Abstract
Chronic congestive heart failure (HF) occurs in infants and children as a result of systemic ventricle incompetence. Neurohormonal activation is thought to be the main consequence of cardiac pump failure and cause of further worsening. Several large multicenter randomized trials have demonstrated that beta-adrenergic blocking agents can improve ventricular ejection fraction, symptoms, and survival in adults with chronic congestive HF. Current literature about pediatric HF is very scarce. The only large, multicenter, randomized, placebo-controlled pediatric trial failed to demonstrate any beneficial effect of beta-blockers in infants and children with chronic HF. Other small-size reports showed significant improvement in ejection fraction and/or clinical outcomes. The HF pediatric population is characterized by wide heterogeneicity regarding causes, underlying cardiac disease, drug pharmacokinetics, and interactions, which may account for divergences. Further large-scale studies are needed to elucidate the optimal use (indications and dosages) of beta-blockers in the management of HF in children, with particular attention to the underlying cardiac disease.
Introduction
Chronic congestive heart failure (HF) is an ongoing problem in pediatric patients with cardiac disease, characterized by a high risk for morbidity and mortality. The physiopathological mechanisms of HF have been widely explored in adults (CitationLowes et al 1999; CitationFrancis 2001; CitationBuchhorn et al 2003). Numerous trials have demonstrated the beneficial impact of newer agents on prognosis and survival in the adult HF population (CitationLechat et al 1998). Only scarce literature is available regarding both mechanisms and treatment of HF in the pediatric population. Most of the practice in the management of HF in children is drawn from adult experience. In particular, the advantage of beta-adrenergic receptor antagonists in the pediatric HF population is still a matter of discussion.
The aims of this review are to give an overview of the mechanisms and causes of HF in children, and to assess current knowledge about efficacy and tolerability of beta-receptor antagonist agents in the treatment of pediatric HF. This review will focus on chronic HF due to systemic ventricle systolic dysfunction.
Congestive HF
Definition
Congestive HF is defined as inadequate oxygen delivery by the heart or the circulatory system to meet the demands of the body. It occurs when the compensatory mechanisms of the body are overcome (CitationFrancis 2001). There is a tremendous heterogeneicity regarding the age, the mechanisms, the causes, and the manifestations of HF in children (CitationO’Laughlin 1999; CitationKay et al 2001).
Oxygen delivery is the product of oxygen content in the blood and cardiac output. Oxygen content is the arterial oxygen saturation and cardiac output is the product of heart rate and stroke volume; the latter is a result of preload, afterload, and contractility conditions of the heart. Any alteration of one (or more) these three components may lead to the occurrence of HF.
Incidence
The incidence of HF in children depends on the underlying cardiac disease and the age of the patient. The annual incidence of HF due to cardiomopathy in the first year of life is as high as 4 cases per 100,000 live births (CitationO’Laughlin 1999; CitationKay et al 2001). It seems to be 16 times as high in patients less than 1 year old than in those more than 1 year. The prevalence of HF among patients with structural heart defects is unknown. Failure of the systemic ventricle may occur in patients with systemic right ventricle who had undergone Mustard or Senning operation (atrial baffle switch correction of transposition of the great vessels), or in those with long-term Fontan-type palliation and functionally single ventricle (total cavo-pulmonary derivation).
Causes of HF in children
The causes of HF in children are very heterogeneous (CitationKay et al 2001). Congestive HF due to left to right shunts and/or left heart outflow tract obstruction is mostly accessible to palliative or reparative surgery (CitationAuslender and Artman 2000).
Cardiomyopathy is the main cause of left ventricle failure. In these cases, myocardial dysfunction may be related to myocarditis or anthracycline toxicity or even metabolic diseases, or may be idiopathic and sometimes from genetic inheritance.
Of highest concern are the cases with chronic HF due to dysfunction of the systemic functional ventricle in the context of congenital heart disease, either left ventricle or right ventricle or single ventricle. Failure of the systemic ventricle due to congenital structural abnormalities of the heart is an unique feature of the pediatric HF population. Not only left ventricle, but also systemic right or single ventricle dysfunction may be involved in the mechanisms of HF in children. Failure of reparative surgery, of Fontan-type single ventricle physiology, or of an overworked systemic right ventricle are crucial issues to address (CitationKay et al 2001).
Pathophysiological mechanisms of HF in children
Several mechanisms are activated to compensate for impaired cardiac output. HF results from inadequate tissue oxygen delivery and develops when the compensatory mechanisms are overhelmed or as a consequence of these mechanisms (CitationO’Laughlin MP 1999; CitationFrancis 2001; CitationKay et al 2001).
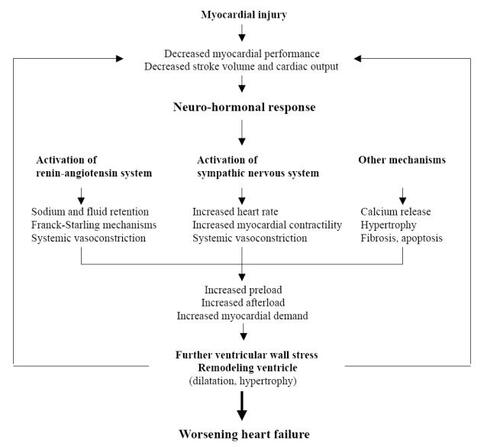
The adaptative mechanisms aim to maintain perfusion of vital organs through: 1) maintenance of systemic pressure by vasoconstriction, 2) restoration of cardiac output by increasing heart rate, contractility, and extracellular volume ().
The neurohormonal activation is thought to be the main adaptative mechanism in HF, but it aggravates the HF through detrimental consequences of activation (CitationHoch and Netz 2005).
In this setting, the activation of the renin-angiotensin system stimulates aldosterone and angiotensin II production. Subsequent salt and water retention results in increased preload and angiotensin-induced peripheral vasoconstriction in increased afterload, in order to compensate for low cardiac output. Volume expansion is effective because increased venous return results in elevation of end-diastolic volume. The Franck Starling mechanism subsequently leads to dilatation of the ventricular cavity which aims to enhance cardiac stroke volume. Additional activation of the sympathic nervous system exacerbates the production of catecholamines and the stimulation of cardiac beta-receptors. Increases in myocardial contractility, heart rate, and wall stress are the main consequences of activation of the sympathetic nervous system. Sustained cardiac adrenergic activation results in desensitization of beta-adrenergic signal transduction mechanisms, downregulation of beta-receptor, and direct damage to cardiac myocytes. Failing hearts show decreased catecholamine sensitivity and impaired beta-adrenergic-receptor density (CitationHoch and Netz 2005).
Other mechanisms combine to aggravate the deleterious consequences of neurohormonal activation, ie:
– Besides its diuretic effect, aldosterone may enhance interstitial collagen and cause fibrosis in long-term HF, leading to alteration of the diastolic function of the heart.
– Angiotensin II is thought to induce myocyte apoptose.
– Abnormal gene expression leads to change in the actin, myosin, and collagen isoforms.
– Lastly, intracellular calcium release may influence systolic and also diastolic function of the heart.
The combination of the above mechanisms results in remodeling of the cardiac structures, through dilation of the cavities, hypertrophy, cell death, decrease in capillary density, and mitochondrial deficiency.
More recent literature provides new insight about the mechanisms of HF. In particular, pulmonary and systemic congestion seem to be important predictors of both mortality and morbidity (CitationDe Luca et al 2006). Elevated left ventricular filling pressures increase pulmonary and right ventricle pressures resulting in pulmonary and systemic congestion. This “hemodynamic congestion” may have deleterious effects on subendocardial coronary perfusion and drainage from coronary veins with subsequent diastolic dysfunction (CitationGeorghiade et al 2006). Pulmonary wedge pressure may be used as a surrogate marker of disease progression (CitationChen and Schrier 2006).
Treatment of HF in children
The rational management of chronic HF in pediatric patients requires comprehensive knowledge of the pathophysiological mechanisms and must be individualized according to age and underlying cardiac disease (Auslander and Artman 2000; CitationClark 2000; CitationRoss 2001).
The beneficial effect of diuretics to reduce congestion and fluid retention is widely accepted. Aldosterone antagonists in association with furosemide not only compensate for potassium loss, but also may prevent fibrosis as a consequence of chronic HF and activation of the renin-angiotensin system.
Several trials have demonstrated the significant benefit of angiotensin converting enzyme inhibitors on survival in adults with chronic HF. These agents can prevent cardiac remodeling through reduction of angiotensin II and aldosterone effects, and decrease myocardial wall stress by decreasing cardiac afterload (CitationStern et al 1990).
There is no evidence that digoxin may improve survival in patients with chronic HF (CitationShaddy 2001). Low dose to achieve levels ranging from 0.5 to 0.8 ng/mL might, however, be beneficial (CitationClark 2000; CitationHoch and Netz 2005). Conversely, the inotropic effect of digoxin is likely to have a deleterious effect by increasing myocardial demand (CitationRoss 2001).
New advances in the treatment of chronic HF in adults have highlighted the advantages of beta-blockade strategy to improve prognosis (CitationQuaife et al 1997; CitationLowes et al 1999). It is currently accepted that third-generation beta-blocker agents like the non-selective carvedilol can affect morbidity and mortality in the adult population (CitationColucci et al 1996; CitationPacker et al 1996; CitationLechat et al 1998). Experience in the pediatric population is still limited and drawn from reported research on adults. Recent reports have brought divergent insights into the beneficial effects of beta-blockade strategy in pediatric HF patients (CitationShaddy 2000, Citation2001).
Beta-blocker agents in chronic congestive HF
Mechanisms of action
The mechanisms of action of beta-blockers agents are not yet clearly defined and probably impact on the neurohormonal component of HF, mainly on the sympathetic activation of chronic HF. Through decreasing the sympathetic activation of chronic HF, they enable a decrease in heart rate and lower myocardial demand.
Studies in adults have demonstrated reversal myocardial remodeling (decreased ventricular volumes and spherical shape) with concurrent improved ejection fraction and shortening fraction (CitationSackner-Bernstein and Mancini 1995; CitationBristow 1997). Not all the beta-receptor antagonists enable reversal of neurohormonal stimulation. The third-generation non-selective carvedilol can achieve this. It provides an additional α-adrenergic receptor blockade responsible for beneficial afterload lowering. From the COMET randomized double-blind trial results, carvedilol had superior hemodynamic effects and reduction of all-cause mortality compared with metoprolol. These data support the advantage of a-adrenergic receptor blockade (CitationPoole-Wilson et al 2003; CitationMetra et al 2005) and were confirmed by experimental study (CitationNikolaidis et al 2006).
The primary mechanism of these agents is to prevent and reverse adrenergically mediated myocardial dysfunction and remodeling (chamber dilation and assumption of a more spherical shape) (CitationSackner-Bernstein and Mancini 1995; CitationBristow 1997; CitationSabbah 1999). Other mechanisms of action have been proposed for the beneficial effects of third-generation beta-blockers in HF, including upregulation of beta-receptors, decreased stimulation of other neurohormonal systems, antiarrhythmic effects, coronary vasodilation, negative chronotropic effects, antioxidant effects, and improved myocardial energetics. In particular, carvedilol has an antioxidative effect that may inhibit catecholamine ability to generate oxygen free radicals in the myocardium (CitationFeuerstein et al 1997; CitationKaye et al 2001; CitationNakamura et al 2002). Both carvedilol and metoprolol have antioxydant and antiapoptotic actions, which are likely to be important in the treatment of HF (CitationKawai et al 2004). However, carvedilol but not metoprolol was found to inhibit the calcium-dependent toxic oxidant species and to increase oxygen consumption (CitationKametani et al 2006). Moreover, the chronotopic effect of beta-blockade was shown to be one of the major determinants of the restoration of contractile function, as demonstrated in an experimental study (CitationNagatsu et al 2000). The impact of beta-blockers on calcium release channel function may also play a significant role in improving myocardial performance (CitationReiken et al 2003). Lastly, carvedilol may inhibit endothelin-1 synthesis in vitro and prevent vascular smooth muscle cell proliferation and intimal migration after vascular injury.
Experience in adults
Most knowledge about beta-blockers efficacy in chronic HF is drawn from randomized trials performed in adults.
The Metoprolol in Dilated Cardiomyopathy (MDC) trial was the first major multicenter, randomized trial of beta-blockers in patients with HF (CitationWaagstein et al 1993). In this study, 383 patients with idiopathic dilated cardiomyopathy were randomized to receive either metoprolol or a placebo. Patients who received metoprolol had increased ejection fractions and exercise times compared with placebo. The Cardiac Insufficiency Bisoprolol Study (CIBIS) compared the effects of bisoprolol with placebo in 641 adults with ischemic and non-ischemic left ventricular dysfunction (CitationCIBIS Investigators and Committees 1994), and showed a 20% reduction in mortality in the bisoprolol group. In 1996, one study demonstrated a 65% reduction in mortality in the carvedilol group compared with placebo (CitationPacker et al 1996). The Multicenter Oral Carvedilol Heart Failure Assessment (MOCHA) randomized patients with HF to receive placebo or carvedilol (CitationBristow et al 1996). In this study, carvedilol was associated with dose-related improvements in left ventricular function and survival.
Subsequent trials with metoprolol (CitationMerit-HF 1999; CitationHjalmarsen et al 2000) and bisoprolol (CitationCIBIS-II 1999) showed similar survival benefits in adults with HF. CIBIS-II included 2647 patients in NYHA class II or IV with left ventricular fraction of 35% or less receiving diuretics with inhibitors of angiotensin-converting enzyme, who were randomly assigned to receive placebo or bisoprolol: all-cause mortality was significantly lower in the treated group (11.8%) compared with placebo (17.3%). Similarly Merit-HF enrolled 3991 patients in NYHA class II to IV, with ejection fraction of 40% or less, to receive either metoprolol or placebo, in addition to standard therapy: results also showed a significant reduction of all-cause mortality (7.2% vs 11%).
From these large-scale randomized double-blind trials, beta-blockers agents were recommended for the treatment of mild to moderate HF, excluding NYHA class IV cases. The COPERNICUS trial (CitationPacker et al 2002) further entered patients with more severe HF: 2289 patients in NYHA class IV, with ejection fraction less than 25%, were randomized to receive either placebo or carvedilol for an average of 10.4 months. The results showed a significant reduction in the number and duration of hospitalizations, and in the occurrence of serious adverse events (including clinical deterioration, sudden death, arrhythmias, and cardiogenic shock).
Experience in children
summarizes the current literature about the use of beta-blockers in the pediatric HF population.
Table 1 Reported studies about beta-receptor antagonists in pediatric heart failure patients
First experience with beta-receptor antagonist agents was reported by CitationShaddy et al (1999) with the use of metoprolol in patients with dilated cardiomyopathy. The authors reviewed data from 3 centers. Fifteen patients aged 8.6 ± 1.3 years and treated over the 1986 to 1997 period were included in this study. They received conventional therapy for 22.5 ± 9 months, and subsequently a mean dose of 1.1 ± 0.1 mg/kg/day metoprolol. Eleven patients were responders and 4 non-responders; mean follow-up was 23.2 ± 7 months. Responders showed a significant improvement in ejection fraction and shortening fraction, while there was no change under conventional therapy (before initiation of beta-blockers). Eight of 11 responders also improved their NYHA class. Since the pre-beta-blocker period was prolonged enough, the authors concluded that improvement was more likely related to the beneficial effect of beta-blockers, rather than to spontaneous recovery of left ventricular function.
With reference to adult trials (CitationLechat et al 1998), carvedilol was highlighted as the beta-blocker of choice to treat chronic HF (CitationSpicer 2001). CitationBruns et al (2001) first reported multicenter (6 centers) retrospective experience with the use of carvedilol in pediatric HF patients, to assess efficacy, safety, and tolerability. The data from 46 patients (aged 3 months to 19 years) with congestive HF due to dilated cardiomyopathy (80%) or chronic heart disease (20%) were collected and analyzed. Third-month evaluation showed improvement in both clinical NYHA class and shortening fraction. Severe adverse outcomes occurred in 30% (death, transplantation, mechanical ventricular support). This study, although limited by retrospective analysis, was the first report of consistent data about carvedilol use in pediatric patients with chronic HF. Since heart transplantation is the only final option in cases with uncontrolled HF, and facing the shortage of donors and serious post-transplant difficulties, one major advantage of beta-blockers is that they enable heart transplant to be delayed. CitationAzeka et al (2002) showed that carvedilol may even allow delisting patients from the waiting list.
Two further retrospective studies also confirmed the beneficial effect of beta-blockers on functional outcomes and echo Doppler left ventricle function (CitationWilliams et al 2002; CitationRusconi et al 2004). One main issue is the difficulty of differentiating beta-blocker effect from the natural favorable evolution of the disease. Left ventricle has been shown to spontaneously recover in dilated cardiomyopathy or myocarditis or after surgery of abnormal origin of the left main coronary artery from the pulmonary artery. Irreversible systemic ventricle dysfunction should not be assumed too soon. Two years is probably the optimal threshold for myocardial function to recover; however, follow-up does not exceed 6 months in most of the available published studies.
Data from prospective studies about carvedilol in children with ventricular dysfunction are very limited. One large multicenter, prospective, double-blind, placebo-controlled study was conducted by CitationShaddy et al (2002). Patients aged 0–17 years were included, diagnosed with ejection fraction less than 40% at least 4 weeks prior to inclusion, and treated with conventional therapy (angiotensin converting enzyme inhibitors with one or more other medications). Patients were designed to receive either placebo or carvedilol, and were followed-up over an 8-month period. Primary end-point was a composite of clinical outcomes, determined as worsened, improved, or unchanged status (CitationShaddy et al 2002). Fifty-five patients were included in the placebo group and 106 in the carvedilol group, recruited from 20 centers. Results showed no difference at 8 months between placebo and treated groups for the primary end-point. This study highlighted a higher-than-expected placebo response rate (CitationCleland et al 2006). Several conclusions can be drawn from these results: 1) the heterogeneicity of the population regarding cardiac disease and age of the patients may have led to excessive disparities; 2) we hypothesize that systemic right or single ventricle may not act like left ventricle in response to beta-blockers; 3) neonates have different cardiac physiology, in particular lower myocardial compliance, that may have influenced the results; 4) cases may have been included that would have recovered spontaneously (myocarditis, ischemic injury in coronary artery disease); and 5) the length of follow-up between diagnosis and onset of beta-blockers may have been too short. Conversely, length of follow-up after onset of beta-blockers may also be too short to draw conclusions on the effectiveness of these agents compared with conventional therapy.
Most recently, CitationBlume et al (2006) reported their results of a single-arm protocol of carvedilol in children with ventricular dysfunction lasting for at least 3 months on maximal medical treatment including angiotensin converting enzyme inhibitors. Twenty carvedilol-treated patients were recruited according to clinical and echocardiographic criteria, and were compared with historical matched controls. Primary end-point was echocardiographic parameters of function measured at the sixth month. This study showed a significant increase in ejection fraction in the treated group with dilated cardiomyopathy. No conclusion could be drawn in the group with congenital heart disease, although some cases experienced echocardiographic improvement similar to patients with dilated cardiomyopathy.
In summary, though beta-blockers have been proven to affect survival in adults, such an evidence is not yet available in the pediatric population. Current practice has nevertheless increasingly added beta-blockers to the armamentarium, in an attempt to optimize therapy for HF in children.
Only limited experience has been reported, mostly from retrospective analysis of heterogeneous data. Prospective studies are very scarce and results are still a matter of discussion. The only large-scale, prospective, double-blind and placebo-controlled study failed to demonstrate the efficacy of carvedilol on a composite end-point of clinical outcomes.However, one may intuitively predict some beneficial impact of these agents in pediatric HF patients. Further progress will be needed to assess this issue.
Current published experience highlights several important points:
– The heterogeneicity of anatomical and pathophysiological factors involved in the development of HF in children. A unique feature of pediatrics is the group of children with congenital heart disease resulting in ventricular dysfunction of a systemic right or single ventricle. Many factors play a role to maintain cardiac output in chronic heart disease with systemic ventricle dysfunction, in particular sinus node (CitationBullinga et al 2005). Moreover chronic hypoxemia and ischemia (as in adults) probably have different pathophysiological effects on myocardial function (CitationBuchhorn et al 2003; CitationGiardini et al 2003).
– The age of the patients that may influence myocardial compliance, or even pharmacokinetics (CitationLäer et al 2002) and drug interference (CitationRatnapalan et al 2003).
– The timing of treatment initiation with beta-blockers. Early onset might prevent ventricular remodeling, whereas late a onset strategy might help to assess potential spontaneous recovery of cardiac function (in the context of postoperative injury or myocarditis, or coronary artery anomaly).
– The length of follow-up after onset of beta-blocker treatment should be long enough to better assess efficacy.
– The difference in HF symptoms between children and adults. Pediatric patients usually do not present with classic symptoms of HF; therefore, relying on clinical symptoms may be not sensitive enough to assess.
Tolerability
Beta-blocker agents have been widely and safely used in children to prevent acute hypoxemia or supraventricular tachycardia, or to treat systemic hypertension. Carvedilol is the gold standard for treatment of HF. It is a non-selective beta-blocker agent with α-adrenergic effect. Despite its impact on systemic resistance, it is usually well tolerated when administred through upgrading doses (CitationKrum et al 2000). Discontinuation of angiotensin converting enzyme inhibitors or of beta-blockers because of low blood pressure is rarely required.
Conclusion
Although no evidence can be drawn from the literature, it is logical to conclude that beta-receptor antagonists may provide consistent improvement in myocardial function, clinical outcomes, and survival in the pediatric HF population. We suggest that further studies are needed to prove carvedilol efficacy in children and select patients who would better benefit from it. Selection of an homogeneous group and longer follow-up would probably help to enhance the power of such studies.
References
- AuslenderMArtmanMOverview of the management of pediatric heart failureProgr Pediatr Cardiol20001123141
- AzekaEFranchini RamiresJABocchiEADelisting of infants and children from the heart transplantation waiting list after Carvedilol treatmentJ Am Coll Cardiol2002402034812475466
- BlumeEDCanterCESpicerRProspective single-arm protocol of carvedilol in children with ventricular dysfunctionPediatr Cardiol2006273364216596434
- BristowMRGilbertEMAbrahamWTCarvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA InvestigatorsCirculation1996942807168941106
- BristowMRMechanism of action of beta-blocking agents in heart failureAm J Cardiol19978026L40L
- BrunsLAKichuk ChrisantMLamourJMCarvedilol as therapy in pediatric heart failure: An initial multicenter experienceJ Pediatr20011385051111295713
- BuchhornRHulpke-WetteMRuschexskiWEffects of therapeutic beta blockade on myocardial function and cardiac remodelling in congenital heart diseaseCardiol Young200313364312691286
- BullingaJRAlharethiRSchramMSChanges in heart rate variability are correlated to hemodynamic improvement with chronic CARVEDILOL therapy in heart failureJ Card Fail200511693916360965
- ChenHHSchrierRWPathophysiology of volume overload in acute heart failure syndromesAm J Med200611912 Suppl 1S11617113395
- CIBIS Investigators and CommitteesA randomized trial of beta-blockade in heart failure The Cardiac Insufficiency Bisoprolol Study_CIBISCirculation1994901765737923660
- CIBIS-IIThe cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trialLancet199935391310023943
- ClarkBJ3rdTreatment of heart failure in infants and childrenHeart Dis200023546111728282
- ClelandJGFColettaAPNikitinNPClinical trials update from the American College of Cardiology: darbepoetin alfa, ASTEROID, UNIVERSE, paediatric carvedilol, UNLOAD and ICELANDEur J Heart Fail20068326916698503
- ColucciWSPackerMBristowMRfor the Carvedilol Heart Failure Study GroupCarvedilol inhibits clinical progression in patients with mild symptoms of heart failureCirculation199694280068941105
- De LucaLAbrahamWTFonarowGCCongestion in acute heart failure syndromes: importance of early recognition and treatmentRev Cardiovasc Med20067697416915125
- FeuersteinGZBrilARuffoloRRJrProtective effects of carvedilol in the myocardiumAm J Cardiol19978041L45L
- FrancisGSPathophysiology of chronic heart failureAm J Med2001110Suppl 7A37S46S11334774
- GheorgiadeMFilippatosGDe LucaLCongestion in acute heart failure syndromes: an essential target of evaluation and treatmentAm J Med200611912 Suppl 1S310
- GiardiniAFormigariRBronzettiGModulation of neurohormonal activity after treatment of children in heart failure with carvedilolCardiol Young200313333614694952
- HjalmarsonAGoldsteinSFagerbergBEffects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF). MERIT-HF study groupJAMA2000283129530210714728
- HochMNetzHHeart failure in pediatric patientsThorac Cardiov Surg200553Suppl 2S129S134
- KametaniRMiuraTHaradaNCarvedilol inhibits mitochondrial oxygen consumption and superoxide production during calcium overload in isolated heart mitochondriaCirc J200670321616501300
- KawaiKQinFShiteJImportance of antioxidant and antiapoptotic effects of β-receptor blockers in heart failure therapyAm J Physiol Heart Circ Physiol2004287H10031215105169
- KayJDColanSDGrahamTPCongestive heart failure in pediatric patientsAm Heart J2001142923811685182
- KayeDMJohnstonLVaddadiGMechanisms of carvedilol action in human congestive heart failureHypertension20013712162111358931
- KrumHNinioDMacDonaldPBaseline predictors of tolerability to carvedilol in patients with chronic heart failureHeart200084615911083738
- LäerSMirTSBehnFCarvedilol therapy in pediatric patients with congestive heart failure: A study investigating clinical and pharmacokinetic parametersAm Heart J20021439162212040358
- LechatPPackerMChalonSClinical effects of beta-adrenergic blockade in chronic heart failure : a meta-analysis of double-blind, placebo-controlled randomized trialsCirculation1998981184919743509
- LowesBDGillEAAbrahamWTEffects of carvedilol on left ventricular mass, chamber geometry and mitral regurgitation in chronic heart failureAm J Cardiol1999831201510215284
- Merit-HFEffects of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF)Lancet19993532001710376614
- MetraMTorp-PedersonCSwedbergKInfluence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trialEur Heart J20052622596816040619
- NagatsuMSpinaleFGKoideMBradycardia and the role of β-blockade in the amelioration of left ventricular dysfunctionCirculation2000101653910673258
- NakamuraKKusanoKNakamuraYCarvedilol decreases elevated oxidative stress in human failing myocardiumCirculation200210528677112070115
- NikolaidisLAPoornimaIParikhPThe effects of combined versus selective adrenergic blockade on left ventricular and systemic hemodynamics, myocardial substrate preference, and regional perfusion in conscious dogs with dilated cardiomyopathyJ Am Coll Cardiol20064718718116682315
- O’LaughlinMPCongestive heart failure in childrenPediatr Clin North Am1999462637310218074
- PackerMBristowMRCohnJNThe effects of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study GroupN Engl J Med19963341349558614419
- PackerMFowlerMBRoeckerEBEffects of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) studyCirculation200210621949912390947
- PackerMColucciWSSackner-BernsteinJDDouble-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure. The PRECISE Trial. Prospective randomized evaluation of carvedilol on symptoms and exerciseCirculation199694279398941104
- Poole-WilsonPASwedbergKClelandJGComparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol european trial (COMET): randomised controlled trialLancet200336271312853193
- QuaifeRAGilbertEMChristianPEEffects of carvedilol on systolic and diastolic left ventricular performance in idiopathic dilated cardiomyopathy or ischemic cardiomyopathyAm J Cardiol199778779848857482
- RatnapalanSGriffithsKMoldovan CosteiADigoxin-carvedilol interactions in childrenJ Pediatr2003142572412756393
- ReikenSWehrensXHTVestJAβ-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failureCirculation200310724596612743001
- RossRDMedical management of chronic heart failure in childrenAm J Cardiovasc Drugs20011374414728050
- RusconiPGomez-MarinORossique-GonzalezMCarvedilol in children with cardiomyopathy: 3-year experience at a single institutionJ Heart Lung Transpl2004238328
- SabbahHNThe cellular and physiologic effects of beta blockers in heart failureClin Cardiol199922Suppl 2V162010526699
- Sackner-BernsteinJDManciniDMRationale for treatment of patients with chronic heart failure with adrenergic blockadeJ Am Med Assoc199527414627
- ShaddyRECurtinELSowerBThe pediatric randomized carvedilol trial in children with chronic heart failure: Rationale and design for The Pediatric Randomized Carvedilol Trial in Children with Heart Failure StudyAm Heart J2002144383912228773
- ShaddyRETaniLYGiddingSSBeta-blocker treatment of dilated cardiomyopathy with congestive heart failure in children: a multi-institutional experienceJ Heart Lung Transpl19991826974
- ShaddyREBeta-adrenergic blockers in the treatment of pediatric heart failureProgr Pediatr Cardiol2000121138
- ShaddyREOptimizing treatment for chronic congestive heart failure in childrenCrit Care Med200129SupplS237S24011593067
- SpicerRLCarvedilol – new dimension in pediatric heart failure therapyJ Pediatr2001138457811295704
- SternHWeilJGenzTVogtWCaptopril in children with dilated cardiomyopathy: acute and long-term effects in prospective study of hemodynamic and hormonal effectsPediatr Cardiol1990112282406705
- WaagsteinFBristowMRSwedbergKBeneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy_MDC. Trial Study GroupLancet1993342144167902479
- WilliamsRVTaniLYShaddyREIntermediate effects of treatment with metoprolol or carvedilol in children with left ventricular systolic dysfunctionJ Heart Lung Transpl2002219069