustular psoriasis |
| QOL | = | quality of life |
| s.c. | = | subcutaneous |
| TCR | = | T cell receptor |
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG NO454/1-4, DFG NO454/2-3, and SFB704 TPA4) and a BONFOR grant of the University of Bonn. N.N. is supported by a Heisenberg-Fellowship of the DFG NO454/3-1.
References
- Biogen Idec IncPrescription information: Amevive (Alefacept)2005Cambridge, MA, U S A
- ChristophersEPsoriasis – epidemiology and clinical spectrumClin Exp Dermatol2001263142011422182
- ElderJTNairRPHenselerTThe genetics of psoriasis 2001: the odyssey continuesArch Dermatol200113714475411708947
- EllisCNKruegerGGTreatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytesN Engl J Med20013452485511474662
- EllisCNMordinMMAdlerEYEffects of alefacept on health-related quality of life in patients with psoriasis: results from a randomized, placebo-controlled phase II trialAm J Clin Dermatol20034131912553852
- FeldmanSRMenterAKooJYImproved health-related quality of life following a randomized controlled trial of alefacept treatment in patients with chronic plaque psoriasisBr J Dermatol20041503172614996104
- FinlayAYKhanGKDermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical useClin Exp Dermatol19941921068033378
- FinlayAYSalekMSHaneyJIntramuscular alefacept improves health-related quality of life in patients with chronic plaque psoriasisDermatology20032063071512771471
- GoffeBPappKGrattonDAn integrated analysis of thirteen trials summarizing the long-term safety of alefacept in psoriasis patients who have received up to nine courses of therapyClin Ther20052719122116507377
- GordonKBKruegerGGvan de KerkohfPEfficacy and safety of multiple courses of alefacept in combination with other psoriasis therapiesJ Am Acad Dermatol20055218315627120
- GordonKBLangleyRGRemittive effects of intramuscular alefacept in psoriasisJ Drugs Dermatol20032624814711140
- GordonKBVaishnawAKO’GormanTreatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T cell countsArch Dermatol200313915637014676071
- GottliebABBoehnckeWHDarifMSafety and efficacy of alefacept in elderly patients and other special populationsJ Drugs Dermatol200547182416302557
- GottliebABCasaleTBFrankelECD4+ T cell-directed antibody responses are maintained in patients with psoriasis receiving alefacept: results of a randomized studyJ Am Acad Dermatol2003498162514576659
- HodakEDavidMAlefacept: a review of the literature and practical guidelines for managementDermatol Ther2004173839215379773
- KormanNJMoulDKAlefacept for the treatment of psoriasis: a review of the current literature and practical suggestions for everyday clinical useSemin Cutan Med Surg20052410815900794
- KorverJEvan de KerkhofPCPaschMCAlefacept treatment of psoriatic nail disease: how severe should nail psoriasis be?J Am Acad Dermatol200654742316546613
- KraanMCvan KuijkAWDinantHJAlefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritisArthritis Rheum20024627768412384938
- KrellJMUse of alefacept and etanercept in 3 patients whose psoriasis failed to respond to etanerceptJ Am Acad Dermatol200654109910116713481
- KruegerGKooJLebwohlMThe impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership surveyArch Dermatol2001137280411255325
- KruegerGGPappKAStoughDBA randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasisJ Am Acad, Dermatol2002478213312451365
- KruegerJGThe immunologic basis for the treatment of psoriasis with new biologic agentsJ Am Acad Dermatol20024612311756941
- LangleyRGChermanAMGuptaAKAlefacept: an expert review concerning the treatment of psoriasisExpert Opin Pharmacother2005623273316218892
- LebwohlMPsoriasisLancet2003361119720412686053
- LebwohlMChristophersELangleyRAn international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasisArch Dermatol20031397192712810502
- LiuCMMcKennaJKKruegerGGAlefacept: a novel biologic in the treatment of psoriasisDrugs Today2004409617415645008
- LoweNJGonzalezJBagelJRepeat courses of intravenous alefacept in patients with chronic plaque psoriasis provide consistent safety and efficacyInt J Dermatol2003422243012653922
- LubaKMStulbergDLChronic plaque psoriasisAm Fam Physician2006736364416506705
- MajeauGRMeierWJimmoBMechanism of lymphocyte function-associated molecule 3-Ig fusion proteins inhibition of T cell responses. Structure/function analysis in vitro and in human CD2 transgenic miceJ Immunol19941522753677511625
- MenterACatherJCBakerDThe efficacy of multiple courses of alefacept in patients with moderate to severe chronic plaque psoriasisJ Am Acad Dermatol20065461316384756
- MillerGTHochmanPSMeierWSpecific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responsesJ Exp Med1993178211227686212
- MyersWChristiansenLGottliebABTreatment of palmoplantar psoriasis with intramuscular alefaceptJ Am Acad Dermatol200553S127916021161
- OrtonneJPLebwohlMEm GriffithsGCAlefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasisEur J Dermatol2003131172312695125
- PappKAThe long-term efficacy and safety of new biological therapies for psoriasisArch Dermatol Res200629871516691429
- ParrishCASoberaJORobbinsCMAlefacept in the treatment of psoriatic nail disease: a proof of concept studyJ Drugs Dermatol200653394016673801
- RobertCKupperTSInflammatory skin diseases, T cells, and immune surveillanceN Engl J Med199934118172810588968
- SandersMEMakgobaMWSharrowSOHuman memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma productionJ Immunol1988140140172894392
- ScheinfeldNAlefacept: a safety profileExpert Opin Drug Saf2005a49758516255657
- SweetserMTWoodworthJSwanSResults of a randomized open-label crossover study of the bioequivalence of subcutaneous versus intramuscular administration of alefaceptDermatol Online J200612116638415
- ThaciDPatzoldSKaufmannRTreatment of psoriasis with alefacept in patients with hepatitis C infection: a report of two casesBr J Dermatol200515210485015888169
- VaishnawAKTenHoorCNPharmacokinetics, biologic activity, and tolerability of alefacept by intravenous and intramuscular administrationJ Pharmacokinet Pharmacodyn2002294152612795239
- van de KerkhofPGriffithsCEChristophersEAlefacept in the treatment of psoriasis in patients for whom conventional therapies are inadequateDermatology20052112566316205071
- WongVKLebwohlMThe use of alefacept in the treatment of psoriasisSkin Therapy Lett2003812714610613
- Yeung-YueKAronsonPMurakawaGClinical improvment of palmoplantar pustular psoriasis with alefaceptJ Am Acad Dermatol200552Proceeding of the 63rd Annual Meeting of the American Academy of DermatologyNew Orleans, LA, USA179