Abstract
5-fluorouracil/leucovorin, with or without oxaliplatin or irinotecan, is the most widely used treatment for the metastatic as well for the adjuvant setting of colorectal cancer. These agents are administered intravenously (by bolus or infusion), thereby causing significant inconvenience to patients. Capecitabine, an oral fluoropyrimidine, has been demonstrated to be at least as effective as bolus 5-fluorouracil/leucovorin in terms of time to disease progression, time to treatment failure, and overall survival, but achieves significantly higher response rates and has the advantage of oral administration. In addition, capecitabine has improved tolerability with a significantly lower incidence of stomatitis, nausea, and alopecia than 5-fluorouracil/leucovorin. Clinical trials have shown that combination therapy with capecitabine and either irinotecan or oxaliplatin is effective and well tolerated. The combination of capecitabine plus oxaliplatin, with or without bevacizumab, could represent the new standard of care for metastatic as well as surgically resected high-risk stage II and III colon cancer patients. Some pharmacoeconomic analyses have highlighted that capecitabine plus oxaliplatin results in cost savings compared with 5-fluorouracil/leucovorin plus oxaliplatin.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies occurring in men and women in the Western world, and is associated with a high rate of mortality (CitationJemal et al 2005). Approximately 55% of patients present with advanced disease (ie, cancer that is metastatic or so locally invasive that surgical resection is insufficient for cure). Diagnosis of CRC at a later stage results in 5-year survival rates of 67% in patients whose cancer has spread to regional lymph nodes, and 10%–30% for those in whom CRC has spread to distant organs such as the liver.
For patients with metastatic disease, surgery has a limited role, because only when it allows a complete resection of organ metastases does it have a relevant impact on survival of patients. In the remaining cases, chemotherapy, although not curative, may increase the time to disease progression and the overall survival.
5-fluorouracil (5FU), the fluorinated analogue of uracil originally synthesized in 1957 as an anticancer drug, has shown activity in a variety of solid tumours. Cytotoxicity of 5FU resulted from both the incorporation of 5FU into RNA, and the depletion of thymidine following the binding of 5FU with the enzyme thymidilate synthase (TS). The binding of 5FU to TS has been demonstrated to increase and stabilize in the presence of leucovorin (LV).
The combination of 5FU plus LV has represented the mainstay for the treatment of metastatic colorectal cancer for some decades. Indeed, several trials have investigated different doses and schedules of delivery of such combination in comparison with single agent 5FU. A recent meta-analysis of such trials revealed that the addition of LV to 5FU was able to significantly increase not only the response rate (RR), but also the overall survival (OS) of patients treated with the combination (CitationThe Meta-Analysis Group in Cancer 2004).
As for the adjuvant setting, up to few years ago a treatment including 5FU 450 mg/m2 plus LV 20 mg/m2, both given i.v. for 5 days monthly for 6 cycles (Mayo Clinic regimen), represented the standard adjuvant treatment for surgically resected stage III and high-risk stage II colon cancer (CitationGill et al 2004). More recently, a biweekly regimen with LV 200 mg/m2 infused over 2 hours plus mixed bolus (400 mg/m2) and infusional (600 mg/m2 in 22 hours) 5FU for 2 days (LV5FU2 regimen) has replaced in clinical practice (at least in Europe) the bolus regimen, because of its better tolerability (CitationAndré et al 2003).
Capecitabine as single-agent in the management of metastatic CRC
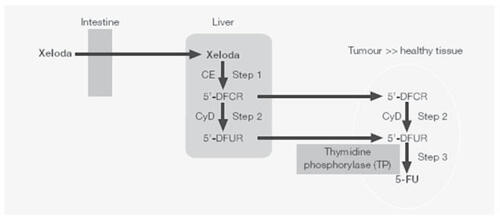
Capecitabine is a pro-drug of 5FU. Assumed orally, its bioavailability is almost 100%, and it exhibits a linear increase of its Cmax and AUC with dosage increases. After a standard dosage of 1250 mg/m2, the peak plasma concentration is achieved in 1.5–2 hours. Capecitabine is metabolized to 5FU through 3 metabolic steps. Once the drug is absorbed through the intestine, the carboxylesterase of the liver cells converts capecitabine to 5′-deoxy-5-fluorocytidine (5′-DFCR). 5′-DFCR is then metabolized to 5′-deoxy-5-fluorouridine (5′-DFUR) by cytidine deaminase, an ubiquitous enzyme with high concentration in the liver, plasma, and tumour tissue. Finally, 5′-DFUR is converted to the active drug 5FU by thymidine phosphorylase, which is present in amounts 3–10 times higher in various solid tumours compared with the normal adjacent tissue (). The higher concentration of thymidine phosphorylase in tumour tissues leads to a final concentration of 5FU that is 3 times higher than in normal tissues (CitationIshikawa et al 1998; CitationMiwa et al 1998).
Figure 1 Enzymatic conversion of capecitabine to 5FU.
Abbreviations: CE, carboxylesterase; CyD, cytidine deaminase.
Based on these assumptions, capecitabine has been assessed in patients affected by metastatic CRC. After a phase II randomized trial, defining the recommended dosage and schedule for capecitabine as 1250 mg/m2 twice daily for 2 consecutive weeks and 1 week of rest every 3 weeks (CitationVan Cutsem et al 2000), 2 phase III randomized trials compared capecitabine as a single agent with the standard 5FU/LV treatment (Mayo Clinic) regimen in patients with metastatic CRC. Both trials had the same primary end-point, ie, to demonstrate that capecitabine was at least as effective as 5FU/LV in terms of RR. Capecitabine was assumed at the recommended total daily dose of 2500 mg/m2 for 2 weeks of treatment and 1 week of rest, while 5FU was given at 425 mg/qm2 i.v. preceded by LV 20 mg/m2 i.v., for 5 days, recycling every 4 weeks. In one of these trials, capecitabine actually resulted to produce a significantly greater RR than the Mayo Clinic regimen (25.8% vs 11.6%) (CitationHoff et al 2001). Median failure-free survival (FFS) (4.1 vs 3.1 months), progression-free survival (PFS) (4.3 vs 4.7 months), and OS (12.5 vs 13.3 months) were not statistically different, supporting the conclusion that capecitabine is at least as effective as the 5FU/LV regimen also for these outcomes. As for safety, patients treated with capecitabine were less likely to experience clinical grade 4 toxicity than those treated with 5FU/LV (2.7% vs 4.8%). Incidence of severe diarrhea was similar (14% vs 15%). Conversely, patients treated with 5FU/LV experienced more severe stomatitis (16% vs 3%), while capecitabine led to a higher incidence of hand and foot syndrome (18% vs 1%). In the other trial (CitationVan Cutsem et al 2001), RR was 18.9% for capecitabine and 15% for 5FU/LV, proving that capecitabine was at least equivalent to the Mayo Clinic regimen. No difference in median FFS (4.2 vs 4.0 months) or PFS (5.2 vs 4.7 months) were observed between the two groups. Survival was also equivalent, with a median of 13.2 months for the capecitabine group, and 12.1 months for 5FU/LV group. Also in this trial, severe stomatitis occurred more frequently with 5FU/LV (13.3% vs 1.3%), while proportion of patients suffering from severe diarrhea (10.7% vs 10.4%) was similar. Grade 3 hand-foot syndrome was seen in 16.2% vs 0.3% of patients. A pooled analysis of these two studies underlined that significantly fewer patients required hospitalization for treatment-related adverse events (11.6% vs 18.8%), and fewer physician visits were required for treatment administration with capecitabine than with 5FU/LV (4 vs 15 in a 12-week period) (CitationVan Cutsem et al 2004) ().
Table 1 Summary of randomized trials comparing capecitabine with 5FU/LV (Mayo Clinic) regimen in metastatic colon cancer
Furthermore, capecitabine as been specifically assessed for the treatment of elderly (aged ≥70 years) patients in a Spanish phase II trial. The dosage was the same as for younger patients (ie, 1250 mg/m2 twice daily orally on days 1–14 every 3 weeks), but it was reduced to 950 mg/m2 in the presence of a creatinine clearance of 30–50 mL/min. The RR was 24% among 51 treated patients, and median times to disease progression and overall survival were 7 months and 11 months, respectively. Treatment was extremely well tolerated, with grade 3 and 4 adverse events (mainly, diarrhea, hand-foot syndrome, and thrombocytopenia) observed in only 6 patients (12%). Of note, 14 patients (40%) of 35 assessable patients had a clinical benefit for a median length of 4 months (CitationFeliu et al 2005).
These findings, along with the good compliance of patients for an oral therapy, represented a strong rationale for combining capecitabine with either oxaliplatin or irinotecan in the first line treatment of metastatic colorectal cancer.
Indeed, irinotecan was the first drug that, in combination with 5FU/LV, was demonstrated to increase the RR, and to improve the PFS and OS of patients, in comparison with 5FU/LV alone. In the Saltz et al study (CitationSaltz et al 2000), a weekly for 4 weeks every 6 weeks regimen of irinotecan 125 mg/m2 plus 5FU 500 mg/m2 and LV 20 mg/m2, given on the same day, was compared with the standard daily for 5 consecutive days every 4 weeks (Mayo Clinic) regimen. In this trial, the combination regimen produced a significantly greater RR (39% vs 21%), and a significantly longer median PFS (7.0 vs 4.3 months) and OS (14.8 vs 12.6) than the Mayo Clinic regimen. Another trial compared the addition of irinotecan (either 80 or 180 mg/m2) with the weekly (AIO regimen) or biweekly (LV5FU2) infusional regimen, respectively: most patients were treated with the LV5FU2 regimen, while 25% of them received the weekly AIO regimen (with or without irinotecan) (CitationDouillard et al 2000). Addition of irinotecan to both regimens significantly increased the proportion of confirmed RR (41% vs 23%), the median PFS (6.7 vs 4.4 months), and OS (17.4 vs 14.1 months).
Subsequently, several randomized trials have compared the combination of oxaliplatin with LV5FU2. In the study of de Gramont et al, the FOLFOX4 (oxaliplatin 85 mg/m2 on day 1 added to LV5FU2 regimen) obtained a significantly greater RR (51% vs 22%), and a longer PFS (9.0 vs 6.2 months) than the LV5FU2 regimen (Citationde Gramont et al 2000). CitationPluzanska et al (2005) reported at the 2005 ASCO Meeting that adding oxaliplatin significantly increased the RR (54% vs 30%) and PFS (8.0 vs 6.0 months) in comparison with infusional 5FU/LV regimens. However, in neither trial was OS significantly prolonged (median, 16.2 vs 14.7 months; and 15.9 vs 15.2 months, respectively). Moreover, CitationGrothey et al (2002) added oxaliplatin to the AIO weekly infusional 5FU regimen, and reported a significantly higher RR (48.3% vs 22.6%) and PFS (7.9 vs 5.3 months) in comparison with the Mayo Clinic regimen. Also in this case median OS was not significantly improved, probably because of effective salvage therapy (19.7 vs 16.1 months).
Capecitabine in combination regimens for treating metastatic CRC
On the basis of such evidences, capecitabine was assessed in combination with oxaliplatin in a large, multicenter, international phase II trial. In a population of 96 patients, recruited with no upper age-limit, the combination of oxaliplatin 130 mg/m2 given on day 1, and capecitabine 2000 mg/m2 daily for 2 weeks, recycling every 21 days, obtained a RR in 55% of patients, with a median PFS of 7.7 months, and a median OS of 19.5 months. This treatment was extremely well tolerated, causing severe hematologic toxicity in a negligible proportion of patients (neutropenia, 7%; thrombocytopenia, 4%), while the main non-hematologic side effects were diarrhea (16%), vomiting 13%, and neuropathy (16%) (CitationCassidy et al 2004). Interestingly, a retrospective analysis on the activity and toxicity of this regimen showed no difference between patients aged less than 65 years and elderly patients (CitationTwelves et al 2005a). Furthermore, a phase II trial specifically addressed the tolerability and activity of the XELOX regimen in elderly (aged ≥70 years) patients. An intra-patient dose escalation of both drugs over the first 3 cycles was planned, starting with absolutely safe initial dosages (oxaliplatin 85 mg/m2, capecitabine 2000 mg/m2 for 2 weeks, recycling every 3 weeks), in order to avoid unexpected toxicity. However, after an interim analysis on the first 35 patients, the design of the study was modified, and the delivery of capecitabine was kept unchanged (2000 mg/m2) over the whole treatment, while oxaliplatin was escalated from an initial dose of 85 mg/ m2 to 130 mg/ m2 over the first 3 cycles. Forty per cent of patients showed a major response, regardless of the schedule employed, and for the whole series of 76 patients, the median PFS was 8.5 months, and the median OS was 14.4 months. The amended schedule was extremely well tolerated, only 7% of patients complaining of severe diarrhea (CitationComella et al 2005a). Comparable results were reported by other investigators with similar combinations of oxaliplatin and capecitabine in phase II trials, regardless of the age of patients (CitationScheithauer et al 2003; CitationComella et al 2005b; CitationFeliu et al 2006).
These findings prompted the activation of prospective, randomized trials to assess the substitution of 5FU with capecitabine in combination with oxaliplatin (). A German trial compared the FUFOX regimen, in which 5FU 2000 mg/m2 (infused over 24 hours), LV 500 mg/m2, and oxaliplatin 50 mg/m2 were given weekly for 4 weeks and 2 weeks of rest, with the CAPOX regimen, in which oxaliplatin 70 mg/m2 on days 1 and 8, and capecitabine 2000 mg/m2 daily for 2 weeks, were recycled every 3 weeks. In this study, in which 476 patients were enrolled, no difference in RR (47% vs 49%), median PFS (7.0 vs 8.0 months) or OS (16.3 vs 17.2 months) were seen between patients treated with CAPOX and FUFOX (CitationArkenau et al 2005).
Table 2 Summary of randomized phase III trials about the combination of capecitabine plus oxaliplatin in metastatic colon cancer
A Spanish trial compared the standard XELOX regimen given every 3 weeks with a weekly 48-hour infusion of 5FU 2250 mg/m2 plus oxaliplatin 85 mg/m2 given biweekly. RR (37% vs 45%) as well as was median PFS (8.9 vs 9.5 months) and OS (18.8 vs 21.2 months) were not significantly different. The XELOX regimen produced a slightly lower occurrence of severe diarrhea (14% vs 24%) (CitationMassuti et al 2006).
In the TREE-1 study, patients were randomly allocated to receive either the mFOLFOX (oxaliplatin 85 mg/m2 and LV 350 mg/m2 on day 1, 5FU 400 mg/m i.v. bolus and 2400 mg/sqm2 i.v. 46-hour infusion) every 2 weeks, the bFOL regimen (oxaliplatin 85 mg/m2 on day 1, and 5FU 500 mg/m2 plus LV 20 mg/m2 i.v. on days 1 and 8, every 2 weeks), or the XELOX regimen every 3 weeks. In this study, 147 patients were enrolled. The RR yielded by each regimen was 43%, 22%, and 35%. The median PFS were 8.7, 6.9, and 5.9 months, while the corresponding median OS were 19.2, 17.9, and 17.2 months, respectively. However, it should be noted that the XELOX regimen produced more frequently a severe dehydration (27%) as opposed to the mFOLFOX (8%) or bFOL regimen (12%), while severe diarrhea had a similar occurrence with all the regimens (31%, 33%, 26%). The safety advantage of the XELOX regimen was limited to the occurrence of neutropenia, which was much lower (15%) than that reported with mFOLFOX (53%) or bFOL (18%). Therefore, when bevacizumab was added to these treatments (5 mg/kg i.v biweekly with mFOLFOX and bFOL regimens, 7.5 mg/kg i.v. triweekly with XELOX) in the second part of the study (TREE-2 study), the dosage of capecitabine in the XELOX was reduced from 2000 to 1750 mg/m2 daily for 2 weeks. Additional 213 patients were included in the TREE-2 study, and the addition of bevacizumab improved all the efficacy parameters in comparison with the previous part of the study (mFOLFOX + bevacizumab: RR, 53%; PFS, 9.9 months, OS, 26.0 months; bFOL + bevacizumab: RR, 41%; PFS, 8.3 months, OS, 20.7 months; XELOX + bevacizumab: RR, 48%; PFS, 10.3 months, OS, 27.0 months). The reduction of capecitabine significantly reduced the occurrence of dehydration (8%) produced by XELOX + bevacizumab in this cohort of patients (CitationHochster et al 2006) ().
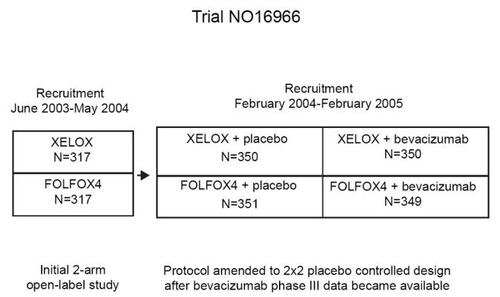
At the 2007 ASCO Gastrointestinal Cancer Symposium, the results of the NO16966 trial were reported. This study was initially aimed at demonstrating the non-inferiority of the XELOX regimen in terms of PFS in comparison with the FOLFOX4 regimen in metastatic CRC. When bevacizumab became available for clinical use, the trial design was amended, and patients were also randomized to receive either bevacizumab (5 mg/kg biweekly with FOLFOX4, or 7.5 mg/kg triweekly with XELOX) or placebo in addition to the assigned regimens (with unchanged dosages) (). This amended design allowed to demonstrate the superiority of the treatments containing bevacizumab in comparison with those including placebo. Patients treated with XELOX, with or without bevacizumab, had a median PFS of 8.0 months in comparison with a median of 8.5 months for patients treated with FOLFOX4 with or without bevacizumab, and the hazard ratio for progression was 1.04 (97.5% CI, 0.93–1.16), demonstrating the non-inferiority of the XELOX treatment. RR (independently confirmed) was 39% for FOLFOX4, and 37% for XELOX. XELOX produced a significantly lower occurrence of severe neutropenia (7% vs 44%), but more severe diarrhea (20% vs 11%) than the FOLFOX4 regimen (CitationCassidy et al 2007). On the other hand, bevacizumab added to FOLFOX4 or XELOX did not increase the RR, which indeed was the same (38%) with bevacizumab or placebo. However, bevacizumab significantly prolonged the overall PFS (from 8.0 to 9.4 months) in comparison with placebo (hazard ratio = 0.83, p = 0.0023). Excluding the occurrence of severe hypertension (3.7% vs 1.2%), toxicity was not significantly worsened by the addition of bevacizumab (CitationSalts et al 2007).
Other investigators assessed the combination of capecitabine with irinotecan in metastatic patients. Bajetta et al explored in a randomized phase II trial two schedules of administration for this combination: a total of 140 patients received capecitabine 2500 mg/m2 daily on days 2 to 15, and irinotecan at a dose of either 300 mg/m2 on day 1 (arm A) or 150 mg/m2 on days 1 and 8 (arm B), recycling every 3 weeks. However, during the course of the study, capecitabine dosage was reduced to 2000 mg/m2/day in both arms, and irinotecan was reduced to 240 mg/m2 (arm A), or to 120 mg/m2 (arm B) in order to decrease the occurrence of severe diarrhea. RR was 47% in arm A, and 44% in arm B, while median PFS was similar in either arm (8.3 and 7.6 months) (CitationBajetta et al 2005). Similarly, the Swiss Group for Clinical Cancer Research randomly assessed two different schedules of irinotecan, either 70 mg/m2 weekly for 5 consecutive weeks (arm A), or 300 mg/m2 (reduced to 240 mg/m2 in the course of the trial) every 3 weeks (arm B), in combination with capecitabine 1000 mg/m2 twice daily, days 1–14 and days 22–35, every 6 weeks. RR was comparable with the two regimens (34% and 25%, respectively). However, median PFS (6.9 vs 9.2 months) and OS (17.4 vs 24.7months) were both in favor of arm B, which also caused less grade 3/4 diarrhea (arm A: 34%, arm B: 19%) (CitationBorner et al 2005).
More recently, the combination of capecitabine and irinotecan has been assessed in some randomized phase III trials. In one such trial, 430 patients were randomly assigned to receive one of 3 regimens: FOLFIRI (biweekly irinotecan 180 mg/m2, LV 400 mg/m2, 5FU 400 mg/m2 i.v. bolus plus 2400 mg/m2 46-hour i.v. infusion); mIFL (irinotecan 125 mg/m2, LV 20 mg/m2 and 5FU 500 mg/m2 for 2 weeks every 3); or CapIRI (irinotecan 250 mg/m2 on day 1 and capecitabine 2000 mg/m2 for 14 days, every 3 weeks). In all arms, patients were also randomized to receive or not celecoxib (a COX-2 inhibitor) given orally at 400 mg bid. The addition of celecoxib had no effect on activity and toxicity of each regimen. However, it is relevant to note that the CapIRI regimen produced an unacceptably higher occurrence of severe diarrhea and dehydration (48% and 19%) than either FOLFIRI (13% and 6%) or mIFL (19% and 7%) regimens. This observation led to the closure of this arm of the trial when bevacizumab was subsequently added (5 mg/kg biweekly, or 7.5 mg/kg triweekly) to the regimens on study. Therefore, in the second part of this trial, 117 patients were randomly allocated to receive either FOLFIRI + bevacizumab or mIFL + bevacizumab. Both regimens showed an increased activity in comparison with that previously reported without bevacizumab (RR was 57% and 69%, and PFS 9.9 and 8.3 months, respectively) () (CitationFuchs et al 2006).
The results of a similar trial of the EORTC were presented at the 2005 ASCO annual meeting: 85 patients were randomized to receive FOLFIRI or CapIRI ± celecoxib. The trial was early closed after this initial accrual due to the occurrence of 8 deaths not due to progressive disease. Six deaths (5 treatment-related) occurred in the CapIRI arm, and 2 deaths (both treatment-related) in the FOLFIRI arm. In addition, 61% of patients starting the CapIRI treatment required dose reduction as opposed to only 7% of the FOLFIRI arm (CitationKöhne et al 2005).
Capecitabine in the primary management of rectal cancer
The management of locally advanced rectal cancer has consistently changed in the last few years. Indeed, there is now a general agreement that a complete resection of the mesorectum (total mesorectal excision, TME) could reduce the risk of local relapse for patients operated with curative intent. However, even when an R0 resection with a TME is performed, a pre-operative short-term radiotherapy (RT) of the pelvis (5 Gy/day for 5 days) has been demonstrated to further reduce the 2-year risk of local failure after surgery in comparison with TME alone (2.4% vs 8.2%) (CitationKapiteijn et al 2001). The benefit of preoperative RT on OS is still debated, but recent meta-analyses on this issue have shown a modest but significant reduction of the overall and cancer-specific mortality for patients receiving RT compared with those treated with surgery alone (CitationCammà et al 2000; CitationColorectal Cancer Collaborative Group 2001).
Table 3 Comparative severe toxicity reported in the TREE-1 and TREE-2 trials (CitationHochster et al 2006)
Following this observation, a German trial has demonstrated that 2 cycles of 5FU 1000 mg/m2 delivered as continuous i.v. infusion for 5 days during the 1st and 5th week of preoperative RT (for a total dose of 50.4 Gy) was able to further reduce the local relapse (6% vs 13%) in comparison with the same postoperative chemo-irradiation treatment, although it produced no survival benefit (CitationSauer et al 2004). Therefore, in many centers, preoperative chemo-radiotherapy is considered the standard treatment for poor-risk rectal cancer, on the basis of a reduced acute and chronic toxicity caused by the preoperative as opposed to the postoperative approach.
Based on this assumption, some investigators have assessed the replacement of 5FU with capecitabine during preoperative RT for rectal cancer. CitationDas et al (2006) have retrospectively compared the safety and efficacy of capecitabine delivered during RT in 89 patients with rectal cancer with those reported in a matched series of 89 patients previously treated with infusional 5FU, reporting a similar low occurrence of grade 3–4 toxicity, and comparable local and distant failure rates. CitationDe Paoli et al (2006) prospectively evaluated such combined treatment, with capecitabine given 825 mg/m2 twice daily during pelvic RT (50.4 Gy in 28 fractions) for high risk rectal cancer. A downstaging was reported in 57% of patients, and a pCR in 24%. Treatment was feasible, with only 6 patients (11%) suffering from grade 3 toxicity. The same combination of capecitabine 825 mg/m2 twice daily and pelvic RT (52.5 Gy) was assessed in 54 patients (51 underwent surgery): 9 patients (24%) achieved a pCR, and 12 patients (24%) a pTmic. Diarrhea occurred in 2% of patients (CitationKhrishnan et al 2006). Finally, CitationKim et al (2006) reported on the comparative activity of 5FU/LV or capecitabine during preoperative RT for rectal cancer (50.4 Gy) in 278 patients. A complete (11.3% vs 16.1%) or nearly complete (12.9% vs 12.9%) tumor regression occurred in similar proportions of patients (Table 5).
Other investigators are now assessing the combination of other active drugs such as oxaliplatin or irinotecan with infusional 5FU or oral capecitabine to primary rectal RT.
CitationGérard et al (2003) have reported on the administration of oxaliplatin 130 mg/m2 plus a 5-day i.v. infusion of 5FU 350 mg/m2 daily and L-LV 100 mg/m2 daily for 2 cycles on the 1st and 5th week of pelvic RT (50 Gy) in 40 patients. This regimen was well tolerated, and no residual tumor was seen in 15% of patients). CitationAschele et al (2005) in a phase I-II study found that the recommended dose for weekly oxaliplatin in addition to 5FU (225 mg/m2 daily in continuous infusion during pelvic RT) was 60 mg/m2. They treated 25 patients with this regimen, and reported a 28% pathologic complete response (pCR) with only mild (grade 3) diarrhea in 16% of them. A similar phase I-II study was also conducted by CitationRyan et al (2006) on behalf of the Cancer and Leukemia Group B: also in this case, the recommended dose for oxaliplatin was 60 mg/m2 weekly in combination with 5FU 200 mg/m2/day as continuous i.v. infusion during pelvic preoperative RT (50.4 Gy). Among 32 patients treated with these dosages in the phase II study, a pCR was reported in 25% of them; however, 12 patients (38%) experienced grade 3 or 4 diarrhea.
The combination of oxaliplatin and capecitabine, delivered during preoperative pelvic RT for poor-risk rectal cancer, has been explored by several investigators in phase I-II trials. All these studies reported encouraging pCR rates, but conflicting results about tolerability. Indeed, CitationRödel et al (2003) conducted a phase I/II study to define the optimal oxaliplatin dose to deliver on days 1 and 8 of a 2-week administration of capecitabine (825 mg/m2 twice daily) for 2 cycles during pelvic RT (50.4 Gy). The recommended dosage for oxaliplatin was 50 mg/m2, and 26 patients were treated with this regimen: severe diarrhea occurred in only 8% of them.
CitationMachiels et al (2005) have treated 40 patients with oxaliplatin 50 mg/m2 weekly for 5 weeks plus capecitabine 825 mg/m2 twice daily during pelvic RT (total dose, 45 Gy); pCR was seen in 14% of patients with such treatment; however, severe diarrhea occurred in 30% of treated patients.
CitationGlynne-Jones et al (2005) reported on a phase II trial assessing the addition of two doses of oxaliplatin 130 mg/m2, 4 weeks apart, during pelvic RT (45 Gy in 5 weeks) associated with a continuous assumption of capecitabine (650 mg/m2 twice daily), followed by surgery. Ninety-five patients were treated, and 85 underwent surgery: a pCR was achieved in 16 (19%) patients, and a Tmic was found at the pathologic examination in additional 21 (25%) patients.
CitationRutten et al (2006) presented the results of the CORE (capecitabine, oxaliplatin, radiotherapy, and excision) study. Eighty-five patients with poor-risk rectal cancer were treated with weekly oxaliplatin 50 mg/m2 and twice daily capecitabine 825 mg/m2, followed by TME, and 6 postoperative cycles of XELOX. These investigators reported a pCR in 11.5% of patients, and stressed the good tolerability of such treatment, with grade 3–4 diarrhea reported in 16% of patients.
A phase II trial published by Chau et al reported the safety and activity of 4 cycles of XELOX (oxaliplatin 130 mg/m2 plus capecitabine 2000 mg/m2 daily for 2 weeks), followed by capecitabine 1650 mg/m2 daily during pre-operative RT in 77 patients affected by rectal cancer, most of whom with poor risk factor for recurrence. Among 67 patients who underwent surgical resection, pT0 was reported in 18 patients (27%), and pathologically negative lymph nodes were found in 29 patients with initial clinical nodal involvement (CitationChau et al 2006). The NSABP R-04 trial is currently comparing, in a 2 × 2 factorial design, the combination of 5FU in continuous i.v. infusion vs capecitabine, with or without oxaliplatin, and preoperative RT for poor risk rectal cancer.
On the other hand, the addition of irinotecan to 5FU during preoperative pelvic RT for locally advanced rectal cancer has been assessed by the Radiation Therapy Oncology Group (RTOG) trial 0012. In this study, 106 patients randomly received either hyperfractionated bid pelvic RT (total dose, 45.6 Gy plus boost of 9.6 Gy) with 5FU (225 mg/m2 daily) continuous i.v. infusion, or a single daily fraction of pelvic RT (total dose, 45 Gy plus boost of 5.4 Gy) with 5FU (225 mg/m2 daily i.v. infusion for 5 days a week) and irinotecan (50 mg/m2 weekly × 4 weeks). The same proportion of pCR (26%) was reported in either arm, and no substantial difference in occurrence and/or severity of toxicity was reported (CitationMohiuddin et al 2006). This observation prompted other investigators to assess the combination of weekly irinotecan 50 mg/m2 with capecitabine during three-dimensional conformal pelvic RT (50.4 Gy). Indeed, CitationHofheinz et al (2005) performed a dose-finding trial for this combination added to pelvic RT. They identified as the dose limiting toxicity the occurrence of severe diarrhea, and recommended a dosage of capecitabine of 500 mg/m2 bid for subsequent evaluation. Thirty-six patients were thereafter treated with this regimen, and 28 patients underwent surgery: a pCR was reported in 5/28 (18%) patients (CitationWilleke et al 2007). Diarrhea was the dose limiting toxicity also in the phase I/II trial of CitationKlautke et al (2006), in which the recommended dose was 750 mg/m2 twice daily for capecitabine, and 40 mg/m2 weekly for irinotecan. In this study, a pCR was reported in 15% of patients. CitationGollins et al (2006) also conducted a phase I/II trial, recommending 60 mg/m2 i.v. weekly for 4 weeks for irinotecan, and 650 mg/m2 twice daily for oral capecitabine; these investigators reported that 9 of 40 (22.5%) patients eligible for resection showed a pCR with this combination.
Table 4 Comparative activity and toxicity of the assessed regimens before and after the addition of bevacizumab (CitationFuchs et al 2006)
Capecitabine in the adjuvant management of colon cancer
For several years, a 6-month treatment with the Mayo Clinic regimen has been considered the standard adjuvant management for patients who underwent curative surgery for high-risk stage II and for stage III colon cancer, because is has been proven to reduce the risk of recurrence and death. This treatment appeared as effective in young as in elderly patients, although older patients are less likely to receive an adjuvant treatment, because of concern regarding tolerability on this group.
Based on the superior RR and improved safety in metastatic patients, capecitabine has also been evaluated in the adjuvant setting. The Capecitabine Adjuvant Chemotherapy for Colon Cancer Trial (X-ACT) evaluated capecitabine 1250 mg/m2 twice daily, from day 1 to 14, every 21 days vs the Mayo Clinic regimen: LV 20 mg/m2 followed by 5FU 425 mg/m2, administered as an i.v. bolus on days 1–5 every 28 days in resected stage III colon cancer. Total treatment duration was 24 weeks. The primary end-point of the trial was to show that disease-free survival is at least as equivalent with capecitabine as with 5FU/LV. This result was clearly met, because the hazard ratio was 0.87 (95% CI, 0.75–1.00). Compared with the targeted upper limit of the CI of 1.20, capecitabine resulted equivalent to 5FU/LV (p < 0.001). Moreover, capecitabine showed a strong trend to superior disease-free survival (p = 0.0528). At 3 years, 3.6% more patients receiving capecitabine were disease-free compared with the 5-5FU/LV group. Adjuvant capecitabine offered significantly superior relapse-free survival (hazard ratio = 0.86, p = 0.0407) vs 5FU/LV. There was also a trend toward superior overall survival with capecitabine (hazard ratio = 0.84, p = 0.0706). The safety of adjuvant capecitabine was greater, with a low rate of treatment-related mortality (0.3%). The only clinical adverse event more commonly seen with capecitabine was the hand-foot syndrome, which, however, was never life threatening (CitationTwelves et al 2005b).
The XELOX regimen has been compared with standard 5FU/LV regimens (Mayo Clinic or Roswell Park) as adjuvant treatment in stage III colon cancer. Safety analysis of this study has recently been published: occurrence of grade ≥3 toxicity was in favor of the XELOX regimen for neutropenia (5.3% vs 10.9%), febrile neutropenia (0.2% vs 3.8%), and severe stomatitis (0.6% vs 79%); however, the XELOX produced more skin (3.6% vs 0.2%) and neurosensory toxicity (8.1% vs 0%) (CitationSchmoll et al 2007).
Given the findings of the MOSAIC trial, showing a 3-year reduced risk of recurrence with a 6-month adjuvant FOLFOX4 treatment in operated stage II and III colon cancer patients (CitationAndré et al 2004), this regimen now represents the new backbone on which to build up new adjuvant strategies. Indeed, on the basis of the above-mentioned safety results of XELOX in the adjuvant setting (CitationSchmoll et al 2007), the AVANT trial is currently evaluating the addition of bevacizumab (biweekly or triweekly) to either FOLFOX4 or XELOX adjuvant treatment in stage II-III colon cancer.
Oral therapy is preferred by patients and is cost-effective
The preference of patients for an oral therapy, provided that it is equally effective as i.v. therapy, has already been reported (CitationLiu et al 1997; CitationBorner et al 2002; CitationTwelves et al 2006). Moreover, several pharmacoeconomic analyses conducted in different countries and from different healthcare perspectives have shown that capecitabine is associated with reduced costs compared with 5FU/LV in both the adjuvant and palliative setting (CitationTwelves et al 2001; CitationCassidy et al 2006; CitationEgginton et al 2006; CitationWard et al 2006). Most cost savings were attributable to the reduced administration costs. These data support the inclusion of capecitabine in the clinical armamentarium for the treatment of metastatic as well as adjuvant CRC. The additional cost of the combination oxaliplatin with capecitabine could be counterbalanced by the lower incidence of some treatment-related adverse events with this regimen in comparison with the FOLFOX4 regimen. Future research should attempt to elucidate the optimal role of bevacizumab in addition to XELOX or FOLFOX in the adjuvant as well in the palliative setting in order to achieve the maximum level of clinical benefit.
References
- AndréTColinPLouvetCSemimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a Randomized TrialJ Clin Oncol200321289690312885807
- AndréTBoniCMounedji-BoudiafLOxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancerN Engl J Med200435023435115175436
- ArkenauHSchmollHKubickaSInfusional 5-fluorouracil/folinic acid plus oxaliplatin (FUFOX) versus capecitabine plus oxaliplatin (CAPOX) as first line treatment of metastatic colorectal cancer (MCRC): Results of the safety and efficacy analysisProceedings American Society of Clinicial Oncology Annual Meeting2005 23, 16s (abstract 3507)
- AscheleCFrisoMLPucciarelliSA phase I-II study of weekly oxaliplatin, 5-fluorouracil continuous infusion and preoperative radiotherapy in locally advanced rectal cancerAnn Oncol2005161140615894548
- BajettaEDi BartolomeoMMarianiLRandomized multicenter phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinomaCancer20051527987
- BornerMMSchoffskiPde WitRPatient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancerEur J Cancer2002383495811818199
- BornerMMBernhardJDietrichDA randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: efficacy, quality-of-life and toxicityAnn Oncol200516282815668285
- CammàCGiuntaMFioricaFPreoperative radiotherapy for resectable rectal cancer. A meta-analysisJAMA200028410081510944647
- CassidyJTaberneroJTwelvesJXELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancerJ Clin Oncol20042220849115169795
- CassidyJDouillardJ-YTwelvesCPharmacoeconomic analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in Dukes’C colon cancer: the X-ACT trialBr J Cancer2006941122916622438
- CassidyJClarkeSDiaz RubioEXELOX vs FOLFOX4: Efficacy results from XELOX-1/NO16966, a randomized phase III trial in first-line metastatic colorectal cancer (MCRC)Proceedings ASCO Gastrointestinal Cancer Symposium2007 abstract 270
- ChauIBrownGCunninghamDNeoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging–defined poor-risk rectal cancerJ Clin Oncol2006246687416446339
- Colorectal Cancer Collaborative GroupAdjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomised trialsLancet2001358129130411684209
- ComellaPNataleDFarrisACapecitabine plus oxaliplatin for the first-line treatment of elderly patients with metastatic colorectal cancer. Final results of the Southern Italy Cooperative Oncology Group (SICOG) trial 0108Cancer2005a104282915948167
- ComellaPMassiddaBPalmeriSBiweekly oxaliplatin combined with oral capecitabine (OXXEL regimen) as first-line treatment of metastatic colorectal cancer patients, A Southern Italy Cooperative Oncology Group phase II studyCancer Chemother Pharmacol2005b56481615902461
- DasPLinEHBhatiaSPreoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: A matched-pair analysisInt J Radiat Oncol Biol Phys20066613788317056196
- de GramontAFigerASeymourMLeucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancerJ Clin Oncol20001829384710944126
- De PaoliAChiaraSLuppiGCapecitabine in combination with preoperative radiation therapy in locally advanced, respectable, rectal cancer: a multicentric phase II studyAnn Oncol200672.651
- DouillardJYCunninghamDRothADIrinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicenter randomised trialLancet20003551041710744089
- EggintonSTappendenPPandorACost-effectiveness of oxaliplatin and capecitabine in the adjuvant treatment of stage III colon cancerBr J Cancer200695119520117031407
- FeliuJEscuderoPLlosaFCapecitabine as first-line treatment for patients older than 70 years with metastatic colorectal cancer: an Oncopaz Cooperative Group studyJ Clin Oncol20052331041115860870
- FeliuJSaludAEscuderoPXELOX (capecitabine plus oxaliplatin) as first-line treatment for elderly patients over 70 years of age with advanced colorectal cancerBr J Cancer2006949697516552438
- FuchsCMarshallJMitchellEA randomized trial of first-line irinotecan/fluoropymidine combinations with or without celecoxib in metastatic colorectal cancer (BICC-C)Proceedings American Society of Clinicial Oncology Annual Meeting2006 24, 18S, (abstract 3506)
- GérardJ-PChapetONemozCPreoperative concurrent chemoradiotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: The Lyon R0–04 phase II trialJ Clin Oncol20032111192412637479
- GillSLoprinziCLSargentDJPooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much?J Clin Oncol200415179780615067028
- Glynne-JonesRSebag-MontefioreDSamuelLSocrates phase II study results: capecitabine (CAP) combined with oxaliplatin (OX) and preoperative radiation (RT) in patients (pts) with locally advanced rectal cancer (LARC) [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2005 23, 16S (abstract 3527)
- GollinsSWMyintSLevineERadiotherapy plus concurrent irinotecan (CPT-11) and capecitabine (CAP) as preoperative downstaging treatment for locally advanced inoperable rectal cancer: A phase I/II study [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2006 24, 18S (abstract 13519)
- GrotheyADeschlerBKroeningHPhase III study of bolus 5-fluorouracil (5-FU)/ folinic acid (FA) (Mayo) vs weekly high-dose 24h 5-FU infusion/FA + oxaliplatin (OXA) (FUFOX) in advanced colorectal cancer (ACRC) [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2002 21, (abstract 512)
- HochsterHSHartLLRamanathanRKSafety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): Final analysis of the TREE-Study [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2006 24, 18S (abstract 3510)
- HoffPMAnsariRBatistGComparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients metastatic colorectal cancer: results of a randomized phase III studyJ Clin Oncol20011922829211304782
- HofheinzR-Dvon Gerstenberg-HelldorfBWenzFPhase I trial of capecitabine and weekly irinotecan in combination with radiotherapy for neoadjuvant therapy of rectal cancerJ Clin Oncol2005231350715684318
- IshikawaTUtohMSawadaNTumor selective delivery of 5-fluorouracil by capecitabine, a new oral fluoropyrimidine carbamate, in human cancerBiochem Pharmacol199855109179605432
- JemalAMurrayTWardECancer statisticsCA Cancer J Clin200555103015661684
- KapiteijnEMarijnenCAMNagtegaalIDPreoperative radiotherapy combined with total mesorectal excision for resectable rectal cancerN Engl J Med20013456384611547717
- KhrishnanSJanjanNASkibberJMPhase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancerInt J Radiat Biol Phys20066676271
- KimDYJungKHKimTHComparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancerInt J Radiat Oncol Biol Phys2006118 Epub ahead of print
- KlautkeGKüchenmeisterUFoitzikTConcurrent chemoradiation with capecitabine and weekly irinotecan as preoperative treatment for rectal cancer: results from a phase I/II studyBr J Cancer2006949768116552435
- KöhneCde GreveJBokemeyerCCapecitabine plus irinotecan versus 5FU/FA/irinotecan +/- celecoxib in the first line treatment of metastatic colorectal cancer: safety results of the prospective multicenter EORTC phase III study 40015 [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2005 23, 16s (abstract 3525)
- LiuGFranssenEFitchMIPatient preferences for oral versus intravenous palliative chemotherapyJ Clin Oncol19971511058996131
- MachielsJ-PDuckLHonhonBPhase II study of preoperative oxaliplatin, capecitabine and external beam radiotherapy in patients with rectal cancer: The RadiOxCape studyAnn Oncol200516189890516219623
- MassutiBGómezASastreJRandomized phase III trial of the TTD Group comparing capecitabine and oxaliplatin (XELOX) vs. oxaliplatin and 5-fluorouracil in continuous infusion (FUFOX) as first line treatment in advanced or metastatic colorectal cancer (CRC) [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2006 24, 18S (abstract 3580)
- MiwaMUraMNishidaMDesign of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissueEur J Cancer1998381274819849491
- MohiuddinMWinterKMitchellERandomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012J Clin Oncol200624650516446336
- PluzanskaAMainwaringPCassidyJFinal results of a randomized phase III study evaluating the addition of oxaliplatin first-line to 5-FU followed by irinotecan at progression in advanced colorectal cancer (LIFE study) [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2005 23, 250s (abstract 3517)
- RödelCGrabenbauerGGPapadopoulosTPhase I/II trial of capecitabine, oxaliplatin, and radiation for rectal cancerJ Clin Oncol200321309810412915600
- RuttenHSebag-MontefioreDGlynne-JonesRCapecitabine, oxaliplatin, radiotherapy, and excision (CORE) in patients with MRI-defined locally advanced rectal adenocarcinoma: Results of an international multicenter phase II study [abstract]Proceedings American Society of Clinicial Oncology Annual Meeting2006 24, 18S (abstract 3528)
- RyanDPNiedzwieckiDHollisDPhase I/II study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B 89901J Clin Oncol20062425576216636336
- SaltzLBCoxJVBlankeCIrinotecan plus fluorouracil and leucovorin for metastatic colorectal cancerN Engl J Med20003439051411006366
- SaltsLBClarkeSDiaz RubioEBevacizumab (Bev) in combination with XELOX or FOLFOX4: Efficacy results from XELOX-1/NO16966, a randomized phase III trial in the first-line metastatic colorectal cancer (MCRC) [abstract]Proceedings ASCO Gastrointestinal Cancer Symposium2007 abstract 238
- SauerRBeckerHHohenbergerWPreoperative versus postoperative chemoradiotherapy for rectal cancerN Engl J Med200435117314015496622
- ScheithauerWKornekGVRadererMRandomized multicenter phase II trial of two different schedules of capecitabine plus oxaliplatin as first-line treatment in advanced colorectal cancerJ Clin Oncol20032113071212663719
- SchmollHJCartwrightTTaberneroJMPhase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancerJ Clin Oncol200725102717194911
- The Meta-Analysis Group in CancerModulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: An updated meta-analysisJ Clin Oncol20042237667515365073
- TwelvesCBoyerMFindlayMCapecitabine (Xeloda) improves medical resource use compared with 5-fluorouracil plus leucovorin in a phase III trial conducted in patients with advanced colorectal carcinomaEur J Cancer20013759760411290435
- TwelvesCButtsCACassidyJCapecitabine/oxaliplatin, a safe and active first-line regimen for older patients with metastatic colorectal cancer: post hoc analysis of a large phase II studyClin Colorectal Cancer2005a5101716098250
- TwelvesCWongANovackiMPCapecitabine as adjuvant treatment for stage III colon cancerN Engl J Med2005b352269670415987918
- TwelvesCGollinsSGrieveRA randomised cross-over trial comparing patient preference for oral capecitabine and 5-fluorouracil/leucovorin regimens in patients with advanced colorectal cancerAnn Oncol200672394516344278
- Van CutsemEFindlayMOsterwalderBCapecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II studyJ Clin Oncol20001813374510715306
- Van CutsemETwelvesCCassidyJOral capecitabine compared with fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III studyJ Clin Oncol200119409710611689577
- Van CutsemEHoffPMHarperPOral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomized, phase III trialsBr J Cancer2004901190715026800
- WardSEKaltenthalerECowanJThe clinical and economic benefits of capecitabine and tegafur with uracil in metastatic colorectal cancerBr J Cancer200695273416804526
- WillekeFHorisbergeKKraus-TiefenbacherUA phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri-RT) as Neoadjuvant treatment of locally advanced rectal cancerBr J Cancer200796912717325705